Abstract
Pollutants, including synthetic organic materials and heavy metals, are known to adversely affect physiological systems in all animal species studied to date. While many individual chemicals can perturb normal functions, the combined actions of multiple pollutants are of particular concern because they can exert effects even when each individual chemical is present at concentrations too low to be individually effective. The biological effects of pollutants differ greatly between species reflecting differences in the pattern of exposure, routes of uptake, metabolism following uptake, rates of accumulation and sensitivity of the target organs. Thus, understanding of the effects of pollutants on wildlife and ecosystems will require detailed study of many different species, representing a wide range of taxa. However, such studies can be informed by knowledge obtained in more controlled conditions which may indicate likely mechanisms of action and suitable endpoint measurements. Responses may be exacerbated by interactions between the effects of pollutants and environmental stressors, such as under-nutrition or osmotic stresses and so changes in such variables associated with climatic changes may exacerbate physiological responses to pollutant burdens.
Keywords: endocrine-disrupting compounds, pollutants, ecosystems, sustainability
1. Introduction
Anthropogenic pollution is not new—humans have contributed to the environmental burden since they learned to control fire and smelt metals. However, the nature and distribution of contaminants in the environment has changed in recent history as new compounds have been created. Most early anthropogenic pollution was localized although early metal smelting, even 2000 years ago, resulted in hemispheric-scale pollution (Hong et al. 1996). The industrial revolution concentrated people in cities and resulted in increased pollution of the air, as a result of the burning of fossil fuels, and of rivers, with organic pollutants in the form of sewage. These caused disease and illness in humans and killed fish and other wildlife in the rivers (Halliday 1999; Davis et al. 2002).
The reduction in the rate of release into our environment of known toxins, through use of improved sewage processing and ‘Clean Air Acts’, roughly paralleled the development of completely novel organic substances that were used in the manufacture of a host of products including pesticides, plastics and fire retardants and the increased use of fossil fuels in transport and power generation, resulting in changes in the nature of the pollutant burden. The environment seemed to become cleaner because the new pollutants were generally invisible, present only at low concentrations, and did not exert acute effects on exposed animals or humans. New chemicals of many classes, including halogenated organic compounds, phthalates, alkyl phenols and polycyclic aromatic hydrocarbons (PAHs) were manufactured for use domestically and in industry and agriculture and inevitably were released into the environment (Colborn et al. 1993). The ‘old pollutants’ clearly had adverse effects on human health and wildlife populations but we are only now beginning to understand the effects of the ‘new pollutants’. Their effects may be equally, or more, harmful.
This review focuses primarily on terrestrial ecosystems and aims to address some key features of anthropogenic pollutants, in particular organic endocrine-disrupting compounds (EDCs) and heavy metals. Arguably, heavy metals are also endocrine disruptors but since they have some properties that are different from organic EDCs, they are often considered to be a separate category. The review will focus mostly on reproductive function in individual animals but will also address ecosystem health and sustainability. Since reproductive success depends not only on the normal function of the hypothalamic–gonadal axis but also on the individual's health and ultimate survival, effects on other physiological systems will also be considered.
It is important to note that while many principles pertaining to pollutants in general, and organic endocrine disruptors in particular, apply in both terrestrial and aquatic ecosystems, the risks associated with certain classes of compounds may differ considerably with the environmental matrix. This is partly attributable to the fact that many EDCs are relatively insoluble in water and so many of the pollutants present in water are the more water-soluble substances, such as the steroid hormones associated with the human contraceptive pill (Bowman et al. 2003), while in terrestrial systems other compounds bound to organic and mineral matter are more important (e.g. polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs)). In addition, differences in the relative hydrophobicity and lipophilicity of compounds mean that they are differentially accumulated in the tissues of species extracting oxygen from air and water (Kelly et al. 2007).
2. What are they, where are they from and why should we be concerned?
The definition of anthropogenic pollutants is not entirely clear. Some organic pollutants, such as the PAHs, are derived primarily from the incomplete combustion of fossil fuels but can also result from natural forest fires (Yunker et al. 2002; Bostrom et al. 2002), while heavy metal pollutants such as lead and copper are naturally occurring elements but are concentrated by artificial means such as through smelting. On the other hand, other organic compounds such as PCBs, PBDEs and various organochlorine pesticides are synthetic compounds but, in common with the other classes of chemical, have the capacity to perturb endocrine function. Recently, nitrates which are commonly used as fertilizers, have been implicated in the disruption of animal physiology (Edwards et al. 2006) although they are chemically very different from compounds that are normally of concern. For the purpose of this review, all these categories of pollutants will be considered ‘man-made’ and will be loosely classed as endocrine disruptors, although the mechanisms of action of heavy metals are frequently different from those of organic pollutants.
The main groups of organic and metal pollutants, some of their key properties, mechanisms of action and the sources of these compounds have been reviewed previously (IEH 1999; Wilkinson et al. 2003; Rhind 2005, 2008). Briefly, most of the organic pollutants are hydrophobic and lipophilic, and neither the organic nor metal pollutants are readily degraded; accordingly, they can accumulate in animal tissues. There they can be biologically active at concentrations well below that at which they are toxic in the conventional sense. Many different pollutants can act additively (Rajapakse et al. 2002; Brian et al. 2005; Kortenkamp 2007), so that biological responses may be induced even when the concentrations of individual chemicals appear to be too low to exert an effect. Animals are especially sensitive at early stages of development but some effects exerted at these stages may be expressed only in adult life or even in subsequent generations.
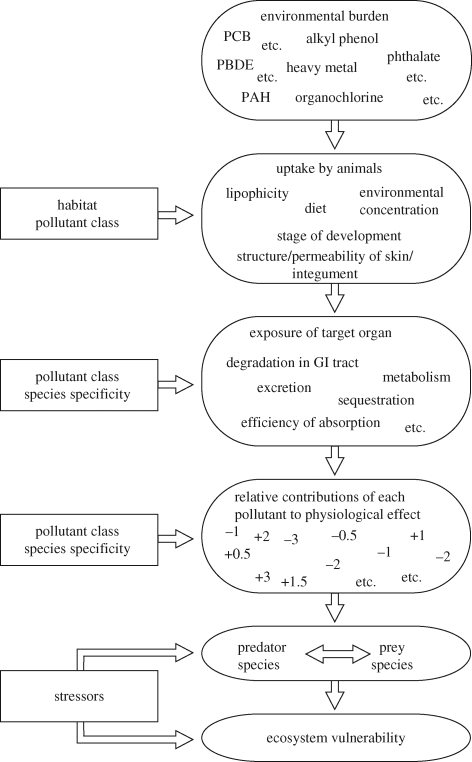
Exposure of target organs and tissues (figure 1) depends on the species and associated habitat (terrestrial, aquatic, soil, sediment), diet composition, age and stage of development (Rhind 2008), as well as the chemical class and associated properties. Exposure can be the result of uptake through skin, gills or lungs, through food and drink, and through maternal blood (foetus) or milk (neonate). Effects of exposure to organic and metal pollutants have been reported for animals of every group so far tested, from bacteria (Fox 2004) to humans (Toppari et al. 1996) and many phyla in between (Colborn et al. 1993; IEH 1999; Oehlmann et al. 2007). The precise nature of these effects depends on the extent to which they are taken up by the animals and then on the extent to which they are degraded, excreted or metabolized (figure 1); each of these factors depends on the individual animal species and compound class. Finally, the overall effect of exposure depends on the combined actions of all of the pollutants together and their interactions with other stressors.
Figure 1.
Some determinants of EDC uptake, target organ exposure and physiological responses of animals and of effects on ecosystem sustainability.
Concern about their effects stems, in part, from an anthropocentric viewpoint, i.e. there is concern about the reproductive success of humans, at least in the developed world (Evers 2002), and significant medical resources are directed towards the treatment of infertility. However, at a more fundamental level, since a wide range of species is susceptible to these compounds, the potential for major disruption of ecosystem function, and for compromising long-term sustainability, is considerable. Damage to the ecosystems on which all life depends threatens not only the survival of the human race but that of many other species. Effects could be direct if, in extreme cases, exposure resulted in the death of some animals in the population or reduced breeding success. Indirect effects are also possible if, say, the reduction or loss of a prey species resulted in a reduction in food supply. Either way, there is at least a theoretical risk to ecosystem health and/or sustainability.
3. What is the evidence that endocrine-disrupting compounds and other pollutants can be a problem?
Interestingly, it has been known for over 70 years that a number of synthetic organic compounds which have specific chemical structures could have oestrogenic effects, at least in theory (Dodds & Lawson 1938). However, it required the mass production and use of many novel chemicals for problems to be identified in practice. Some of these effects, such as effects of pollutants on egg shell thinning in birds (Faber & Hickey 1973) and associated declines in the populations of the affected species have been very well publicized. These early observations were followed by large numbers of epidemiological (based on field observations) and empirical (laboratory based) studies of many species and taxa, involving many classes of chemicals; a representative selection of examples is given in table 1 to illustrate the range of chemical types, taxa and physiological responses. Such disruption of reproductive activity or function in any one species, or group of species, may have disastrous consequences for ecosystem sustainability owing to changes in the balance of predator and prey species.
Table 1.
Selected examples of perturbation of various components of reproductive systems following exposure of animals representing different taxa to a range of classes of anthropogenic pollutants.
| species | chemical | observed effect(s) | reference |
|---|---|---|---|
| white-footed mouse (Peromyscus leucopus) | PCB | reduced testis size | Batty et al. (1990) |
| seagull (Larus californicus) | DDT | feminization of embryos | Fry & Toone (1981) |
| alligator (Alligator mississippiensis) | dicofol, DDT | abnormal testes, phalli and testosterone | Guillette et al. (1994) |
| flathead minnow (Pimephales promelas) | methyl mercury | expression of multiple genes | Klaper et al. (2006) |
| dogwhelk (Nucella lapillus) | tributyltin | imposex | Gibbs & Bryan (1987) |
| mayfly (Cloeon dipterum) | esfenvalerate | reduced survival | Beketov & Liess (2005) |
| annelids | toxic metals | survival and reproductive development | Spurgeon et al. (1994) |
| nematodes (Caenorhabditus elegans) | many (individually) | reduced fecundity | Hoss & Weltje (2007) |
| bacteria | mixture (sewage sludge) | altered communities | Kuntz et al. (2008) |
Many of the historical problems associated with the use of relatively high concentrations of single, highly potent chemicals have been recognized and addressed. Examples include the perturbation of reproductive processes in marine gastropod molluscs exposed to organotins, used in marine anti-fouling paints (Smith 1981) and, in human males exposed to chemical defoliants during the Vietnam war (Wolfe et al. 1995); legislative changes have resulted in the use of such chemicals being restricted. Approximately half a century on from the first major production of many of the pollutants, pesticide use, factory discharges and the specific types of chemicals used in manufactured products are all the subject of regulation and legislation. Accordingly, the problem might be considered to be resolved.
However, empirical studies designed to investigate the mechanisms of action of some of the pollutants have demonstrated more subtle perturbation of underlying processes, which are not necessarily apparent in the field observations but may seriously compromise animal health or productivity. For example, PBDEs have been shown to exert oestrogenic and anti-oestrogenic effects, in vitro (Meerts et al. 2001), while exposure to phthalates induced alterations in foetal and neonatal anatomy and testis weight in rats (Gray et al. 2000), and bisphenol A and octyl phenol disrupted gonadotrophin profiles (Evans et al. 2004). While much has been learned about both the potential effects and the mechanisms of action of individual compounds from such studies, they are usually a poor representation of normal, real-world exposure and are often based on single chemicals, often at very high concentrations and applied for short periods. In practice, massive exposures to single pollutants in the natural environment are rare but long-term exposure to low concentrations of multiple organic and heavy metal chemicals has become commonplace, as indicated by reports of detectable concentrations of some of the classes in air (Lee et al. 2004; Tonne et al. 2004), soil (Smith 1996; Rhind et al. 2005b) and water (IEH 1999). However, controlled studies of the effects of exposures to these low-concentration mixtures are also rare. While it is clear that many pollutants accumulate in many wild species (IEH 1999; Law et al. 2003), in practice, the detailed study of effects of prolonged, low-level exposure is logistically almost impossible because it requires assessment of exposure rates and an understanding of animal physiology, anatomy and behaviour in species that may be intractable, of very small or large size, or rare. Thus, there is a need for studies of more tractable model species with similar physiology, from which extrapolation to their wild counterparts is possible. Accordingly, sheep have been used as a convenient, relatively long-lived, mammalian model for which there is a good basic understanding of both general and reproductive physiology. In the present context they have been the subject of one long-term study involving their exposure to a mixture of EDCs, at environmental concentrations, through the application of sewage sludge to the surface of their pastures.
4. Domestic animal studies: a clue to the threat to wildlife
In this study (Rhind et al. 2005a,b), sheep grazed sludge-treated pastures, or control pastures treated with inorganic fertilizers, for periods ranging from several months to 5 years, with three-week withdrawal periods after each sludge application. Sludge is known to contain relatively large amounts of organic and heavy metal pollutants (Stevens et al. 2003; Harrison et al. 2006; Ghanem et al. 2007), being derived from industrial, agricultural and domestic sources, and it is frequently applied to land as a fertilizer at the rates used in these studies. This experimental approach exposes animals to a cocktail of pollutants, similar to that to which humans and other animals are likely to be exposed, and serves to elucidate a number of important features concerning their accumulation and effects. The sheep are subject to a ‘real-world exposure’ to the pollutants, i.e. to environmental (low) concentrations of multiple pollutants, through physiological routes and at all stages of the life cycle.
In this study, the twice yearly application of sludge to the pastures resulted in only minimal increases in environmental (soil) concentrations of the organic pollutants phthalate and alkyl phenol (Rhind et al. 2002) and variable but generally small increases in PAHs, PCB and PBDE (S. M. Rhind, C. E. Kyle, C. Mackie & L. Macdonald, unpublished observations) and heavy metals (Rhind et al. 2005b), relative to concentrations found in pastures treated with inorganic fertilizer. These results agree with a review of earlier observations by Smith (1996) who reported that even extremely high rates of application resulted in increases in soil concentrations of not more than a few fold.
Studies of tissue concentrations of animals grazing sludge-treated pastures also indicate that tissue concentrations are only slightly increased following exposure, if at all (Rhind et al. 2005a,b, 2009). However, these small increases should be considered against the background of known extreme variability in rates of tissue accumulation in animal tissues, even in animals subject to the same levels of pollutant exposure (Rhind 2008). Notwithstanding the issue of variance, a superficial consideration of these observations might suggest that exposure of animals to multiple pollutants, none of which was present at concentrations within the range known to induce biological effects, was unlikely to be of any biological consequence.
In fact, despite the low level exposure and apparently low tissue concentrations, investigations of the underlying physiology of sludge-exposed animals showed that significant effects occurred. Compared with control animals maintained on fields fertilized with inorganic fertilizer containing minimal amounts of pollutants, exposure to the multiple pollutants in sludge had limited effects on some parameters associated with adult reproductive physiology, e.g. altered rates of hypothalamic–pituitary expression of galanin receptors (Whitelaw et al. 2007) and perturbed mammary development (Fowler et al. 2007). However, of much greater potential concern are changes reported in several foetal organs. Specifically, the testes of foetuses in ewes exposed during gestation exhibited fewer Leydig cells (the source of testosterone) and Sertoli cells (essential for germ cell development) and lower blood concentrations of the hormones testosterone and inhibin (Paul et al. 2005). Reported effects of exposure on the foetal ovary included a reduction in oocyte density and an altered balance of pro- and anti-apoptotic proteins (BAX and BCL2) towards apoptosis (i.e. there was an increased propensity for programmed cell death) (Fowler et al. 2008). There was evidence of both up- and down-regulation of the expression of large numbers of proteins involved in gene expression/transcription and of changes in protein synthesis, phosphorylation and receptor activity in the foetal ovaries of exposed animals (Fowler et al. 2008). Changes in foetal neuroendocrine development were also identified that could potentially mediate later effects on reproductive development and function. The foetuses of sludge-exposed ewes were shown to have reduced hypothalamic expression of galanin (a neuropeptide involved in the regulation of food intake, metabolism and reproduction), GnRH (the brain hormone that drives gonad function) and galanin and GnRH receptor mRNA (Whitelaw et al. 2007), and reduced hypothalamic and pituitary expression of the KISS-1 gene that regulates GnRH neuronal function and puberty (Bellingham et al. 2009). These observations indicate that a number of fundamental physiological processes were disrupted in the foetus. Furthermore, the adult female offspring of ewes exposed during gestation and lactation exhibited a lower proportion of healthy primordial follicles than those derived from control animals (Amezaga et al. 2008). Adult male offspring also exhibited changes in behaviour following exposure which were consistent with demasculinizing effects of EDC exposure (Erhard & Rhind 2004). However, in contrast to the observation of disrupted development in the foetal testis (Paul et al. 2005), studies of the sperm counts in adult male domestic animals (Setchell 1997) and other sperm qualities (G. B. Boe-Hansen, H. G. Pedersen, C. E. Kyle, I. B. Boegh & S. M. Rhind, unpublished observations) have not revealed any abnormalities.
Collectively, these results indicate that prolonged low-level exposure to a cocktail of pollutants can perturb multiple physiological systems directly related to reproductive function even though the final tissue concentrations of the pollutants are not consistently higher than background levels. Furthermore, they support previous observations which indicated that the developmental stages were particularly sensitive to EDC exposure and that the effects exerted on the foetus could be expressed in adult life (e.g. reduced proportion of healthy ovarian follicles). There is evidence from rat studies that perturbations of components of the reproductive system induced in one generation by EDC exposure can be expressed at least up to the F4 generation, even in the absence of any further exposure (Anway & Skinner 2006; Crews et al. 2007; Edwards & Myers 2007). Such transgenerational epigenetic effects of EDC exposure are yet to be demonstrated more widely and the extent to which they occur is likely to depend on the chemical, animal species and physiological system involved. Much more work is needed on this topic to establish the incidence of such potentially important transgenerational effects.
While much of the knowledge of the effects of EDCs and their mechanisms of action is derived from studies on domestic and laboratory species, the principles derived from these studies can be applied to wildlife. These studies, together with many others in a variety of species, show that there is potential for reproductive success to be compromised not only by direct effects of pollutants on the hypothalamic–pituitary–gonadal axis but also by adversely affecting the health status of the individual. For example, EDCs are known to have the potential to perturb processes as diverse as immune function (Vine et al. 2000; Markman et al. 2008), bone structure (Lind et al. 2003, 2004a; Fox et al. 2008; Hermsen et al. 2008), thyroid function (Hansen 1998; Langer et al. 1998), obesogenic systems (Grun et al. 2006; Newbold et al. 2007), mammary structure and function (Fenton 2006; Moral et al. 2008), cardiovascular function (Lind et al. 2004b; Ha et al. 2007) and social and other behaviours (Clotfelter et al. 2004; Markman et al. 2008). Perturbation of any one of these systems has the potential to adversely affect an animal's reproductive success and competitiveness, and therefore the potential viability of the population. In humans, it has been suggested that EDC could be implicated in processes ranging from the increased incidence of breast cancer (Kortenkamp 2006) and a variety of male reproductive effects (Sharpe & Skakkebaek 2008) to metabolic disturbances (see http://jme.endocrinology-journals.org/cgi/content/abstract/JME-08-0132v1).
5. How useful is this model?
Studies of domestic species can provide many clues to the effects of exposure to pollutants and their mechanisms of action because many of them act on physiological processes so fundamental and, in evolutionary terms, so ancient, that they are common to animals of many phyla, e.g. many organic pollutants have oestrogen-like properties and act through the oestrogen receptors (Dodge 1998). Oestrogen receptors are just one member of the large nuclear receptor superfamily (including steroid, thyroid and retinoid receptors). Nuclear receptors are widely expressed throughout the metazoa and the various receptors have fundamental roles in the control of a wide variety of key developmental, metabolic and physiological processes. The action of the various members of the nuclear receptor superfamily is often modulated by relatively hydrophobic ligands and it is to be expected that many of the organic EDCs will also be found to affect the action of many nuclear receptors.
The subtle, underlying physiological changes reported in the sheep, particularly in the foetuses (Paul et al. 2005; Fowler et al. 2008), would not be detected, generally, in conventional breeding systems. It is therefore likely that similar subtle effects are being induced in some wildlife species as a result of exposure to pollutants and that they remain undetected. Indeed, various physiological changes have been reported in some of the few wildlife species that have been studied following exposure to relatively large chemical insults (Faber & Hickey 1973; Guillette et al. 1994). Similarly, there is also evidence from studies of amphibians (McCoy et al. 2008) that chronic, low-level exposure to an undefined mixture of agriculture-related pollutants can perturb gonadal structure and function, a finding that parallels the observations on sheep. Clearly, the absence of a visible, whole-animal response does not necessarily indicate the absence of a response. The studies based on domestic animals may indicate mechanisms and physiological systems that are worthy of investigation in other species.
Environmental, dietary and other exposure to EDC, and rates of uptake from the environment are likely to differ between species and it has been shown that the capacities to metabolize pollutants also differ greatly between species (Eaton & Klaassen 2001; Norstrom 2002). Thus, although the mechanism of action of the pollutant may be similar across species (e.g. through an oestrogen receptor), there can be large species differences in target organ exposure, owing to differences in uptake, metabolism, sequestration and excretion (figure 1) and so in the rate of delivery to the target receptor. This problem is further magnified by the extreme complexity of the relationships between pollutant, individual genotype and phenotypic response, including post-translational control of gene expression (Nebert 2005). Consequently, extrapolation of the results obtained in sheep or laboratory rodent studies to wildlife species, and indeed to humans, requires extreme caution. Understanding the effects of pollutants on entire ecosystems will require detailed study of each species or at least of each species group. Such studies can be informed, however, by knowledge obtained in more controlled conditions.
While the sheep studies begin to define patterns of environmental exposure, tissue concentrations and some physiological effects in one specific species, it should be emphasized that tissue measurements are necessarily restricted to one or a few time points and may not reflect biologically important but transient increases in animal exposure. Neither do they elucidate the interactions between the many chemical classes that may contribute to the observed responses (figure 1), nor the effects of different doses, variable exposure or exposure at different stages of development. These are extremely important issues that remain to be pursued in further research.
6. Environmental changes and environmental stressors
At present the overall reproductive capacity of domestic animals does not appear to be compromised by exposure to EDCs, at least in males (Setchell 1997). However, while the global human population continues to grow, evidence of possible effects of EDCs on human fertility is beginning to accumulate. This may reflect the constant exposure to a wide variety of pollutants through contaminated air (Lee et al. 2004; Tonne et al. 2004) and food (Norstrom 2002). The importance of EDCs with respect to declining sperm counts in humans is debated (Sharpe et al. 1995; de Kretser 1998), but it is becoming clear that human sperm quality and fertility are declining, at least in some regions (Nordstrom Joensen et al. 2009), while an increased incidence of testicular dysgenesis syndrome (Sharpe & Skakebaek 2008), premature menopause and reduced female fertility (Sharara et al. 1998; Hruska et al. 2000) have been reported. Understanding the role of EDCs in such human conditions remains a distant objective as it depends on a carefully balanced interpretation of epidemiological data from humans exposed to multiple pollutants and controlled experimental studies in animal models. In relation to the ‘real world’, the results obtained with sludge-exposed sheep reflect responses in well-nourished, unstressed animals, but wildlife species are frequently subject to additional environmental insults and stressors that are known to interact adversely with pollutant burdens. For example, under-nutrition has been shown to result in increased liver concentrations of pollutants in some bird species (Wienburg & Shore 2004); this may reflect an increased mobilization of fat depots and associated release of stored EDCs which may then affect the function of other systems. In fish, tolerance to salinity can be compromised by EDC exposure (McCormick et al. 2005), making them more susceptible to osmotic stresses. It seems likely that other stressors, including social stressors in group-living species, may induce endocrine, metabolic and physiological responses that may also interact with pollutant burdens to affect animal health and productivity. Such effects of stressors are not confined to vertebrates. For example, drought can compromise survival in soil-living collembolans exposed to pollutants (Holmstrup 1997) and food restriction interacts adversely with pollutants in mayfly larvae (Beketov & Liess 2005), while temperature extremes affect physiological responses to copper contamination in shore crabs (Camus et al. 2004). Thus, if the risks associated with exposure to pollutants are to be properly assessed, it is essential that their actions are considered in conjunction with the effects of additional physiological stressors.
Interestingly, while a combination of environmental stressors or insults might be expected to have uniformly adverse effects, this is not necessarily the case. For example, while exposure to pollutants can suppress immune activity and thus increase parasite burden (Sures 2006) and therefore reduce an animal's health status, more favourable outcomes are also possible because some parasites can modify physiology such that tissue burdens of heavy metals are reduced (Sures 2006). It is also noteworthy that some parasites of species as diverse as fish and molluscs have the capacity to exert an endocrine-disrupting effect, unrelated to environmental pollutants, which causes suppression of gonad development (Morley 2006; Sures 2006). Clearly, this has implications for studies of the effects of exposure to environmental EDCs since there may be commonality of mechanism, and therefore parasite burden may confound effects of EDCs.
Collectively, these observations indicate that the effects of pollutants are dependent, in part, on the actions of other environmental stressors. Any change in the environment in the form of increased or reduced air, water or soil temperatures, altered rainfall/humidity or soil waterlogging/drying or associated changes in plant growth or predator or prey populations, all of which might be expected as a result of climate change, has the potential to exert new stresses on individual animals or on populations and ecosystems (figure 1). Animals living near to the limits of their natural range, and therefore at the extremes of their physiological tolerances, may be especially vulnerable to such influences, and loss of such species may allow invasion by new species, with potential consequences for ecosystem sustainability (Schiedek et al. 2007).
It is important to recognize that changes in human activities or climate can also alter the nature of the ‘insults’ through changes in the patterns of manufacture and use of specific EDCs. For example, changing climatic conditions may alter the requirements for EDC-containing products in agriculture, industry and personal use (e.g. pesticides, fungicides and sunscreens) with associated changes in the inputs of such compounds into the environment (Schiedek et al. 2007).
7. Which species and ecosystems may be most vulnerable?
It would appear from some of the early studies of effects of pollutants, and particularly EDCs, that the most vulnerable species of animals are those near the top of the food chain which tend to accumulate particularly high concentrations of pollutants owing to the effects of biomagnification, e.g. birds of prey (Naert et al. 2007) and alligators (Guillette et al. 1994). On the other hand, the studies of sheep suggest that even species low in the food chain can accumulate limited amounts of pollutants and are susceptible to their effects. While there have been few studies that link exposure, accumulation and biological effects in invertebrate species, it is clear that tissue accumulation of many classes of pollutants occurs in many species including various aquatic insects (Kovats & Ciborowski 1989; Corkum et al. 1997), earthworms (Markman et al., 2007), amphipod crustaceans (Landrum et al. 2001) and molluscs (Markich et al. 2002). In addition to any direct effects of low levels of multiple pollutants on these species, both aquatic and terrestrial predators that feed on such species will be exposed to levels greater than those present in the environment through their consumption of the contaminated prey.
The relative vulnerability of ecosystems to pollutant exposure is difficult to assess. While there appears to be a positive relationship between species diversity and ecosystem stability, other factors also affect stability (Ives & Carpenter 2007) and so it is not possible to conclude that simpler ecosystems (fewer species; less complex food webs) such as those of the Arctic and Antarctic regions are especially vulnerable. Nevertheless, many organic pollutants, in particular, accumulate in the food chains in these regions. Thus, it is suggested that such ecosystems may be the first to show signs of instability as a result of increased pollutant burden and particularly if changes in climate result in additional stressors on such species. The ecosystems of the polar regions may be particularly vulnerable to climatic changes, not only because of the relative simplicity of the ecosystems but also because of the albedo effect (as the ice melts, more heat is absorbed and still more ice melts causing further increases in temperature). Thus, particularly rapid increases in temperature and associated effects on pollutant availability and action, combined with high pollutant burdens may make it particularly difficult for individual species to adapt to the changes. In the case of the larger species, these effects may be further compounded by additional factors such as hunting pressure and/or habitat loss.
It is concluded that since many fundamental physiological systems can be perturbed by very low levels of organic and heavy metal pollutants, particularly when acting in combination, all species and ecosystems may be vulnerable to their effects. Much basic work is required to characterize the risks fully, and even more is needed to begin to resolve the problems already identified.
8. What should be done?
The question ‘What do we do about it?’ is frequently posed in discussions about endocrine disruptors but, since knowledge of them is so incomplete, the answer is inevitably complex. Identification of the problem of environmental pollutants in relation to environmental sustainability is relatively easy. Understanding it is much more difficult because of the many, inter-related facets addressed above, including the rates and duration of exposures, processing of chemicals by the exposed individuals, the sensitivity of the target organs and interactions with other environmental stressors. Solving the problems of exposure may be even more difficult.
The introduction of new European Community Regulations on chemicals and their safe use (EC 2006 Regulation) which deals with the Registration, Evaluation, Authorization and Restriction of Chemical substances (REACH) is a recent initiative that aims to identify the most potent pollutants. This programme will coordinate detailed evaluations of chemicals and is intended to provide basic toxicity data on each individual chemical which are intended to provide a sound basis for future research.
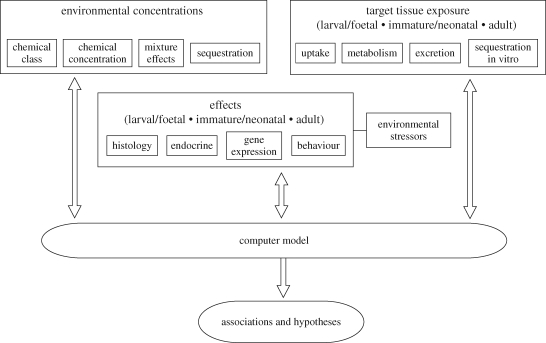
Attempts to understand the underlying actions of such chemicals on individuals and on ecosystems will demand a suite of investigative approaches (figure 2). Firstly, environmental concentrations of each pollutant and their bioavailability must be determined. Secondly, uptake, metabolism and excretion must be investigated in a number of carefully selected species, to take account of age and species differences in effects on target tissues. Finally, a range of appropriate endpoints must be identified for the quantification of responses (figure 2). These may include not only population changes but also behavioural, physiological, histological, endocrine and molecular responses.
Figure 2.
Some major components of a model of pollutant exposure and action.
Criteria have been proposed for the selection of sentinel species and for the choice of endpoint measurements (IEH 1999), e.g. they should be common, ecologically or economically important, amenable to laboratory maintenance and have a range of diets and habitats. However, the species selected for study must be determined by the specific question posed.
Individual investigators often favour one particular experimental approach to address specific questions concerning biochemical or physiological effects. This is entirely appropriate for the elucidation of components of the system but diverse experimental approaches are essential for understanding of the ecosystem as a whole because no single approach can address all questions. For example, controlled laboratory studies, involving single chemicals, sometimes at pharmacological doses, are necessary for the investigation of the mechanisms of action and effective doses of each compound. On the other hand, field studies of effects on animal populations of exposure to multiple pollutants, at low but variable doses, are essential to the understanding of ‘real-world’ effects. Both types of study may benefit from the incorporation of genomic, proteomic and metabolomic techniques to elucidate the actions of pollutants. In order to optimize progression of understanding, it is vital that the results of these contrasting types of study are brought together, so that effects of low concentrations of mixtures of chemicals on multiple species can be modelled and so that factors critical to ecosystem function can be identified. Such critical factors will include the most potent chemical insults, the most sensitive species and stages of development, and the most significant additional stressors. It may be possible to begin this process through the construction of computer models designed, ultimately, to integrate all types of relevant information, including exposure rates, tissue accumulation and animal and population responses (figure 2). Eventually, such an approach may also allow models of climate change and associated changes to be linked to models of ecosystem function so that the combined effects of exposure to pollutants and environmental stressors can be predicted.
It is concluded that endocrine-disrupting pollutants do pose threats to ecosystem sustainability and to long-term human health and wellbeing. However, determination of the precise nature and magnitude of the threats will require significant further investigation. The cost of the required research and the social cost of the lifestyle changes that may be required to reduce the impact of environmental pollutants are likely to be high. However, such costs should be considered in the context of the potential cost of failure to address damage to the marine, freshwater and terrestrial ecosystems that support our food production and wildlife.
Footnotes
One contribution of 11 to a Theme Issue ‘Impacts of environmental change on reproduction and development in wildlife’.
References
- Amezaga M. R., Bellingham M., Evans N. P., Cotinot C., Sharpe R. M., Rhind S. M., McNeilly A. S., Fowler P. A.2008. Ewes exposed to a cocktail of environmental chemicals as fetuses have fewer primordial follicles. Proc. Soc. Reprod. Fertil., 29 June–1 July 2008, Edinburgh, UK, pp. 68 See http://www.srf-reproduction.org/meetings/conf2008/progabstractbook.pdf; accessed 02 September 2009 [Google Scholar]
- Anway M. D., Skinner M. K.2006Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147, S43–S49 (doi:10.1210/en.2005-1058) [DOI] [PubMed] [Google Scholar]
- Batty J., Leavitt R. A., Biondot N., Polin D.1990An ecotoxicological study of a population of the white footed mouse (Peromyscus leucopus) inhabiting a polychlorinated biphenyls-contaminated area. Arch. Environ. Contam. Toxicol. 19, 283–290 (doi:10.1007/BF01056098) [DOI] [PubMed] [Google Scholar]
- Beketov M. A., Liess M.2005Acute contamination with esfenvalerate and food limitation: chronic effects on the mayfly, Cloeon dipterum. Environ. Toxicol. Chem. 24, 1281–1286 (doi:10.1897/04-256R1.1) [DOI] [PubMed] [Google Scholar]
- Bellingham M., Fowler P. A., Amezaga M. R., Rhind S. M., Cotinot C., Mandon-Pepin B., Sharpe R. M., Kyle C. E., Evans N. P.2009Exposure to a complex cocktail of environmental endocrine disrupting compounds disturbs the KiSS-1/GPR54 system in ovine hypothalamus and pituitary gland. Environ. Health Perspect See http://ehp.niehs.nih.gov/docs/2009/0900699/abstract.html. (doi:10.1289/ehp.0900699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom C. E., et al. 2002Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 110Suppl. 3, 451–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J. C., Readman J. W., Zhou J. L.2003Sorption of the natural endocrine disruptors, oestrone and estradiol-17B in the aquatic environment. Environ. Geochem. Health 25, 63–67 (doi:10.1023/A:1021296831175) [DOI] [PubMed] [Google Scholar]
- Brian J. V., et al. 2005Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ. Health Perspect. 113, 721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus L., Davies P. E., Spicer J. I., Jones M. B.2004Temperature dependent physiological response of Carcinus maenas exposed to copper. Mar. Environ. Res. 58, 781–785 (doi:10.1016/j.marenvres.2004.03.093) [DOI] [PubMed] [Google Scholar]
- Clotfelter E. D., Bell A. M., Levering K. R.2004The role of animal behaviour in the study of endocrine disrupting chemicals. Anim. Behav. 68, 665–676 (doi:10.1016/j.anbehav.2004.05.004) [Google Scholar]
- Colborn T., Vom Saal A. M., Soto A. M.1993Developmental effects of endocrine disrupting chemicals in wildlife and human. Environ. Health Perspect. 101, 378–384 (doi:10.2307/3431890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum L. D., Ciborowski J. J. H., Lazar R.1997The distribution and contaminant burdens of adults of the burrowing mayfly, Hexagenia, in Lake Erie. J. Great Lakes Res. 23, 383–390 [Google Scholar]
- Crews D., Gore A. C., Hsu T. S., Dangleben N. L., Spinetta M., Schallert T., Anway M. D., Skinner M.2007Transgenerational epigenetic imprints on mate preference. Proc. Natl Acad. Sci. USA 104, 5942–5946 (doi:10.1073/pnas.0610410104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. L., Bell M. L., Fletcher T.2002A look back at the London smog of 1952 and the half century since. Environ. Health Perspect. 110, A734–A735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kretser D. M.1998Are sperm counts really falling? Reprod. Fertil. Dev. 10, 93–95 [DOI] [PubMed] [Google Scholar]
- Dodds E. C., Lawson W.1938Molecular structure in relation to oestrogenic activity. Compounds without phenanthrene nucleus. Proc. R. Soc. Lond. B 118, 222–232 [Google Scholar]
- Dodge J. A.1998Natural and anthropogenic environmental oestrogens: the scientific basis for risk assessment. Structure/activity relationships. Pure Appl. Chem. 70, 1725–1734 (doi:10.1351/pac199870091725) [Google Scholar]
- Eaton D. L., Klaassen C. D.2001Casarett and Doull's toxicology, the basic science of poisons (eds Casarett L. J., Klaassen C. D., Doull J.), ch. 2, pp. 11–34, 6th edn Highstown, NJ: McGraw Hill [Google Scholar]
- EC 2006 Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No. 793/93 and Commission Regulation (EC) No. 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. [Google Scholar]
- Edwards T. M., Myers J. P.2007Environmental exposures and gene regulation in disease etiology. Environ. Health Perspect. 115, 1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T. M., Miller H. D., Guillette L. J.2006Water quality influences reproduction in female mosquitofish (Gambusia holbrooki) from eight Florida springs. Environ. Health Perspect. 114, 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard H., Rhind S. M.2004Prenatal and postnatal exposure to environmental pollutants in sewage sludge alters emotional reactivity and exploratory behaviour in sheep. Sci. Total Environ. 332, 101–108 [DOI] [PubMed] [Google Scholar]
- Evans N. P., North T., Dye S., Sweeney T.2004Differential effects of the endocrine-disrupting compounds bisphenol-A and octylphenol on gonadotrophin secretion, in prepubertal ewe lambs. Dom. Anim. Endocr. 26, 61–73 (doi:10.1016/j.domaniend.2003.09.005) [DOI] [PubMed] [Google Scholar]
- Evers J. H. L.2002Female subfertility. Lancet 360, 151–159 (doi:10.1016/S0140-6736(02)09417-5) [DOI] [PubMed] [Google Scholar]
- Faber R., Hickey J.1973Eggshell thinning, chlorinated hydrocarbons and mercury in inland aquatic bird eggs, 1969 and 1970. Pesticide Monit. J. 7, 27–36 [PubMed] [Google Scholar]
- Fenton S. E.2006Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology 147, S18–S24 (doi:10.1210/en.2005-1131) [DOI] [PubMed] [Google Scholar]
- Fowler P. A., Gordon K. L., Thow C. A., Cash P., Miller D. W., Lea R. G., Rhind S. M.2007. Exposure to sewage sludge disrupts ewe mammogenesis. Proc. of Society for the Study of Reproduction, April 2007, York, UK See http://www.srf-reproduction.org/meetings/progbook2007.pdf; accessed 02 September 2009 [Google Scholar]
- Fowler P. A., et al. 2008In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol. Human Reprod. 14, 269–280 (doi:10.1093/molehr/gan020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E.2004Chemical communication threatened by endocrine-disrupting compounds. Environ. Health Perspect. 112, 648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. A., Lundberg R., Wejhededen C., Lind L., Larsson S., Orberg J., Lind P. M.2008Health of herring gulls (Larus argentatus) in relation to breeding location in the early 1990s. III. Effects on the bone tissue. J. Toxicol. Environ. Health Part A 71, 1–9 [DOI] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K.1981DDT-induced feminization of gull embryos. Science 213, 922–924 (doi:10.1126/science.7256288) [DOI] [PubMed] [Google Scholar]
- Ghanem A., Bados P., Estaun A. R., de Alencastro L. F., Taibi S., Einhorn J., Mougin C.2007Concentrations and specific loads of glycophosphate, diuron, atrazine, nonylphenol and metabolites thereof in French urban sewage sludge. Chemosphere 69, 1368–1373 (doi:10.1016/j.chemosphere.2007.05.022) [DOI] [PubMed] [Google Scholar]
- Gibbs P., Bryan G.1987TBT paints and the demise of the dog-whelk, Nucella lapillus (Gastropoda). Oceans 19, 1482–1487 (doi:10.1109/OCEANS.1987.1160635) [Google Scholar]
- Gray L. E., Ostby J., Furr J., Price M., Veeramachaneni D. N. R., Parks L.2000Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365 (doi:10.1093/toxsci/58.2.350) [DOI] [PubMed] [Google Scholar]
- Grun F., et al. 2006Endocrine disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 20, 2141–2155 (doi:10.1210/me.2005-0367) [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Gross T. S., Masson J. R., Matter J. M., Percival H. F., Woodward A. R.1994Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect. 102, 680–688 (doi:10.2307/3432198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M.-H., Lee D.-H., Jacobs D. R.2007Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ. Health Perspect. 115, 1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday 1999The great stink of London: Sir Joseph Bazalgette and the cleansing of the Victorian metropolis Thrupp, Stroud, Gloucestershire: Sutton Publishers; [PubMed] [Google Scholar]
- Hansen L.1998Stepping backward to improve assessment of PCB congener toxicities. Environ. Health Perspect. 106, 171–189 (doi:10.2307/3433919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E. Z., Oakes S. R., Hysell M., Hay A.2006Organic chemicals in sewage sludge. Sci. Total Environ. 367, 481–497 [DOI] [PubMed] [Google Scholar]
- Hermsen S. A. B., et al. 2008In utero, lactational exposure to 2,3,7,8–tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology 253, 147–152 (doi:10.1016/j.tox.2008.09.005) [DOI] [PubMed] [Google Scholar]
- Holmstrup M.1997Drought tolerance in Folsomia candida Willem (Collembola) afterexposure to sublethal concentrations of three soil-polluting chemicals. Pedobiologia 41, 361–368 [Google Scholar]
- Hong S., Candelone J.-P., Patterson C. C., Boutron C. F.1996History of ancient copper smelting pollution during Roman and medieval times recorded in Greenland ice. Science 272, 246–249 (doi:10.1126/science.272.5259.246) [Google Scholar]
- Hoss S., Weltje L.2007Endocrine disruption in nematodes: effects and mechanism. Ecotoxicology 16, 15–28 (doi:10.1007/s10646-006-0108-y) [DOI] [PubMed] [Google Scholar]
- Hruska K. S., Furth P. A., Seifer D. B., Shahara F. I., Flaws J. A.2000Environmental factors in infertility. Clin. Obstet. Gynecol. 43, 821–829 (doi:10.1097/00003081-200012000-00014) [DOI] [PubMed] [Google Scholar]
- IEH 1999IEH assessment of the ecological significance of endocrine disruption: effects on reproductive function and consequences for natural populations (assessment A4) Leicester, UK: MRC Institute for Environment and Health [Google Scholar]
- Ives A. R., Carpenter S. R.2007Stability and diversity of ecosystems. Science 317, 58–62 (doi:10.1126/science.1133258) [DOI] [PubMed] [Google Scholar]
- Kelly B. C., Ikonomou M. G., Blair J. D., Morin A. E., Gobas F. A. P. C.2007Food web-specific biomagnification of persistent organic pollutants. Science 317, 236–238 (doi:10.1126/science.1138275) [DOI] [PubMed] [Google Scholar]
- Klaper R., Rees C. B., Drevnick P., Weber E. D., Sandheinrich M., Carvan M. J.2006Gene expression changes related to endocrine function and decline in reproduction in fathead minnow (Pimephales promelas) after dietary methylmercury exposure. Environ. Health Perspect. 114, 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A.2006Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int. J. Androl. 29, 193–198 (doi:10.1111/j.1365-2605.2005.00613.x) [DOI] [PubMed] [Google Scholar]
- Kortenkamp A.2007Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 115Suppl. 1, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats Z. E., Ciborowski J. H.1989Aquatic insect adults as indicators of organochlorine contamination. J. Great Lakes Res. 15, 623–634 [Google Scholar]
- Kuntz J., Nassr-Amellal N., Lollier M., Schmidt J. E., Lebeau T.2008Effect of sludges on bacteria in agricultural soil. Analysis at laboratory and outdoor lysimeter scale. Ecotoxicol. Environ. Saf. 69, 277–288 (doi:10.1016/j.ecoenv.2007.05.013) [DOI] [PubMed] [Google Scholar]
- Landrum P. F., Tigue E. A., Driscoll S. K., Gossiaux D. C., Van Hoof P. L., Gedeon M. L., Adler M.2001Bioaccumulation of PCB congeners by Diporeia spp.: kinetics and factors affecting bioavailability. J. Great Lakes Res. 27, 117–133 [Google Scholar]
- Langer P., Tajtakova M., Fodor G., Kocan A., Bohov P., Michalek J., Kreze A.1998Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated biphenyls. Eur. J. Endocrinol. 139, 402–409 (doi:10.1530/eje.0.1390402) [DOI] [PubMed] [Google Scholar]
- Law R. J., Alaee M., Allchin C. R., Boon J. P., Lebeuf M., Lepom P., Stern G. A.2003Levels and trends of polybrominated diphenylethers and other brominated flame retardants in wildlife. Environ. Int. 29, 757–770 (doi:10.1016/S0160-4120(03)00110-7) [DOI] [PubMed] [Google Scholar]
- Lee R. G. M., Thomas G. O., Jones K. C.2004PBDEs in the atmosphere of three locations in Western Europe. Environ. Sci. Technol. 38, 699–706 (doi:10.1021/es035042c) [DOI] [PubMed] [Google Scholar]
- Lind P. M., Bergman A., Olsson M., Orberg J.2003Bone mineral density in male Baltic seal (Halichoerus grypus). Ambio 32, 385–388 [DOI] [PubMed] [Google Scholar]
- Lind P. M., Milnes M. R., Lundberg R., Bermudez D., Orberg J., Guillette L. J.2004aAbnormal bone composition in female juvenile American alligators from a pesticide-polluted lake (Lake Apopka, Florida). Environ. Health Perspect. 112, 359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P. M., Orberg J., Edlund U.-B., Sjoblom L., Lind L.2004bThe dioxin-like pollutant PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol. Lett. 150, 293–299 (doi:10.1016/j.toxlet.2004.02.008) [DOI] [PubMed] [Google Scholar]
- Markich S. J., Jeffree R. A., Burke P. T.2002Freshwater bivalve shells as archival indicators of metal pollution from a copper-uranium mine in tropical Northern Australia. Environ. Sci. Technol. 36, 821–832 (doi:10.1021/es011066c) [DOI] [PubMed] [Google Scholar]
- Markman S., Guschina I. A., Barnsley S., Buchanan K. L., Pascoe D., Muller C. T.2007Endocrine disrupting chemicals accumulate in earthworms exposed to sewage effluent. Chemosphere 70, 119–125 (doi:10.1016/j.chemosphere.2007.06.045) [DOI] [PubMed] [Google Scholar]
- Markman S., Leitner S., Catchpole C., Barnsley S., Muller C. T., Pascoe D., Buchanan K. L.2008Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PLoS ONE 3, e1674 (doi:10.1371/journal.pone.0001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. D., O'Dea M. F., Moeckel A. M., Lerner D. T., Bjornsson B. T.2005Endocrine disruption of parr-smolt transformation and seawater tolerance of Atlantic salmon by 4-nonylphenol and 17β-estradiol. Gen. Comp. Physiol. 142, 280–288 [DOI] [PubMed] [Google Scholar]
- McCoy K. A., Bortnick L. J., Campbell C. M., Hamlin H. J., Guillette L. J., St Mary C. M.2008Agriculture alters gonadal form and function in the toad Bufo marinus. Environ. Health Perspect. 116, 1526–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts I. A. T. M., Letcher R. J., Hoving S., Marsh G., Bergman A., Lemmen J. G., van der Berg B., Brouwer A.2001In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 109, 399–407 (doi:10.2307/3454900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moral R., Wang R., Russo I. H., Lamartiniere C. A., Pereira J., Russo J.2008Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J. Endocrinol. 196, 101–112 (doi:10.1677/JOE-07-0056) [DOI] [PubMed] [Google Scholar]
- Morley N. J.2006Parasitism as a source of potential distortion in studies on endocrine disrupting chemicals in molluscs. Mar. Pollut. Bull. 52, 1330–1332 (doi:10.1016/j.marpolbul.2006.08.025) [DOI] [PubMed] [Google Scholar]
- Naert C., Van Peteghem C., Kupper J., Jenni L., Naegeli H.2007Distribution of polychlorinated biphenyls and polybrominated diphenyl ethers in birds of prey from Switzerland. Chemosphere 68, 977–987 (doi:10.1016/j.chemosphere.2007.01.009) [DOI] [PubMed] [Google Scholar]
- Nebert D. W.2005Inter-individual susceptibility to environmental toxicants—a current assessment. Toxicol. Appl. Pharmacol. 207, S34–S42 [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Padilla–Banks E., Snyder R. J., Phillips T. M., Jefferson W. N.2007Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod. Toxicol. 23, 290–296 (doi:10.1016/j.reprotox.2006.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom Joensen U., Skakkebaek N. E., Jorgensen N.2009Is there a problem with male reproduction? Nat. Clin. Pract. Endocrinol. Metab. 5, 144–145 [DOI] [PubMed] [Google Scholar]
- Norstrom R. J.2002Understanding bioaccumulation of POPs in food webs. Environ. Sci. Pollut. Res. 9, 300–303 (doi:10.1007/BF02987570) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., Di Benedetto P., Tillmann M., Duft M., Oetken M., Schulte-Oehlmann U.2007Endocrine disruption in prosobranch molluscs: evidence and ecological relevance. Ecotoxicology 16, 29–43 (doi:10.1007/s10646-006-0109-x) [DOI] [PubMed] [Google Scholar]
- Paul C., Rhind S. M., Kyle C. E., Scott H., McKinnell C., Sharpe R. M.2005Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge. Environ. Health Perspect. 113, 1530–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N., Silva E., Kortenkamp A.2002Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ. Health Perspect. 110, 917–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind S. M.2005Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod. Dom. Anim. 40, 282–290 (doi:10.1111/j.1439-0531.2005.00594.x) [DOI] [PubMed] [Google Scholar]
- Rhind S. M.2008Endocrine disruptors and other food contaminating environmental pollutants as risk factors in animal reproduction. Reprod. Dom. Anim. 43Suppl. 2, 15–22 [DOI] [PubMed] [Google Scholar]
- Rhind S. M., Smith A., Kyle C. E., Telfer G., Martin G., Duff E., Mayes R. W.2002Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants. J. Environ. Monitor. 4, 142–148 (doi:10.1039/b107539j) [DOI] [PubMed] [Google Scholar]
- Rhind S. M., Kyle C. E., Telfer G., Duff E. I., Smith A.2005aAlkyl phenols and diethylhexyl phthalate in tissues of sheep grazing pastures fertilised with sewage sludge or inorganic fertilizer. Environ. Health Perspect. 113, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind S. M., Kyle C. E., Owen J.2005bAccumulation of potentially toxic metals in the liver tissue of sheep grazed on sewage sludge-treated pastures. Anim. Sci. 81, 107–113 [Google Scholar]
- Rhind S. M., Kyle C. E., Mackie C., McDonald L.2009Accumulation of endocrine disrupting compounds (EDCs) in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge. J. Environ. Monitor. 11, 1469–1476 [DOI] [PubMed] [Google Scholar]
- Schiedek D., Sundelin B., Readman J., Macdonald R. W.2007Interactions between climate change and contaminants. Mar. Pollut. Bull. 54, 1845–1856 (doi:10.1016/j.marpolbul.2007.09.020) [DOI] [PubMed] [Google Scholar]
- Setchell B.1997Sperm counts in semen of farm animals 1932–1995. Int. J. Androl. 20, 209–214 (doi:10.1046/j.1365-2605.1997.00054.x) [DOI] [PubMed] [Google Scholar]
- Sharara F. I., Seifer D. B., Flaws J. A.1998Environmental toxicants and female reproduction. Fertil. Steril. 70, 613–622 (doi:10.1016/S0015-0282(98)00253-2) [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E.2008Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil. Steril. 89Suppl. 1, e33–e38 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Fisher J. S., Millar M. M., Jobling S., Sumpter J. P.1995Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ. Health Perspect. 103, 1136–1143 (doi:10.2307/3432610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. S.1981Male characteristics on female mud snails caused by antifouling paints. J. Appl. Toxicol. 1, 22–25 (doi:10.1002/jat.2550010106) [DOI] [PubMed] [Google Scholar]
- Smith S. R.1996Organic pollutants. In Agricultural recycling of sewage sludge and the environment, ch. 10, pp. 207–236 Wallingford, UK: CAB International [Google Scholar]
- Spurgeon D. J., Hopkin S. P., Jones D. T.1994The effects of cadmium, copper, lead and zinc on growth, reproduction and survival of the earthworm Eisenia fetida (Savigny): assessing the environmental impact of point-source metal contamination in terrestrial ecosytems. Environ. Pollut. 84A, 123–130 [DOI] [PubMed] [Google Scholar]
- Stevens J. L., Northcott G. L., Stern G. A., Tomy G. T., Jones K. C.2003PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in UK sewage sludge: survey results and implications. Environ. Sci. Technol. 37, 462–467 (doi:10.1021/es020161y) [DOI] [PubMed] [Google Scholar]
- Sures B.2006How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J. Helminthol. 80, 151–157 (doi:10.1079/JOH2006346) [DOI] [PubMed] [Google Scholar]
- Tonne C. C., Whyatt R. M., Camann D., Perera F. P., Kinney P. L.2004Predictors of personal polycyclic aromatic hydrocarbon exposure among pregnant minority women in New York City. Environ. Health Perspect. 112, 754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J., et al. 1996Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 104Suppl. 4, 741–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine M. F., Stein L., Weigle K., Schroeder J., Degnan D., Chiu-kit J. T., Hanchette C., Backer L.2000Effects on the immune system associated with living near a pesticide dump site. Environ. Health Perspect. 108, 1113–1124 (doi:10.2307/3434822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw C. M., Rhind S. M., Kyle C. E., Evans N. P.2007. Expression of the mRNAs for galanin and its receptors is suppressed by exposure to endocrine disrupting compounds during ovine foetal development. Proc. British Soc. Neuroendocrinol, 10–11 September 2007, Nottingham, UK [Google Scholar]
- Wienburg C. L., Shore R. F.2004Factors influencing liver PCB concentrations in sparrowhawks (Accipiter nisus), kestrels (Falco tinnunculus) and herons (Ardea cinerea) in Britain. Environ. Pollut. 132, 41–50 (doi:10.1016/j.envpol.2004.03.027) [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Hill J., Phillips J. C.2003The accumulation of potentially toxic metals by grazing ruminants. Proc. Nutr. Soc. 62, 267–277 [DOI] [PubMed] [Google Scholar]
- Wolfe W. H., Michalek J. E., Miner J. C., Rahe A. J., Moore C. A., Needham L. L., Patterson D. G.1995Paternal serum dioxin and reproductive outcomes among veterans of Operation Ranch Hand. Epidemiology 6, 17–22 [DOI] [PubMed] [Google Scholar]
- Yunker M. B., Macdonald R. W., Vingarzan R., Mitchell R. H., Goyette D., Sylvestre S.2002PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 33, 489–515 (doi:10.1016/S0146-6380(02)00002-5) [Google Scholar]