Abstract
To test the impact of increased mitochondrial oxidative stress as a mechanism underlying aging and age-related pathologies, we generated mice with a combined deficiency in two mitochondrial-localized antioxidant enzymes, Mn superoxide dismutase (MnSOD) and glutathione peroxidase-1 (Gpx-1). We compared life span, pathology, and oxidative damage in Gpx1−/−, Sod2+/−Gpx1+/−, Sod2+/−Gpx1−/−, and wild-type control mice. Oxidative damage was elevated in Sod2+/−Gpx1−/− mice, as shown by increased DNA oxidation in liver and skeletal muscle and increased protein oxidation in brain. Surprisingly, Sod2+/−Gpx1−/− mice showed no reduction in life span, despite increased levels of oxidative damage. Consistent with the important role for oxidative stress in tumorigenesis during aging, the incidence of neoplasms was significantly increased in the older Sod2+/−Gpx1−/− mice (28–30 months). Thus, these data do not support a significant role for increased oxidative stress as a result of compromised mitochondrial antioxidant defenses in modulating life span in mice and do not support the oxidative stress theory of aging.
Keywords: Oxidative stress, Longevity
THE free radical or oxidative stress theory of aging has been among the most widely accepted theories in aging research (1). The theory proposes that the physiological declines associated with aging are due to an accumulation of oxidative damage over the life span of the organism. Despite the attractive logic of this theory, existing evidence in direct support of this theory has remained essentially correlative. One approach to probe this theory has been to use animal models with altered antioxidant defenses. A variety of animal models ranging from invertebrates to mammals with deficient or enhanced expression of major components of the antioxidant defense system have been studied (2). Overexpression of some primary antioxidant genes has been shown to extend life span in lower organisms including Caenorhabditis elegans and Drosophila (3–7); yet, altering the expression of these genes does not universally increase life span in mouse models. For example, studies from our laboratory and others have shown that increased expression of the major antioxidant enzymes, catalase, Cu/Zn superoxide dismutase (SOD), and MnSOD alone or in combination, does not extend maximum life span, which is in contradiction to the oxidative stress theory of aging (8–10).
Mice with genetically reduced levels of individual components of the antioxidant defense system have also been studied, including SOD (MnSOD and Cu/ZnSOD; 11–13), glutathione peroxidase (Gpx-1, Gpx-2, and Gpx-4; 14–16), catalase (17), peroxiredoxin (18,19), and thioredoxin (18,20). Complete ablation of individual components of antioxidant defense often leads to embryonic lethality (e.g., Gpx4 (21), Trx2 (20), MnSOD (11)), but surprisingly, a reduction of one allele (∼50% loss in activity) in heterozygous knockout mice (Sod1+/−, Sod2+/−, Gpx4+/−) does not result in reduced life span (22–24). Homozygous deletion of Cu/ZnSOD in Sod1−/− mice does in fact lead to a reduction in life span (∼30%); however, this reduction in life span is associated with a high incidence of hepatocellular carcinoma, and thus, the impact on aging per se cannot readily be interpreted (24). In light of these studies, we were interested in determining whether disrupting oxidative stress specifically within the mitochondrial compartment would alter oxidative damage and reduce life span.
MnSOD and Gpx-1 are two primary oxidative stress defense enzymes in mitochondria. MnSOD is critical for detoxifying superoxide anions generated by the electron transport chain in the mitochondrial matrix. The hydrogen peroxide formed by the dismutation of superoxide is detoxified by glutathione peroxidase and other peroxidases such as peroxiredoxins in the mitochondria. Homozygous deletion of MnSOD is either embryonic or neonatal lethal, depending on the genetic background of the mouse strain (11,12,25). Although heterozygous MnSOD mice are viable and not short lived, these mice have altered mitochondrial function, increased sensitivity to apoptosis, increased cancer incidence, and susceptibility to oxidative stress (23,26,27). In contrast, homozygous deletion of Gpx-1 in mice does not affect embryonic development, and Gpx-1 null mice develop without an obvious deleterious phenotype (14,28). Adult Gpx-1-deficient mice show decreased mitochondrial function in liver (14), decreased resistance to oxidative stress (29), and defects in cell proliferation and differentiation in certain tissues (30,31). Because MnSOD and Gpx-1 sequentially detoxify superoxide anions generated in the mitochondria, deletion of either one of them would be predicted to disturb the balance between the generation of reactive oxygen species (ROS) and ROS inactivation. Deficiencies in both enzymes would in theory lead to even greater ROS accumulation and damage to the cell. In support of this prediction, we previously showed that deficiency of both MnSOD and Gpx-1 in young adult Sod2+/−Gpx1−/− mice resulted in a dramatic increase in sensitivity to oxidative stress induced by ,-irradiation or paraquat treatment compared with a deficiency of either MnSOD or Gpx-1 individually (32).
Because mouse models in which the expression of individual proteins in the antioxidant defense system is altered have not definitively revealed the relationship between oxidative stress and longevity, we wanted to determine whether a dual deficiency in both MnSOD and Gpx-1 would have an adverse effect on mouse life span. We hypothesized that because these two primary antioxidant enzymes are located in mitochondria, deficiencies in these protective proteins would render the mitochondria more compromised, resulting in increased levels of cellular oxidative stress and ultimately reducing life span and increasing pathology in the mice as would be predicted by the mitochondrial theory of aging (1,33). To test this hypothesis, we have compared the life span and incidence of pathology in wild-type control, Gpx1−/−, Sod2+/−Gpx1+/−, and Sod2+/−Gpx1−/− mice.
METHODS
Animals
The combined Sod2+/−Gpx1−/− knockout mice were generated by breeding the two parent knockout models. Mice heterozygous for the Sod2 gene (Sod2+/− mice) were kindly provided by Dr Charles J. Epstein (University of California, San Francisco, CA), and mice heterozygous for the Gpx1 gene (Gpx1−/− mice) were obtained from Dr Ye-Shih Ho (Wayne State University, Detroit, MI). Details regarding the generation and characterization of these two mouse models have been previously described (11,34,35). Sod2+/− mice were bred to Gpx1−/− mice to produce mice heterozygous for both genes (Sod2+/−Gpx1+/−). To generate Sod2+/−Gpx1−/− mice, the Sod2+/−Gpx1+/− mice were bred to Gpx1−/− mice. Wild-type, Sod2+/−, and Gpx1−/− mice were generated by crossing Sod2+/−Gpx1+/−mice with each other. The Sod2 genotype of each mouse was determined by the polymerase chain reaction (PCR) analysis of DNA obtained from tail clips as described by Li and colleagues (11). The Gpx1 genotype of the mice was determined by PCR using the following primer pairs: mGpx1sh-5′-ACA CAg gAC ACA CAA ACA ATT CTg-3′, mGpx1wild type-5′-AAT AAA TgC CTT TgT CTT CCC TAg-3′, and mGpx1neo-5′-AAg AAC gAg ATC AgC AgC CTC T-3′.
All mice were fed a standard NIH-31 chow (Teklad Diet LM485; Harlan Teklad, Madison, WI) ad libitum and maintained under barrier conditions in microisolator cages on a 12-hour dark/light cycle. For tissue collection, animals were killed by CO2 inhalation followed by cervical dislocation, and the tissues were immediately excised and placed on ice. All tissues following collection were stored at −80°C and analyzed within 30 days. All procedures involving the mice were approved by the Subcommittee for Animal Studies at the Audie L. Murphy Veterans Administration Hospital.
Life Span Study
For the life-span experiments, wild-type mice, Gpx1−/− mice, Sod2+/−Gpx1+/−, and Sod2+/−Gpx1−/− mice (mixed males and females) were housed four animals per cage starting at 2 months of age. Mice were assigned to survival groups at 4–8 months of age and were allowed to live out their life span, that is, there was no censoring in all groups of mice when measuring survival. Life spans for mice were determined by recording the ages of spontaneous death of the mice. The mean, median, 10% (the mean life span of longest lived 10% animals), and maximum (the age of death for the longest lived mouse in the cohort) life spans were calculated from the survival data for each genotype. The survival curve shown for the wild-type mice (n = 121) was derived from combined data collected from several survival studies on male and female C57BL6 mice conducted by our laboratory over the past 10 years.
Measurement of DNA Oxidation and Protein Oxidation
Oxidative damage in tissues from mice of different genotypes was assessed by measuring the oxidation of DNA, specifically the increase of oxidized guanidine 8-oxo-dG. DNA was isolated from tissues by the NaI method using the DNA Extractor WB Kit obtained from Wako Chemicals USA, Inc. (Richmond, VA). Our laboratory had previously shown that the NaI isolation method greatly reduces oxidative damage to DNA during extraction, as compared with the phenol extraction method (36). The level of 8-oxo-dG was measured as described (32,36). Results are expressed as the ratio of nanomoles of 8-oxo-dG to 105 nmol of 2-deoxyguanosine.
Protein oxidation was determined by measuring the level of protein carbonyls in the brain using a method described by Chaudhuri and colleagues (37). In brief, brain tissues were homogenized in 50 mM phosphate buffer (pH 6.0), 0.5 mM MgCl2, and 10 mM ethylenediaminetetraacetic acid containing a cocktail of protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 1 mg/mL leupeptin, and 1 mg/mL aprotinin). The tissue homogenate was centrifuged at 4°C for 1 hour at 100,000g. The extract was incubated with 1% streptomycin sulfate for 10 minutes at 37°C, followed by centrifugation at 16,000g for 10 minutes. The protein concentration of the supernatant was measured by the Bradford method (Bio-Rad, Hercules, CA). The proteins (1 mg/mL) were incubated with 1 mM fluorescein-5-thiosemicarbazide (FTC) in deaerated phosphate buffer (pH 6.0) with 0.3 M guanidine for 2 hours at 37°C in the dark. The proteins were then precipitated by addition of an equal volume of 20% trichloroacetic acid on ice for 10 minutes. After a 10-minute centrifugation at 16,000g at room temperature, protein pellets were broken up and washed five times with ethanol/ethyl acetate (1:1; vol/vol) to remove free FTC. The protein pellets were resuspended in 20 mM phosphate buffer (pH 8.0) containing 8 M urea, and equal amounts of proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After electrophoresis, the image of the resolved fluorescent-labeled proteins was captured using a Typhoon 9400 (excitation wavelength of 532 nm, emission filter at 526 nm, 40-nm bandpass). The fluorescence intensity for each lane of the gel (from top to the bottom) was calculated using ImageQuant 5.0 (Molecular Dynamics, Amersham, Piscataway, NJ) software. The gel was then stained with Coomassie R-250 (Sigma, St Louis, MO) and destained with 10% acetic acid and 20% methanol solution, and the image of the Coomassie-stained proteins was captured with an AlphaImage™ 3400 for quantification.
Measurement of Carboxymethyl-Lysine Residues
Levels of carboxymethyl-lysine (CML) have previously been shown to increase with age in rodent models and the increase is attenuated by caloric restriction, which is known to retard aging in rodents (38). To determine whether levels of this marker were increased in mice deficient in MnSOD and Gpx-1 to a greater degree than in wild-type mice, which would indicate an acceleration of age-related changes, we measured CML levels in insoluble skin collagen by isotope dilution gas chromatography–mass spectrometry as previously described (39).
Analysis of Pathology
Mice in the cross-sectional and survival groups were subjected to pathological analysis. The mice were necropsied for gross pathological lesions. Organs and tissues were excised and preserved in 10% buffered formalin. The fixed tissues were processed conventionally, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. For each mouse, a list of pathological lesions that included both neoplastic and non-neoplastic diseases was constructed. Based on these histopathological data, the tumor burden, disease burden, and severity of each lesion in each mouse were assessed (40). The severity of neoplastic and nephrologic lesions was assessed using the grading system previously described (41,42). For example, glomerulonephritis was graded in the order of increasing severity: grade 0, no lesions; grade 1, minimal change in glomeruli (minimal glomerulosclerosis); grade 2, grade 1 with a few (<10) casts in renal tubules; grade 3, grade 1 with more than 10 casts in renal tubules; and grade 4, grade 3 with interstitial fibrosis. For neoplastic diseases, cases that had grade 3 or 4 lesions were categorized as death by neoplastic lesions. For non-neoplastic diseases, cases that had a severe lesion, for example, grade 4, associated with other histopathological changes (pleural effusion, ascites, congestion, and edema in lung) were categorized as death by non-neoplastic lesions. In more than 90% of the cases, there was agreement between two pathologists. In cases in which there was not agreement or in which no one disease was considered severe enough, the cause of death was evaluated as undetermined.
RESULTS
Body and Tissue Weights in Wild-Type and Sod2+/−Gpx1−/− Mice
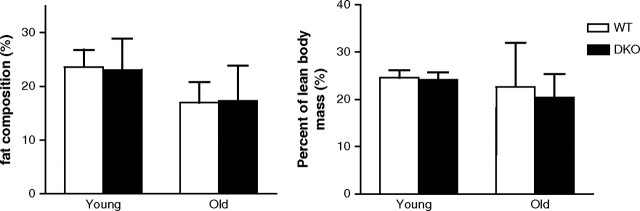
To investigate whether the deficiency in both MnSOD and Gpx-1 affects animal development and growth, we compared the body weights among mice of different genotypes. As shown in Table 1, the body weight of double knockout mice (Sod2+/−Gpx1−/−) was not different from that of wild-type mice from young to old ages, indicating that deficiency of both MnSOD and Gpx-1 did not grossly affect animal growth and development. Likewise, individual tissue weights (liver, heart, and brain) were not affected by the reduction in mitochondrial antioxidant enzymes, as shown in Table 1. Also, the fat composition and lean body mass were not affected, as shown in Figure 1.
Table 1.
Body and Tissue Weights of Young and Old Wild-Type and Sod2+/−Gpx1−/− Mice
| Age (mo) | Body Weight (g) | Liver (g) | Heart (g) | Brain (g) | |
| Wild type | 5–6 | 27.4 ± 0.86 | 1.13 ± 0.03 | 0.13 ± 0.01 | 0.45 ± 0.01 |
| 28–29 | 22.2 ± 2.69 | 1.09 ± 0.17 | 0.13 ± 0.02 | 0.46 ± 0.02 | |
| Sod2+/−Gpx1−/− | 5–6 | 25.60 ± 0.96 | 1.14 ± 0.07 | 0.12 ± 0.01 | 0.41 ± 0.01 |
| 28–29 | 22.92 ± 1.24 | 1.35 ± 0.14 | 0.135 ± 0.01 | 0.46 ± 0.01 |
Body weight and tissue weight for young (5–6 months) and old (28–29 months) wild-type and Sod2+/−Gpx1−/− mice. Values shown are mean ± standard error of the mean for six mice per group. No statistical difference between wild-type and Sod2+/−Gpx1−/− mice at either young or old age (one-way analysis of variance, p > .05).
Figure 1.
Body composition of young and old wild-type (WT) and Sod2+/−Gpx1−/− (DKO) male mice. Lean body mass (lbm) and fat composition were measured by dexascan and calculated as percent of total body mass.
Oxidative Damage Is Increased in Sod2+/−Gpx1−/− Mice
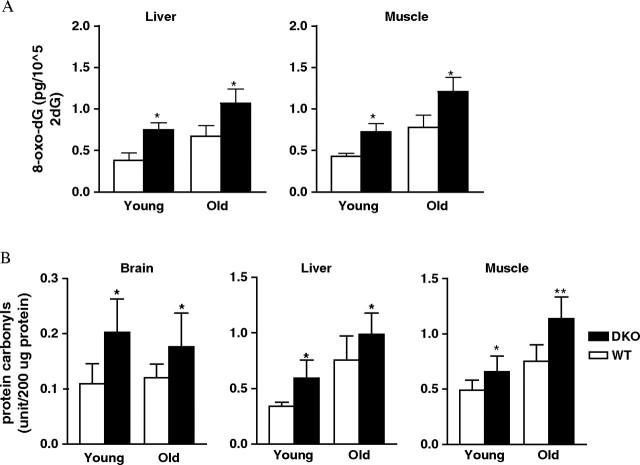
Deficiency of antioxidant enzymes could lead to increased levels of ROS in vivo, resulting in increased oxidation of macromolecules such as DNA, protein, and lipid. Increased levels of oxidative damage have been observed in both Sod2+/− and Gpx1−/− mice (14,23). In this study, we measured the levels of DNA oxidation in liver and skeletal muscle from both young and old mice. We found that Sod2+/−Gpx1−/− mice showed a significant increase in 8-oxo-dG, a marker of DNA oxidation (Figure 2). As the data indicate, the elevation of DNA oxidation was significant in liver and skeletal muscle from young Sod2+/−Gpx1−/− mice (6 months): 60% and 50% increase over the wild-type control in liver and skeletal muscle, respectively. The trend of increased DNA oxidation was maintained at advanced ages (27–29 months): 30% increase in liver and 50% increase in skeletal muscle compared with age-matched wild-type mice. We also measured protein oxidation as protein carbonyls in brain, liver, and skeletal muscle from young and old wild-type and Sod2+/−Gpx1−/− mice. Protein carbonyl levels in brain were significantly elevated in young Sod2+/−Gpx1−/− mice and remained high in all three tissues from the older mice. These data demonstrate the importance of MnSOD and Gpx-1 in maintaining ROS homeostasis and minimizing oxidative damage in tissues.
Figure 2.
Oxidative damage in young and old wild-type (WT) and Sod2+/−Gpx1−/− (dKO) mice. (A) DNA oxidation (level of 8-oxo-dG) was quantified in liver and skeletal muscle samples as described in the Methods section (n = 6). *p < .001, as determined by analysis of variance (ANOVA). (B) Protein oxidation was measured in homogenates from brain, liver, and skeletal muscle (n = 6). *p < .05, as determined by ANOVA. Open bars represent values from wild-type mice; filled bars, values from Sod2+/−Gpx1−/− mice.
Life Span of Sod2+/−Gpx-1−/− Double-Deficient Mice
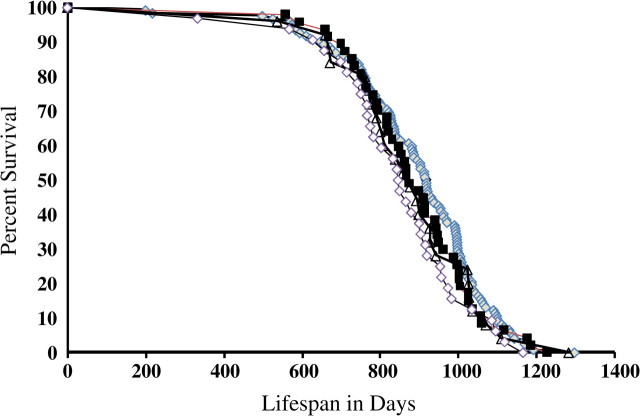
According to the oxidative stress theory of aging, a deficiency in antioxidant enzymes would lead to increased oxidative damage, thereby accelerating the aging process. Our laboratory previously showed that mice deficient in MnSOD (heterozygous deletion) exhibited no change in life span (23). To further address the question of how a deficiency in oxidative stress defenses affects the aging process, we generated mice that were deficient in both MnSOD and Gpx-1, mitochondria-located antioxidants (32). Hypothetically, a deficiency in both enzymes would lead to a greater ROS accumulation in vivo, which could have a significant impact on the aging process or even on embryonic development. Figure 3 shows the life span of mice deficient in antioxidant defenses. Data are shown for Gpx1−/− mice as well as for mice heterozygous for both Gpx1 and Sod2 deficiency (Sod2+/−Gpx1−/−) and mice heterozygous for MnSOD deficiency and homozygous null for Gpx1 (Sod2+/−Gpx1−/−). As shown in Table 2, we observed no significant differences in the mean, median, or 10% (when 90% of the mice have died) survival of any of the genotypes compared with wild-type mice. In addition, we observed no differences in the survival curves when statistically compared by the log-rank test (43). Furthermore, we also analyzed the data according to mouse genders and did not detect any significant differences in longevity between different genotypes associated with sex (data not shown).
Figure 3.
Life span of Sod2+/−Gpx1−/− mice. Survival curves are shown for wild-type (121 mice, solid diamond), Gpx1−/− (47 mice, solid square), Sod2+/−Gpx1+/− (25 mice, solid circle), and Sod2+/−Gpx1−/− (32 mice, open diamond). In these survival experiments, we combined both male and female mice. The mean (± standard error of the mean [SEM]), median, maximum, and top 10% (± SEM) survival of mice were determined from the age at death as described in the Methods section.
Table 2.
Life-Span Characteristics of Antioxidant Deficient Mice*
| Genotype |
|||||
| WT | Gpx1−/− | Sod2+/− | Sod2+/−Gpx1+/− | Sod2+/−Gpx1−/− | |
| Median (d) | 892 (868–916) | 903 (884–922) | 940 | 877 (791–943) | 911 (833–983) |
| Mean (d) | 892 ± 24 | 908 ± 19 | 918 ± 23 | 880 ± 33 | 905 ± 31 |
| 90% (d) | 1,092 (1,040–1,188) | 1,063 (1,031–1,183) | 1,088 (1,084–1,107) | 1,057 (1,027–1,283) | 1,121 (1,069–1,248) |
| Maximum (d) | 1,298 | 1,226 | 1,239 | 1,283 | 1,166 |
Life-span data for the wild-type (WT), Gpx1−/−, Sod2+/−, Sod2+/−Gpx1+/−, and Sod2+/−Gpx1−/− mice. Data shown for the Sod2+/− mice are taken from Van Remmen and colleagues (32). For medians and 90th, percentiles the parenthesized values are the lower and upper bounds of the 95% confidence interval. The means are shown with ± standard error of the mean. There are no statistical differences among the different groups for any parameters listed in the right column of the table (p > .05). The log-rank test was used to compare survival distributions, with the p value adjusted based on the empirical null distribution from 2,000 permutations of the data (43,55). Means of the log-transformed survival times were compared using the Student's t test. The medians and 90th percentiles were compared using a modified version of a score test described by Wang and colleagues (56). These statistical tests were done using custom scripts written in the R statistical language (57).
Pathological Changes in Sod2+/−Gpx1−/− Mice
Increased incidence of tumorigenesis is a common phenomenon in most established mouse models with disrupted antioxidant defenses compared with wild-type control mice, especially at advanced ages (14,23–35). Because we observed a significant increase in oxidative damage in the Sod2+/−Gpx1−/− mice, we asked whether the increased damage was associated with pathological changes. As shown in Table 3, the incidence of fatal neoplastic lesions was similar in all groups, which is consistent with our previous results comparing wild-type and Sod2+/− mice (23). However, the overall incidence of tumors increased significantly in older Sod2+/−Gpx1−/− mice (28–32 months of age), when compared with age-matched wild-type control mice, and the tumor burden (the average number of tumor/mouse) also was significantly higher in the Sod2+/−Gpx1−/− mice compared with control mice. These data are also consistent with our previous observations of Sod2+/− mice. Therefore, the pathology results indicated that increased oxidative damage plays an important role in earlier stage of tumorigenesis with minimal effects on the growth of tumors to become fatal.
Table 3.
Age-Related Pathology in Wild-Type and Sod2+/−Gpx1−/− Mice
| Genotype (Sod2/Gpx1) |
|||
| +/+ +/+ | +/+ −/− | +/− −/− | |
| Probable cause of death (end-of-life pathology) | |||
| Neoplasm | 12 | 12 | 14 |
| Lymphoma | 8 | 10 | 9 |
| Hemangioma | 1 | 1 | 0 |
| Adenocarcinoma (lung) | 3 | 0 | 4 |
| Others | 0 | 1 | 1 |
| Non-neoplasm | 2 | 5 | 2 |
| Glomerulosclerosis | 1 | 4 | 0 |
| Thrombus | 0 | 0 | 1 |
| Others | 1 | 1 | 1 |
| Undetermined | 2 | 6 | 3 |
| Total | 16 | 23 | 19 |
| Tumor incidence at old age (28–32 mo) | n = 21 | n = 10 | n = 19 |
| Lymphoma | 9 | 4 | 9 |
| Hemangioma | 0 | 0 | 1 |
| Pituitary adenoma | 1 | 0 | 4 |
| Others | 0 | 1 | 3 |
| Tumor burden | 0.48 | 0.5 | 0.89* |
Tumor incidence at old age is statistically different between Sod2+/−Gpx1−/− mice and either wild-type or Gpx1−/− mice (p < .05), whereas there is no difference between Gpx1−/−and wild-type mice (p > .05) by Wilcoxon rank test.
Aging Biomarker Changes in Wild-Type and Sod2+/−Gpx1−/− Mice With Age
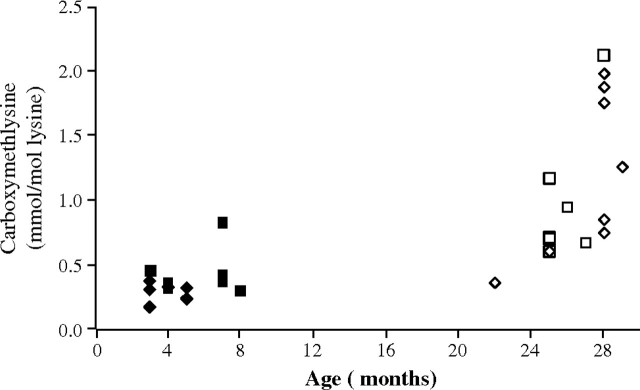
In addition to the life-span data, we measured the age-sensitive biomarker, CML, residues in skin collagen. The level of CML increased with age but did not differ between wild-type and Sod2+/−Gpx1−/− mice, as shown in Figure 4. This result is also consistent with our previous study with Sod2+/− mice that showed an age-related increase in CML in skin but no change in life span.
Figure 4.
Changes in carboxymethyl-lysine level (CML), a biomarker for aging. Data are results for skin collagen taken from five to seven young and old mice. The level of CML clearly increased with age for the wild-type and Sod2+/−Gpx1−/− mice but was not different between the two groups. Diamond-shaped symbols represent data from the wild-type mice; squares, the Sod2+/−Gpx1−/− mice.
DISCUSSION
The oxidative stress theory of aging was first proposed just more than 50 years ago and has been the focus of many studies investigating potential basic underlying mechanisms of aging. According to this theory, a deficiency in the detoxification of ROS due to reduced levels of antioxidant enzymes could lead to an accumulation of ROS and oxidative damage and thereby shorten life span. In the subsequent years, the potential role of the mitochondria in this process was highlighted and the theory extended to suggest that ROS released from the mitochondrial respiratory chain damages macromolecules, especially mitochondrial DNA (mtDNA), resulting in an accumulation of mtDNA mutations, defective mitochondrial respiration, and further increases in ROS generation and oxidative damage. To investigate the effect of reduced mitochondrial antioxidant defenses on life span, we generated mice with deficiencies in both MnSOD and Gpx-1, two primary mitochondrial ROS-scavenging enzymes. Contrary to the predicted role of mitochondrial oxidative stress in aging, in the current study we show that mice lacking both MnSOD and Gpx-1 had no reduction in life span.
Many vertebrate and invertebrate animal models have been developed to test the role of oxidative stress in aging. Although some studies in which antioxidant defenses have been increased in lower organisms such as C. elegans and Drosophila have been successful in extending life span, many studies using models of altered antioxidant defenses in mammals have not been supportive of this theory. Thus, whereas deletion of MnSOD in mice, the major mitochondrial enzyme that detoxifies superoxide, is either embryonic or postnatal lethal depending on the genetic background of the strain, heterozygous (Sod2+/−) knockout mice, which have approximately 50% of the level of MnSOD activity found in wild-type mice, develop normally and do not exhibit a reduction in life span. However, the Sod2+/− mice do have increased oxidative damage to DNA, alterations in liver and heart mitochondrial function, and increased sensitivity to apoptosis (23,26,34). These results seem to contradict the predictions of the oxidative stress theory of aging and have raised questions about the role of oxidative stress and damage in modulating longevity. However, the aging process per se may not be simply an issue of living or dying but rather an issue of functioning well versus functioning poorly, that is, the issue of healthy life span or healthy aging (44). From this point of view, the increase of oxidative stress due to the deficiency of Sod2 does affect the aging process in Sod2+/− mice as evidenced by the decrease of resistance to oxidative stress and increase of tumor incidence.
Gpx-1 is an important antioxidant enzyme that detoxifies hydrogen peroxide. Gpx-1 is located in both the cytosol and the mitochondria and thus could play an important role in detoxifying hydrogen peroxide formed by removal of superoxide in the mitochondrial matrix by MnSOD. Homozygous deletion of Gpx1 has no effect on mouse development (28), but to date, no longevity study has been conducted with Gpx1−/− mice. We had previously shown that cells and tissues from Gpx1−/− mice are more sensitive to oxidative stress compared with wild-type control mice (32,45). In the current study, we demonstrated that the lack of Gpx-1 does not alter life span. No differences were observed in median, mean, and maximum life span between Gpx1−/− and wild-type mice, suggesting that Gpx-1 had no significant impact on mouse longevity. These data, again inconsistent with the oxidative stress theory of aging, also suggest that deficiency of one gene may not be enough to affect longevity.
Because the antioxidant defense system is a complex and integrated system, it is possible that deficiency of a single antioxidant enzyme may not compromise the system to a magnitude sufficient to alter longevity. To address this possibility, we generated mice with a double deficiency in MnSOD and Gpx-1. Because mice null for MnSOD are not viable, we generated mice heterozygous for Sod2 and null for Gpx1. In a previous study, we had shown that young Sod2+/−Gpx1−/− mice are very sensitive to ,-irradiation and paraquat treatment, and cells isolated from these mice are more susceptible to oxidative stress than those deficient in either MnSOD or Gpx-1 alone (32). These results demonstrate that a deficiency in these two primary defense enzymes has a dramatic ability at the cellular and whole organism levels to increase sensitivity to oxidative insults.
In the present study, we examined whether deficiency of both MnSOD and Gpx-1 in mice affects aging and longevity. Our data demonstrated that Sod2+/−Gpx1−/− and Sod+/−Gpx1+/− mice have a life span that is not different from that of the wild-type mice, suggesting there is no additive effect on aging with deficiency of both enzymes. Our finding is similar to that of the Marklund's group, which demonstrated no life-span difference between mice with a single deletion of Cu/ZnSOD and mice with double knockout of extracellular SOD (EC-SOD) and Cu/ZnSOD (46). In that study, deletion of EC-SOD affected neither the life span of the wild-type mice nor the life span of the Cu/ZnSOD null mice, suggesting no functional overlap between EC-SOD and Cu/ZnSOD in terms of modulating longevity. The same may be true for our study, which did not show overlapping roles between MnSOD and Gpx-1 in accelerating mouse life span.
Although we did not find a reduction in life span, we did observe a significant increase in oxidative damage in liver, skeletal muscle, and brain from Sod2+/−Gpx1−/− mice when compared with wild-type mice. The Sod2+/−Gpx1−/− mice also developed more pathology than wild-type and Gpx1−/− mice, similar to our previous results in the Sod2+/− mice in which we found increases in tumor incidence and tumor burden in the Sod2+/− mice with age (32). These results indicate that there is no additive effect of deleting Gpx1 in the presence of simultaneous reduction in MnSOD. It is possible that other peroxide detoxification enzymes (e.g., peroxiredoxins) may compensate for the lack of Gpx-1. Further studies will be required to address this question.
Although the sample size was somewhat small, the incidence of fatal tumors was similar among the groups. However, the overall incidence of tumors and tumor burden were significantly higher in the old (28–32 months of age) Sod2+/−Gpx1−/− mice, when compared with age-matched wild-type control mice. The lack of increase in fatal tumors in the Sod2+/−Gpx1−/− mice suggests that increased oxidative damage played a more important role in the early stages of carcinogenesis initiation, potentially through increased DNA oxidation that is followed by increased mutation and so forth, and may have a minimal effect on the growth and induction of tumors to become fatal. The rate of tumor growth could have a significant impact on longevity in mice because mice naturally have a high incidence of cancer (42). In support of a role for oxidative damage in tumor progression, pathological analysis of long-lived Ames dwarf mice demonstrated that these mice have a similar incidence of cancer compared with the wild-type control but also have retarded growth of tumors, which is correlated with extended longevity (41). Thus, the fact that the Sod2+/−Gpx1−/− mice showed no changes in life span in spite of increased oxidative damage could be explained by the minimal effects of oxidative stress on tumor growth.
According to the oxidative stress theory of aging, a reduction in antioxidant protection, especially in mitochondria, would be predicted to have negative effect on longevity. However, our studies using both single- and double-deficient mice do not support this conclusion. In fact, we show that increased oxidative damage was not correlated to a change in life span. However, it should be noted that ROS generation and its detoxification in vivo must be maintained in a delicate balance for the animal to age normally, that is, to reach maximal life span. The effect of a partial reduction in MnSOD even in the face of a lack of Gpx-1 may not be enough to disturb oxidative homeostasis and affect longevity. Furthermore, ROS are not solely harmful to cell structure and function. They also serve a critical function as signaling molecules. Therefore, disruption of ROS homeostasis may have profound effects on multiple signaling systems that may play an important role in regulating aging and longevity, including the insulin-like growth factor–mammalian target of rapamycin axis and critical damage removal processes such as autophagy. Therefore, simple manipulation of the ROS generation/detoxification may not be enough to achieve the expected results due to the complexity of the signaling networks.
There is another important layer of complexity when studying the effects of oxidative stress on aging. Mammalian cells have developed redundant pathways for detoxifying ROS. Thus, it is possible that the experimental effect of altering one part of the defense system may be masked by unknown compensatory pathways or factors. Furthermore, the expression and distribution of different ROS detoxification enzymes vary from tissue to tissue with age. Simple deletion or overexpression of the gene in the whole animal may not be the best approach for longevity studies. The data from studies overexpressing catalase in mitochondria proved that targeted expression of genes may be a way of extending life span (47). Thus, there may yet be other unidentified compensatory mechanisms that could reduce the effect of the loss of MnSOD and Gpx-1. Still, it is also possible that deficiency of these two enzymes may be insufficient to affect life span. In addition, it is possible that the deficiency in Sod2 and Gpx1 might also affect functional parameters such as muscle strength or cognitive function, which may affect the health span of the mice without altering the life span.
Finally, the understanding of oxidative stress theory of aging is further complicated by some recent studies. Several investigators have reported that mildly increasing oxidative stress or increase of oxidative stress under certain genetic background after removing SODs in C. elegans could actually increase longevity (48–50). Others observed no change of longevity in mammals including mice when ROS level was reduced by overexpressing antioxidant enzymes (10). These observations obviously contradict the prediction by the oxidative stress theory of aging. However, they are in agreement with a recently proposed hormesis theory of aging that states that mild stress-induced stimulation of protective mechanisms in cells and organisms results in biologically beneficial effects on aging (51–54). As has been noted, most of the initial data supportive of the oxidative stress theory of aging are correlative observations among different species. However, the effect of oxidative stress on aging and longevity in each species may differ from each other. Moreover, in higher organisms, oxidative stress may have differential effects on various tissues during aging. Thus, whole body deletion of or overexpression of antioxidant enzymes may not achieve the expected results. Finally, whether oxidative stress is a result of the aging process or a cause of aging is still an unresolved issue.
In conclusion, although our data do not provide support for the oxidative stress theory of aging, it does not definitely rule it out either. Additional carefully designed animal models may be required to better define the full role of oxidative stress in aging, whether it is a cause or result of the aging process. Such studies could include the use of tissue- or age-specific and dose-controlled gene expression/knockout models.
FUNDING
The Department of Veterans Affair Merit Grants (H.R.R., A. C., Y.I., and A.R.); Glenn Foundation (Y.I.); The National Institute of Health grant (DK19971 to J.W.B.)
Acknowledgments
We thank Dr. Ye-Shih Ho from Wayne State University for providing the Gpx1−/− mice used in this study. In addition, we acknowledge the excellent animal husbandry and life-span study support of the Animal Core of the Nathan Shock Center for Excellence in the Biology of Aging headed by Vivian Diaz. We also thank Corinne Price for editing the manuscript.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Taub J, Lau JF, Ma C, Hahn JH, Hoque R, Rothblatt J, Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 4.Svensson MJ, Larsson J. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas. 2007;144:25–32. doi: 10.1111/j.2007.0018-0661.01990.x. [DOI] [PubMed] [Google Scholar]
- 5.Radyuk SN, Michalak K, Klichko VI, et al. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J. 2009;2:437–445. doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 7.Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. Faseb J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 8.Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J Gerontol A Biol Sci Med Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 9.Youngmok C, Jang VP, Song W, et al. Overexpression of Mn Superoxide dismutase (MnSOD) protects against oxidative stress but does not increase lifespan. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2008;8(1):73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 12.Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo T, Reaume AG, Huang TT, et al. Edema formation exacerbates neurological and histological outcomes after focal cerebral ischemia in CuZn-superoxide dismutase gene knockout mutant mice. Acta Neurochir Suppl. 1997;70:62–64. doi: 10.1007/978-3-7091-6837-0_19. [DOI] [PubMed] [Google Scholar]
- 14.Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. 2000;28:754–766. doi: 10.1016/s0891-5849(00)00161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran Q, Van Remmen H, Gu M, et al. Embryonic fibroblasts from Gpx4+/- mice: a novel model for studying the role of membrane peroxidation in biological processes. Free Radic Biol Med. 2003;35:1101–1109. doi: 10.1016/s0891-5849(03)00466-0. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Cheng WH, Porres JM, Ross DA, Lei XG. Knockout of cellular glutathione peroxidase gene renders mice susceptible to diquat-induced oxidative stress. Free Radic Biol Med. 1999;27:605–611. doi: 10.1016/s0891-5849(99)00104-5. [DOI] [PubMed] [Google Scholar]
- 17.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Kim SU, Yu SL, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid Redox Signal. 2006;8:229–237. doi: 10.1089/ars.2006.8.229. [DOI] [PubMed] [Google Scholar]
- 20.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 22.Ran Q, Liang H, Ikeno Y, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 23.Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 24.Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 25.Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Remmen H, Williams MD, Guo Z, et al. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 27.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 28.Ho YS, Magnenat JL, Bronson RT, et al. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 29.Lei XG. In vivo antioxidant role of glutathione peroxidase: evidence from knockout mice. Methods Enzymol. 2002;347:213–225. doi: 10.1016/s0076-6879(02)47021-8. [DOI] [PubMed] [Google Scholar]
- 30.de Haan JB, Bladier C, Lotfi-Miri M, et al. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Shin HS, Shireman PK, Vasilaki A, Van Remmen H, Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Van Remmen H, Qi W, Sabia M, et al. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Miquel J, de Juan E, Sevila I. Oxygen-induced mitochondrial damage and aging. EXS. 1992;62:47–57. doi: 10.1007/978-3-0348-7460-1_5. [DOI] [PubMed] [Google Scholar]
- 34.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 35.Esworthy RS, Mann JR, Sam M, Chu FF. Low glutathione peroxidase activity in Gpx1 knockout mice protects jejunum crypts from gamma-irradiation damage. Am J Physiol Gastrointest Liver Physiol. 2000;279:G426–G436. doi: 10.1152/ajpgi.2000.279.2.G426. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton ML, Guo Z, Fuller CD, et al. A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic Acids Res. 2001;29:2117–2126. doi: 10.1093/nar/29.10.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55:B522–B529. doi: 10.1093/gerona/55.11.b522. [DOI] [PubMed] [Google Scholar]
- 39.Shaw JN, Baynes JW, Thorpe SR. N epsilon-(carboxymethyl)lysine (CML) as a biomarker of oxidative stress in long-lived tissue proteins. Methods Mol Biol. 2002;186:129–137. doi: 10.1385/1-59259-173-6:129. [DOI] [PubMed] [Google Scholar]
- 40.Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 41.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 42.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PK, Borgan E, Gill RD, Keiding N. Statistical Models Based on Counting Processes. New York: Springer; 1993. [Google Scholar]
- 44.Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64:187–191. doi: 10.1093/gerona/gln061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han ES, Muller FL, Perez VI, et al. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 47.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 48.Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr. 2009;4:59–67. doi: 10.1007/s12263-009-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009:5. doi: 10.1371/journal.pgen.1000361. e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doonan R, McElwee JJ, Matthijssens F, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Masoro EJ. Role of hormesis in life extension by caloric restriction. Dose Response. 2007;5:163–173. doi: 10.2203/dose-response.06-005.Masoro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Fleming TR, Harrington DP. Counting Processes and Survival Analysis. New York: Wiley; 1991. [Google Scholar]
- 56.Wang CX, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Ihaka Ross, Gentleman G. R—a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]