Abstract
Circadian rhythms are endogenous 24 h cycles that persist in the absence of external time cues. These rhythms provide an internal representation of day length and optimize physiology and behaviour to the varying demands of the solar cycle. These clocks require daily adjustment to local time and the primary time cue (zeitgeber) used by most vertebrates is the daily change in the amount of environmental light (irradiance) at dawn and dusk, a process termed photoentrainment. Attempts to understand the photoreceptor mechanisms mediating non-image-forming responses to light, such as photoentrainment, have resulted in the discovery of a remarkable array of different photoreceptors and photopigment families, all of which appear to use a basic opsin/vitamin A-based photopigment biochemistry. In non-mammalian vertebrates, specialized photoreceptors are located within the pineal complex, deep brain and dermal melanophores. There is also strong evidence in fish and amphibians for the direct photic regulation of circadian clocks in multiple tissues. By contrast, mammals possess only ocular photoreceptors. However, in addition to the image-forming rods and cones of the retina, there exists a third photoreceptor system based on a subset of melanopsin-expressing photosensitive retinal ganglion cells (pRGCs). In this review, we discuss the range of vertebrate photoreceptors and their opsin photopigments, describe the melanopsin/pRGC system in some detail and then finally consider the molecular evolution and sensory ecology of these non-image-forming photoreceptor systems.
Keywords: circadian, melanopsin, photopigment, photoreceptor
1. Introduction
The 24 h cycle of light and dark caused by the rotation of the Earth produces dramatic but predictable changes in the light environment. Instead of passively responding to these changes, organisms have evolved an endogenous representation of the 24 h day—a circadian timing system. These circadian clocks set the time or phase at which physiological and behavioural events occur with respect to the external 24 h environmental cycle. In this way, change can be anticipated, and physiology is optimized to the varying demands of night and day. This allows the organism to exploit the changed conditions as soon as they take place; avoiding the time lost in physiological and behavioural adjustments. However, the circadian system will only provide a selective advantage if biological time remains synchronized (entrained) to environmental time. Thus, the circadian oscillator requires a daily synchronization with the external environment via time cues termed zeitgebers (time givers) (Aschoff 1984; Pittendrigh 1993). The systematic daily change in the gross amount of environmental light (irradiance) at dawn or dusk provides the primary indicator of the time of day. As a result, most organisms have evolved to use the twilight transition as their main zeitgeber to adjust circadian time to local time. This process is termed photoentrainment (Roenneberg & Foster 1997).
The study of the photoreceptors mediating irradiance-detection tasks such as photoentrainment has led to the identification of a range of vertebrate opsins, and perhaps most remarkably, the identification of a novel photoreceptor system within the mammalian retina, a subset of retinal ganglion cells (RGCs) that express the photopigment melanopsin (Opn4). In this review, we will consider the range of vertebrate photoreceptors and their opsin photopigments and then provide an overview of the melanopsin/photosensitive retinal ganglion cell (pRGC) system in detail. Finally, we will discuss the molecular evolution of the opsin photopigments and consider the different selective pressures acting upon visual pigments and non-image-forming photopigments in general.
2. Vertebrate photoreceptors
In mammals, both visual and non-visual photoreception is ocular, and enucleation abolishes all responses to light (Nelson & Zucker 1981; Foster et al. 1991). By contrast, non-mammalian vertebrates possess a wide range of photoreceptive sites, including the pineal complex, deep-brain photoreceptors and dermal photoreceptors (Shand & Foster 1999). As well as being anatomically diverse (figure 1), these photoreceptors mediate many different aspects of physiology and behaviour. Identifying the extraretinal opsin photopigments that underlie these responses in non-mammalian photoreceptors has a long and fairly complex history. Many immunocytochemical studies were undertaken on these tissues, using a wide range of antibodies raised to different retinal or visual pigment preparations (Shand & Foster 1999). However, as the epitopes/specificities of many of these antibodies were not known, it was difficult to make any definitive conclusions regarding the molecular identity of the molecules labelled. The ambiguous terms ‘rod-like’ or ‘cone-like’ were frequently used to describe such immunolabelling. The molecular characterization of the extraretinal photopigments became further blurred with the discovery of multiple new opsin families, quite different from the rod and cone opsins. Parallel studies on mammals also produced results that were initially difficult to interpret. Although it was clear that mammals lack extraocular photoreceptors (Nelson & Zucker 1981; Foster et al. 1991), mice lacking rod and cone photoreceptors could still regulate multiple physiological responses to light (Freedman et al. 1999; Lucas et al. 1999). Further, these responses were clearly being mediated by an opsin/vitamin A-based photopigment system (Lucas et al. 2001). Yet until recently the molecular identity of this photoreceptor system remained unknown. In the past decade much new information has emerged regarding the location and function of the vertebrate non-rod, non-cone photoreceptor systems. Here in §2, we take the opportunity to summarize some of these findings, and in §3 we discuss the photopigment biochemistry in detail.
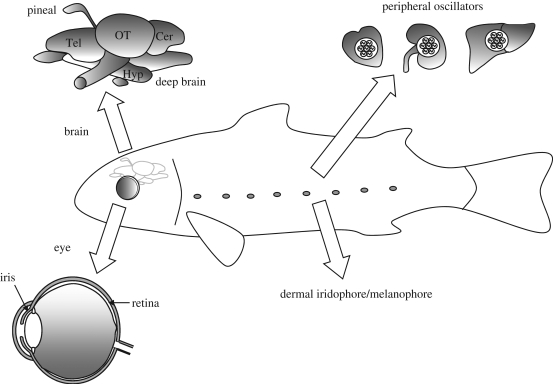
Figure 1.
Photoreceptive sites in the vertebrates. As well as the classical photoreceptors within the retina of the lateral eye, direct photoreception in the isolated iris has also been described. In non-mammalian species the pineal complex also contains photoreceptors, and deep brain photoreceptors may also occur. Dermal photoreception has been described in amphibians and fish. Finally, in the zebrafish peripheral tissues have been shown to be able to entrain their molecular oscillators directly to light. See text for further details.
(a). Lateral eyes
The lateral eyes are the most familiar photoreceptive site in vertebrates. The classical photoreceptors of the vertebrate retina consist of the rods and cones. Rods mediate scotopic (dim light) vision, providing low-resolution but high sensitivity, whereas cones are involved in photopic (bright light) vision, and enable high-resolution colour vision/contrast detection. Rod and cone light detection is characterized by rapid and transient electrical responses. The graded potentials from these receptors are processed by inner retinal neurons prior to advanced visual processing in the brain. Light information reaches the visual centres of the brain via topographically mapped axons of the RGCs that form the optic nerve (Rodieck 1998). But in addition to the ‘classical’ photoreceptors of the outer retina, other retinal cells are now also known to be capable of responding to light. A subset of RGCs (approx. 1% in the mouse) expresses the photopigment melanopsin (Opn4; Hattar et al. 2002) and are capable of responding to light directly (Berson et al. 2002; Sekaran et al. 2003). The identification of these pRGCs is discussed in more detail in §4.
In addition to melanopsin-based pRGCs, the teleost retina (and perhaps other non-mammalian retinae) possesses photosensitive horizontal cells. In the cyprinid retina of the roach (Rutilus rutilus), a subtype of the horizontal cell, termed HC-RSD, expresses both melanopsin and vertebrate ancient (VA) opsin and shows depolarizing responses to light that are maximally sensitive to approximately 477 nm. These cells have longer integration times than rods or cones and maintain their responses when classical photoreceptor inputs are saturated by background light (Jenkins et al. 2003). These cells may signal environmental irradiance as well as modulating rod and cone outputs.
(b). Pineal
Perhaps the best known photoreceptive site outside the retina is the pineal organ (epiphysis cerebri). Here we will use the term pineal complex to refer to the intracranial pineal proper as well as the parapineal and the extracranial ‘third’ eyes found in tuatara (Sphenadon punctatus, Rhynchocephalia), some lizards (Squamata) and frogs (Anura). The intracranial parapineal organ only occurs in some species of fish, and remarkably little is known about the physiological functions of this enigmatic organ (Vollrath 1981; Shand & Foster 1999). The extracranial third eyes can be further subdivided into the frontal organs (or Stirnorgan) of anuran amphibians and the parietal eyes found in lizards. The parietal eye shows remarkably structural similarity to the lateral eyes, with a transparent cornea and lens (Shand & Foster 1999).
Embryologically, the pineal complex is derived from an evagination of the dorsal diencephalon, similar to the retina, and in non-mammalian vertebrates is located near the surface of the brain (Vollrath 1981). In teleost and cyclostome species there is often a translucent window or area of reduced pigmentation overlying the pineal, allowing approximately 10 per cent of the incident light to reach the pineal. In amphibians, reptiles and birds, such a pineal window is less apparent or absent. Despite this, a considerable amount of light is still able to penetrate the overlying tissues, amounting to 0.1 per cent−0.3 per cent of the incident light (Dodt & Meissl 1982). In all non-mammalian vertebrates, the pineal complex is photoreceptive, and the predominant cell type is photoreceptor-like in appearance. In mammals, the pineal organ expresses many elements of the phototransduction cascade (Korf et al. 1985a,b), but lacks photosensitivity and appears exclusively secretory (Foster et al. 1989, 2003). The pineal organ is the primary source of the neurohormone melatonin, which is synthesized in the dark phase of the light/dark cycle, and acts as a signal of darkness to regulate circadian rhythms and photoperiodic responses (Arendt 1998; Korf et al. 1998). Melatonin synthesis is locally regulated by light at the level of the pineal in non-mammalian vertebrates, but in mammals photic information reaches the pineal via a multisynaptic pathway via the retinohypothalamic tract (RHT) and the superior cervical ganglion of the sympathetic nervous system (Korf & Moller 1984; Meissl 1997).
A range of opsins has been detected in the pineal complex of vertebrates (Shand & Foster 1999). One of the first extraretinal opsins to be identified, Pinopsin (P-opsin), was isolated from the avian pineal (Okano et al. 1994; Max et al. 1995). In the teleost pineal, a range of rod and cone opsins along with VA-opsin are expressed (Philp et al. 2000b). However, it appears that the predominant opsin in the fish pineal is a rod-like opsin (exo-rhodopsin/extraretinal rod-like opsin) which differs from that found in the lateral eyes (Mano et al. 1999; Philp et al. 2000a). Despite the lack of information about the structure and function of the parapineal organ a novel opsin photopigment, parapinopsin, has been isolated specifically from the parapineal of the channel catfish (Ictalarus punctatus) (Blackshaw & Snyder 1997) as well as from the lamprey pineal (Koyanagi et al. 2004). Most recently, studies on the parietal eye have identified the expression of two opsins within the same cell, a blue-sensitive pinopsin and a novel green-sensitive opsin named parietopsin (Su et al. 2006).
(c). Deep brain
Deep-brain photoreceptors were first described following studies by Karl von Frisch in 1911 on blinded and pinealectomized European minnows (Phoxinus phoxinus). These fish still demonstrated colour changes in response to light, leading to the suggestion of ‘deep-diencephalic photoreceptors’ (von Frisch 1911). Similarly, studies in blinded pinealectomized European eels (Anguilla anguilla) by van Veen et al. (1976) showed that deep-brain photoreceptors mediate photoentrainment as well as negative phototaxis. The photoperiodic response in birds, whereby gonadal growth is regulated by day length, is also mediated by a deep-brain photoreceptor rather than by the pineal complex or lateral eyes (Benoit 1964). Action spectroscopy provided a clue as to the molecular identity of these photoreceptors. An absorption corrected action spectrum for photoperiodic induction in the Japanese quail described an opsin/vitamin A-based photopigment with a λmax at 492 nm. A recent reanalysis of the original data suggests that the λmax may be closer to 483 nm (S. N. Peirson & R. G. Foster 2008, unpublished data). Although this action spectrum inferred the biochemistry of the photopigment, the precise molecular identity still remains unresolved. Attempts to characterize these photoreceptors have involved the use of immunocytochemical techniques employing antibodies raised against rod and cone photopigment opsins or other elements of the phototransduction cascade. Such approaches either failed to localize opsins within the avian hypothalamus or produced ambiguous results owing to the use of unknown epitopes (Silver et al. 1988).
(d). Iris
Light striking the isolated iris has been reported to produce rapid sphincter pupillae constriction in several non-mammalian species of vertebrate. For example, an opsin/vitamin A type action spectrum has been described from the isolated frog iris (Barr & Alpern 1963) and eel iris (Selinger 1962). In the isolated chick iris, there are marked and rapid responses to light (Tu et al. 2004). In some mammalian species, there have also been reports of an extremely gradual pupil constriction (occurring over at least 20 s to very bright stimuli) that survives both isolation of the iris from the eye and application of atropine (Bito & Turansky 1975; Lau et al. 1992). In all cases, a definitive photopigment characterization for iris photosensitivity is lacking, although in Xenopus laevis melanopsin has been implicated (Provencio et al. 1998).
(e). Dermal
Photoreception by dermal cells mediates colour changes in chromatophores and iridophores. Dermal photoreception has also been linked to the triggering of locomotor activity (Wolken & Mogus 1979; Shand & Foster 1999). Dermal chromatophores are photosensitive in many vertebrates, regulating the aggregation and dispersal of pigment granules within these cells (Weber 1983). Melanopsin, the photopigment of retinal pRGCs, was first isolated from Xenopus laevis melanophores (Provencio et al. 1998) and appears to be the photopigment mediating the pigment dispersal responses within dermal melanophores (Isoldi et al. 2005).
(f). Tissue photoreception
Perhaps the most surprising result in recent years has been the discovery that the circadian clocks located within the peripheral organs (such as the heart and kidney) of zebrafish can be entrained to light after being isolated and maintained in vitro (Whitmore et al. 2000). The photopigment(s) mediating these responses remain poorly understood, although the widespread expression pattern of teleost multiple tissue (TMT) opsin (see below) makes this opsin a strong candidate.
3. Opsin photopigments
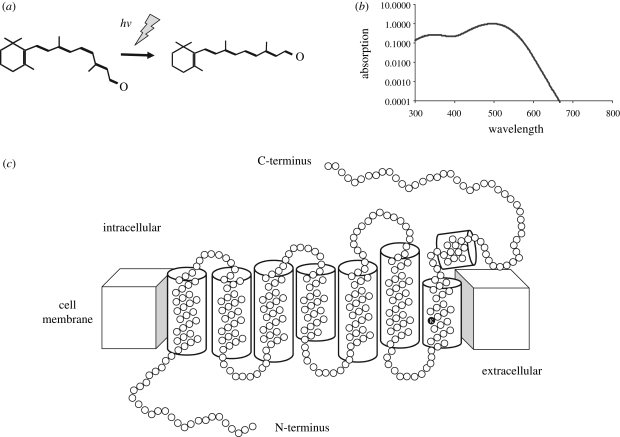
All vertebrate photoreceptors identified to date use an opsin/vitamin A-based photopigment. These photopigments consist of an opsin protein bound to a vitamin-A chromophore. In most terrestrial and marine vertebrates, this chromophore is 11-cis-retinaldehyde (A1), while in many freshwater vertebrates the chromophore is based upon 11-cis-3-dehydroretinal (A2) (Knowles & Dartnall 1977). The first stage of light detection involves the absorption of a photon by the retinal chromophore and the photoisomerization of this molecule to the all-trans state (figure 2a). The conformational change of the chromophore allows the opsin to interact with a G-protein (transducin) and triggers the phototransduction cascade, ultimately giving rise to a change in receptor membrane potential (Hargrave & McDowell 1992; Okada et al. 2001; Pepe 2001; Shichida & Matsuyama 2009). All vitamin A-based photopigments have a characteristic absorption spectrum. This means that although the maximal sensitivity (λmax) of the pigment may vary widely across the visible spectrum (ultraviolet at 360 nm to deep red at 620 nm), all these pigments have the same characteristic shape, a bell-shaped curve (figure 2b) (Dartnall 1953).
Figure 2.
Structure and function of vertebrate photopigments. Vertebrate photopigments consist of an isomer of vitamin A, retinaldehyde, bound to an opsin protein. (a) The primary step in phototransduction is the absorption of a photon of light (hv) by the 11-cis isomer of retinal resulting in isomerization to the all-trans form. (b) All vitamin A/opsin-based photopigments have a characteristic absorption spectrum which can be used as a ‘spectral fingerprint’ to determine the photopigment mediating a given biological response. (c) Opsins consist of a single polypeptide chain forming seven α-helical transmembrane regions connected by cytoplasmic and extracellular loops. The intracellular domains mediate G-protein interactions. The retinal binding site (K) is indicated in the 7th transmembrane domain. Structure based on that of Palczewski et al. 2000.
Opsins are members of the superfamily of G-protein-coupled receptors (GPCR) which function through the activation of a guanine nucleotide binding protein (G-protein) and an effector enzyme. All opsins consist of a single polypeptide chain of 340–500 amino acids that form seven α-helical transmembrane regions connected by cytoplasmic and extracellular loops (figure 2c). These seven α-helices form a bundle within the membrane, creating a hollow cavity on the extracellular side that serves as a binding site for the chromophore, retinal. There are also several other features usually present in an opsin: the retinal attachment site is a lysine residue (at position 296 numbered as in bovine rod opsin) located in the seventh transmembrane domain which binds the chromophore via a protonated Schiff base linkage; a glutamate counterion at position 113 in the third transmembrane domain; a glutamate, arginine and tyrosine (ERY, 134–136) tripeptide for G-protein interaction; and cysteines (C) at positions 110 and 187 for disulfide bridge (Palczewski et al. 2000).
A wide range of opsins have been identified within the vertebrates, with currently 15 distinct gene families (table 1). These are described in detail below and a phylogenetic analysis to illustrate their molecular evolution is shown in figure 3.
Table 1.
Vertebrate opsin subgroups. Table showing the amino acid identity (%) across the ‘core’ region of representatives of the various vertebrate opsin classes. The core region is defined by residues 34–306 of the bovine rod opsin model of Palczewski et al., (2000). The chicken sequences were used where available, other species are stated below. Accession numbers: rod opsin D00702; LWS M62903; MWS M92038; SWS M92037; versus M92039; RGR AY339627; peropsin AY339626; pinopsin U15762; Opn4m AY036061; Opn4x AY882944; Opn5 XM_001130743; catfish parapinopsin AF028014; lizard parietopsin, DQ100320; zebrafish VA1 AB035276; zebrafish VA2, AY996588; pufferfish exo-rod AF201472; human OPN3 NM_014 322; pufferfish TMT AF402774. Abbreviations as follows: exo, exo-rod; per, peropsin; P, pinopsin; PP, parapinopsin; par, parietopsin.
| opsin | rod | LWS | MWS | SWS1 | SWS2 | RGR | per | P | PP | par | VAa | VAb | exo | Opn3 | TMT | Opn4m | Opn4x | Opn5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rod opsin | — | |||||||||||||||||
| LWS opsin | 41 | — | ||||||||||||||||
| MWS opsin | 69 | 42 | — | |||||||||||||||
| SWS1 opsin | 45 | 40 | 49 | — | ||||||||||||||
| SWS2 opsin | 51 | 41 | 53 | 50 | — | |||||||||||||
| RGR | 28 | 22 | 21 | 21 | 23 | — | ||||||||||||
| peropsin | 27 | 24 | 23 | 21 | 24 | 24 | — | |||||||||||
| pinopsin | 46 | 49 | 48 | 47 | 51 | 24 | 26 | — | ||||||||||
| parapinopsin | 40 | 40 | 40 | 40 | 41 | 24 | 28 | 48 | — | |||||||||
| parietopsin | 36 | 35 | 35 | 30 | 37 | 23 | 27 | 42 | 42 | — | ||||||||
| VAa | 38 | 42 | 40 | 41 | 43 | 22 | 27 | 43 | 43 | 41 | — | |||||||
| VAb | 37 | 40 | 40 | 41 | 41 | 20 | 26 | 45 | 46 | 40 | 80 | — | ||||||
| exo-rod | 78 | 42 | 66 | 46 | 49 | 27 | 27 | 48 | 40 | 35 | 39 | 36 | — | |||||
| Opn3 | 30 | 26 | 31 | 27 | 32 | 25 | 29 | 30 | 29 | 29 | 31 | 30 | 30 | — | ||||
| TMT | 33 | 34 | 34 | 35 | 36 | 22 | 29 | 38 | 39 | 37 | 36 | 34 | 30 | 41 | — | |||
| Opn4m | 30 | 30 | 30 | 32 | 27 | 26 | 27 | 28 | 29 | 31 | 29 | 29 | 30 | 27 | 34 | — | ||
| Opn4x | 29 | 33 | 29 | 28 | 26 | 27 | 29 | 29 | 28 | 30 | 28 | 28 | 32 | 31 | 34 | 59 | — | |
| Opn5 | 25 | 24 | 26 | 20 | 25 | 26 | 30 | 26 | 25 | 26 | 26 | 24 | 25 | 28 | 29 | 33 | 29 | — |
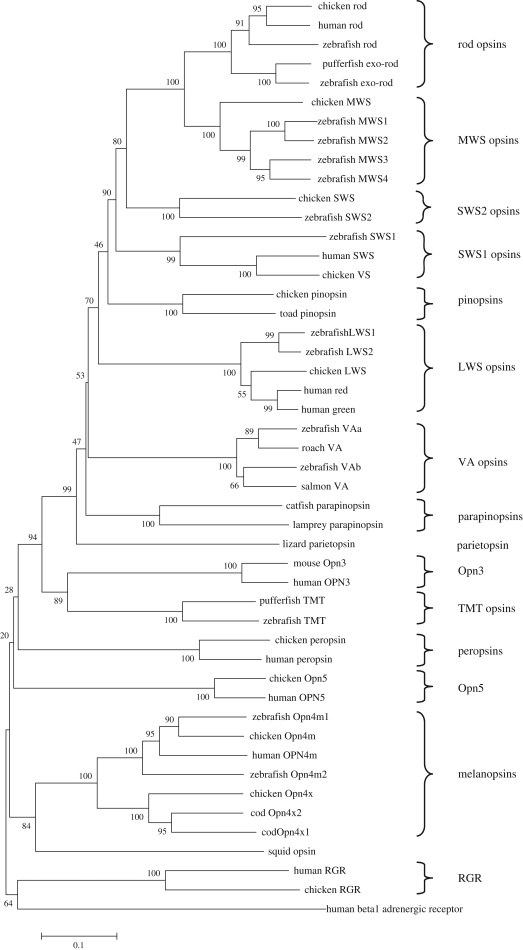
Figure 3.
Phylogenetic tree showing the relationship of the various classes of vertebrate opsins. The entire amino acid sequences were aligned using ClustalW (Higgins et al. 1996) and the tree was generated by the neighbour joining method (Saitou & Nei 1987) using the MEGA4 program (Tamura et al. 2007). Branch confidence levels (% based on 1000 bootstrap replicates) are marked. Scale bar indicates substitutions per site. The human beta 1 adrenergic receptor was used as an outgroup. The major classes of the vertebrate opsins are indicated by parentheses on the right-hand side. The analysis reveals that the exo-rod opsins are a duplication of the rod opsins and that pinopsin has arisen by a duplication of the cone opsins. To date there is only one sequence for parietopsin (see text for details). Opn3 and TMT, both expressed in multiple tissues, seem to share a common ancestor. The Opn4 sequences now quite clearly consist of two families, the mammalian-like ‘m’ form and the Xenopus-like ‘x’ form. Interestingly the opsin from the invertebrate squid also included in the analysis clades with the melanopsin sequences, adding credence to the argument that melanopsin is ‘invertebrate’-like. Finally RGR is the least similar to the visual opsins. Accession numbers: Chicken: rod D00702; LWS M62903; MWS M92038; SWS M92037; versus M92039; RGR AY339627; peropsin AY339626; pinopsin U15762; Opn4m AY036061; Opn4x AY882944; Opn5 XM_001130743. Human: rod NM_000539; red NM_020061; green NM_000513; SWS NM_001708; RGR NM_002921; peropsin NM_006583; OPN3 NM_014322; OPN4M NM_033282; OPN5 NM_181744; beta 1 adrenergic receptor NM_000684. Zebrafish: rod NM_131084; LWS1, NM_001002443; LWS2, NM_131175; MWS1, NM_131253; MWS2, NM_182891; MWS3, NM_182892; MWS4, NM_131254; SWS1, NM_131319; SWS2, NM_131192; exo-rod, AB025312; VA1, AB035276; VA2, AY996588; TMT, AF349947; Opn4m1, AY882945; Opn4m2, AY078161. Pufferfish: exo-rod, AF201472; TMT, AF402774. Salmon VA AF001499. Roach VA AY116411. Catfish parapinopsin AF028014. Lamprey parapinopsin AB116380. Lizard parietopsin DQ100320. Toad pinopsin AF200433. Mouse Opn3 NM_010098. Cod Opn4x1 AF385823. Opn4x2 AY126448. Squid opsin, P09241.
(a). Visual rod/cone opsins (Opn1, Opn2)
The well-characterized photoreceptors of the vertebrate eye are the rods and cones of the outer retina. These cells contain an opsin located in the lamellae of their outer segments which were named after the photoreceptor class in which they have been found, although they have now been given gene symbols, Opn1 for cone opsins and Opn2 for rod opsin. Bovine rod opsin was the first opsin to be cloned and sequenced (Nathans & Hogness 1983) and is still the only vertebrate opsin to have its crystal structure determined (Palczewski et al. 2000). There are four classes of cone opsins, long-wave sensitive (LWS) with a λmax between 500 and 620 nm; medium wave sensitive (MWS) λmax of 480–520 nm; ultraviolet/violet sensitive (SWS1) λmax 355–435 nm and short-wave sensitive (SWS2) λmax 415–470 nm. The various cone classes show 40–50% amino acid identity to each other (table 1). The SWS2 and MWS opsins have been lost in the mammalian lineage (Bowmaker & Hunt 1999).
(b). RPE opsins (RGR, peropsin)
Two opsin classes have been shown to be expressed in the retinal pigment epithelium (RPE), retinal G-protein-coupled receptor (RGR) and peropsin. RGR was originally identified from a bovine RPE library and although it contains the lysine required for chromophore attachment it has a histidine at the position of the potential counterion (Jiang et al. 1993). This sequence and its expression in the RPE and the Müller cells led to suggestions that it may function as an 11-cis-retinal transporter or an all-trans retinal photoisomerase (Jiang et al. 1993; Pandey et al. 1994). Although further studies showed that RGR will bind all-trans-retinal and generate 11-cis-retinal in the RPE (Hao & Fong 1996), more recent work by the same group has shown that the isomerized 11-cis-retinal does not readily dissociate from RGR, so it does not make a significant contribution to the pool of 11-cis-retinal (Hao & Fong 1999).
Peropsin, also called RPE-derived rhodopsin homologue, was isolated in 1997 (Sun et al. 1997) and only shows approximately 27 per cent identity to the visual opsins. It is localized to the RPE and there is some evidence that it shares a common ancestor with RGR. This has led to the speculation that it may also function as a retinal isomerase (Bellingham et al. 2003). Melanopsin has also been shown to be expressed in the RPE (Peirson et al. 2004), although whether this opsin forms a functional photopigment in the RPE remains unclear. Further work is required to clarify the role of these RPE opsins.
(c). Pineal opsins (pinopsin, parapinopsin, parietopsin, exo-rod)
As discussed in §2b, the pineal complex is photosensitive in non-mammalian vertebrates and multiple opsins have been isolated from this part of the brain.
(i). Pinopsin
Pinopsin was the first extraretinal opsin to be cloned and was isolated from the pineal gland of the chicken (Okano et al. 1994; Max et al. 1995). It showed 43–48% amino acid identity to the vertebrate visual opsins (table 1) and in chicken is expressed exclusively in the pineal. Several groups have reported the in vitro expression and reconstitution of pinopsin with 11-cis-retinal. All report the formation of a blue-sensitive pigment, but with slightly different λmax values: approximately 470 nm (Okano et al. 1994); approximately 462 nm (Max et al. 1998); and approximately 460 nm (Nakamura et al. 1999). Pinopsin has also been identified from the Reptilia (Kawamura & Yokoyama 1997) and Amphibia (Yoshikawa et al. 1998). Pinopsin has been localized to the anterior preoptic nucleus of the hypothalamus in the toad (Yoshikawa et al. 1998) and interestingly in both the retina and pineal of a diurnal gecko (Taniguchi et al. 2001). By contrast, a study on the Ruin lizards (Podarcis sicula) suggested that pinopsin was only expressed in the pineal complex (Frigato et al. 2006). Pinopsin orthologues have not, to date, been isolated from either fish or mammals.
(ii). Parapinopsin
Despite the lack of information about the structure and function of the teleost parapineal, a novel opsin photopigment—parapinopsin—has been isolated from this organ. Until recently there was only one reported sequence for parapinopsin isolated from the channel catfish (Ictalurus punctatus; Blackshaw & Snyder 1997). The sequence shows 40 per cent identity to other vertebrate opsins (table 1) and is expressed in a majority of parapinealocytes and a subset of pineal cells. However, a homologue of parapinopsin was isolated from the lamprey pineal complex and appears to form a bi-stable photopigment (Koyanagi et al. 2004). In situ hybridization showed that lamprey parapinopsin is expressed in the photoreceptor cells located in the dorsal region of the pineal and parapineal organs. The authors also demonstrated that lamprey parapinopsin photopigment has a λmax at 370 nm and that UV light causes cis–trans isomerization of its retinal chromophore, forming a stable photoproduct with λmax at 515 nm (Koyanagi et al. 2004). The authors of this paper also report the isolation of parapinopsin sequences from the rainbow trout and the clawed frog which exhibit 61 and 71 per cent amino acid identity, respectively, to the lamprey sequence (Koyanagi et al. 2004).
(iii). Parietopsin
Most recently, studies on another photoreceptive structure, the parietal eye of a lizard, have identified expression of two opsins within the same photoreceptor, a blue-sensitive pinopsin and a novel green-sensitive opsin named parietopsin (Su et al. 2006). These findings are consistent with the observation that the parietal eye photoreceptors have two antagonistic light signalling pathways, a hyperpolarizing pathway maximally sensitive to blue light and a depolarizing pathway maximally sensitive to green light (Solessio & Engbretson 1993). Parietopsin showed the highest degree of amino acid identity (approx. 40%) to parapinopsin (table 1) (Su et al. 2006).
(iv). Exo-rod opsin
Vigh-Teichmann and colleagues first reported the presence of opsin immunoreactivity in the teleost pineal in the early 1980s (Vigh-Teichmann et al. 1982, 1983). However, it was not until the independent isolation of a rod-like opsin from the pineal of the zebrafish (Mano et al. 1999) and from the pufferfish and Atlantic salmon (Philp et al. 2000a) that the molecular identity of this opsin was elucidated. Exo-rod opsins are 74 per cent identical to the retinal rod opsin from the same species suggesting that they diverged early in the teleost lineage (Philp et al. 2000a). Their expression is restricted to the pineal gland and their exact function remains unknown.
(d). VA opsin
VA opsin was first described in the Atlantic salmon (Salmo salar) (Soni & Foster 1997) and was subsequently isolated from several other teleost fish: zebrafish (Danio rerio) (Kojima et al. 2000), the common carp (Cyprinus carpio) (Moutsaki et al. 2000), a smelt fish (Plecoglossus altivelis) (Minamoto & Shimizu 2002) and roach (Rutilus rutilus) (Jenkins et al. 2003). VA opsins show 37–41% identity with the rod and cone opsins and approximately 43 per cent identity to other non-visual opsins such as pinopsin (table 1). Phylogenetic analysis suggests that the VA opsins diverged from a common ancestor before the other known opsin families (Soni & Foster 1997). Lamprey (Petromyzon marinus) pinopsin originally described by Yokoyama and Zhang (Yokoyama & Zhang 1997) is now considered to be a member of the VA family (Moutsaki et al. 2000; Bellingham & Foster 2002).
Functional studies demonstrated that salmon VA opsin can form a photopigment with a λmax between 460 and 480 nm when expressed in vitro and reconstituted with 11-cis-retinal (Soni et al. 1998). Significantly VA opsin was shown to be expressed in a subset of horizontal cells and retinal ganglion cells (Soni et al. 1998). Subsequently VA opsin was shown to be expressed within the pineal organ and epithalamic/hypothalamic regions of the teleost brain (Philp et al. 2000b), sites strongly implicated as photoreceptive in fish. Similar findings were reported in the zebrafish (Kojima et al. 2000).
Two VA opsin isoforms were isolated in zebrafish, a long (VAL) and short (VAS) form, which vary in the length of their C-terminal tails (74 and seven amino acids, respectively). Both isoforms were functionally expressed in human embryonic kidney cells 293S but only VAL appeared capable of forming a photopigment when reconstituted with 11-cis-retinal (Kojima et al. 2000). Studies of several other teleosts, such as carp (Cyprinus carpio) (Moutsaki et al. 2000), smelt (Plecoglossus altivelis) (Minamoto & Shimizu 2002) and roach (Rutilus rutilus) (Jenkins et al. 2003) have confirmed the existence of different isoforms of VA opsins. In all cases, the shorter isoforms appear to be generated by intron retention at a splice site. A comparison of the known teleost sequences indicates that they fall into two groups, one consisting of zebrafish, roach and carp, the other of smelt and salmon. This split might be explained by the identification of a second VA gene in zebrafish, named VAL-opsin B by the authors (Kojima et al. 2008) (table 1). The newly isolated gene clades with the smelt and salmon sequences. The functional significance of this gene duplication in the teleost genome remains unclear.
These results in teleost fish prompted the search for orthologues of VA opsin in other vertebrate classes, but until recently attempts have met with failure. This restricted taxonomic distribution of the VA opsins was puzzling as most other opsins classes span multiple vertebrate taxa. Recent and unpublished studies have led to the isolation of the full-length sequence of chicken VA. The gene contains an open reading frame of 972 base pairs and encodes a predicted protein of 323 amino acids. Further studies have also identified VA-like genes in the Amphibia (Xenopus tropicalis), Reptilia (Anolis carolinensis) and the Elasmobranchii (Callorhinchus milii), but have failed to find any VA homologues within the mammalian lineage (S. Halford & R. G. Foster 2008, unpublished data). This surprising finding raises important questions as to the possible function of this opsin within vertebrate taxa.
(e). Encephalopsin/panopsin (Opn3)
Opn3 was originally termed encephalopsin, and reported to be an extra-ocular opsin with strong expression in mouse brain and testis, with lower levels in the heart, liver and kidney (Blackshaw & Snyder 1999). However, a subsequent study demonstrated that Opn3 was in fact expressed in the retina and in all tissues examined; hence the name ‘panopsin’ was proposed (Halford et al. 2001). A more recent study on human OPN3 confirms the wide tissue distribution and in multiple sites within the retina including the rods and cones, the outer plexiform, inner plexiform and ganglion cell layers of the retina (White et al. 2008). A comparison of Opn3 with the visual opsins shows a low amino acid identity of approximately 30 per cent (table 1). Opn3 contains a lysine, at residue 299, which is required for Schiff base linkage. This residue is equivalent to position 296 in bovine rod opsin but the counterion, usually a glutamate at position 113, is replaced by an aspartate. This does not preclude the formation of a photopigment as an aspartate residue is also present in the UV photopigment of Xenopus. The function of Opn3/OPN3 is further complicated by considerable alternate splicing of the human Opn3 gene (Kasper et al. 2002).
(f). TMT opsin
Isolated organs and cell lines from zebrafish have been shown to exhibit circadian oscillations in clock gene expression that can be entrained to light (Whitmore et al. 2000). These data provide strong evidence for the existence of a photopigment within these cells. TMT opsin was isolated in 2003 as part of a study to identify the photopigment or pigments in these peripheral tissues (Moutsaki et al. 2003). The full-length sequence of TMT opsin was isolated from Fugu and encodes a predicted protein of 402 amino acids, containing all of the essential features of an opsin photopigment including a lysine residue at position 296. Interestingly the Schiff base counterion, usually a glutamate, is substituted by a tyrosine in both Fugu and zebrafish TMT. TMT opsin shows 33–39% identity when compared to other vertebrate opsins (table 1). The gene was given its name because it is expressed in the liver, kidney and heart as well as eye and brain, and to date, has only been isolated from teleost fish. Phylogenetic analysis reveals that it clades with Opn3 (figure 3), which also exhibits a multiple pattern of tissue expression. The function of this gene family remains completely unresolved.
(g). Melanopsin (Opn4)
Melanopsin was originally isolated from the photosensitive melanophores of Xenopus (Provencio et al. 1998). Subsequently, orthologs of melanopsin were isolated from mammals and shown to be expressed in a subset of RGCs (Provencio et al. 2000). In mammals, light information reaches the master circadian pacemaker, the suprachiasmatic nuclei (SCN), through a dedicated monosynaptic pathway that originates in the retina and is called the RHT. The anatomy and distribution of these melanopsin expressing cells was very similar to the RGCs that form the RHT. Subsequent analysis showed that these RGCs, which only account for approximately 1 per cent of the total, are directly photosensitive (pRGCs) (Hattar et al. 2002). See below for further discussion. Mouse Opn4 encodes a predicted protein of 521 amino acids and contains the lysine residue at position 337 necessary for Schiff base formation. Melanopsin has now been isolated from a range of species including zebrafish (Bellingham et al. 2002) and the chicken (Chaurasia et al. 2005). In all cases the Opn4 opsins contain a tyrosine at the counterion position, like peropsin and TMT opsin, rather than a glutamate. The melanopsins show a relatively low level of identity with the photopigment opsins, approximately 27 per cent (table 1). Recently two melanopsin genes, Opn4m and Opn4x, have been described in non-mammalian vertebrates (Bellingham et al. 2006). But to date only one form, Opn4m, has been isolated from the placental and marsupial mammals (Pires et al. 2007). Preliminary in silico analysis also suggests that the monotreme the platypus lacks the Opn4x gene (S. Halford & R.G. Foster unpublished data).
(h). Neuropsin (Opn5)
Neuropsin (Opn5) was identified in 2003 using a bioinformatic approach (Tarttelin et al. 2003). Opn5 encodes a predicted protein of 377 amino acids in the mouse and 354 amino acids in human, with the mouse having a longer C-terminal tail. All of the expected features of an opsin are conserved, but Opn5 shows only 25–30% identity to the vertebrate members of the opsin superfamily (table 1). RT-PCR suggests that Opn5 is expressed in mouse testis, brain and eye and in human retina and brain. Further work is necessary to establish both the type of retinal cells that express Opn5/OPN5 and whether it can function as a photopigment.
4. A novel retinal photoreceptor
The identification of multiple photoreceptors, and the non-rod, non-cone opsin-based photopigments across the vertebrates were not predicted. Indeed, the assumption until the early 1990s was that some form of conventional rod- or cone-like opsin would mediate all forms of vertebrate photoreception, both visual and extraocular. But perhaps the least expected result to emerge has been the discovery of another class of photoreceptor within the eye, quite distinct from the rods and cones. Until recently it was inconceivable to most vision biologists that there could be an unrecognized class of photoreceptor within the vertebrate eye. After all, the eye was the best understood part of the central nervous system. One hundred and fifty years of research had explained how we see: photons are detected by the rods and cones and their graded potentials are assembled into an image by inner retinal neurons, followed by advanced visual processing in the brain. But image detection is very different from the demands of irradiance detection. Rods and cones are highly sensitive radiance detectors, which rapidly adapt and can only integrate signals of a short duration. By contrast, the circadian system is relatively insensitive to light, requiring high intensity and long duration stimuli to bring about photoentrainment. The appreciation that the mammalian eye has to perform two quite radically different sensory tasks triggered a line of enquiry that ultimately led to the discovery of a population of pRGC which use the photopigment melanopsin. The key findings that have led to this discovery are outlined next.
(a). Retinal mutant studies
Initial studies to determine which retinal photoreceptors mediate circadian photoentrainment took advantage of a naturally occurring mutation in mice, termed retinal degeneration (rd/rd). These animals lack rods, and show a greatly reduced number of cones. As might be expected, rd/rd mice fail to show any classical visual responses to light. Studies were then undertaken to determine whether the circadian system of rd/rd mice was similarly impaired. When a mouse is housed under a light–dark cycle, its circadian wheel running behaviour is entrained. As would be expected of a nocturnal species, mice are largely active during the dark and inactive in the light. Under conditions of constant darkness, entrainment is lost and circadian behaviour drifts or freeruns with a recurring period approximately 23.5 h, starting its activity cycle approximately 0.5 h earlier every day. If the mouse is then exposed to a pulse of light shortly after activity onset it will delay the onset of activity the following day. The magnitude of this phase delay (Δϕ) is intensity dependent and can be used to determine the sensitivity of circadian responses to light. Both the wavelength and intensity of the light pulse can be systematically varied and the effect of these treatments on delaying wheel running behaviour can be assessed in a dose-dependent manner to produce a series of irradiance response curves.
Initial studies on photoentrainment in rd/rd mice (C3H) and wild-type controls (C57) demonstrated that these mice could still entrain, although the threshold for entrainment in rd/rd animals was approximately 2 log units higher (Ebihara & Tsuji 1980). The difference in the genetic background in these animals (C3H versus C57) appears to account for this difference in sensitivity (Foster & Helfrich-Forster 2001). Remarkably, in mice of the same genetic background, the massive loss of classical photoreceptors in the rd/rd mutants had little or no effect on the ability of the mice to either entrain to a light–dark cycle or phase-shift circadian rhythms in wheel running behaviour (Foster et al. 1991). Their irradiance response curves were indistinguishable from congenic wild-type controls, while eye loss completely blocked all effects of light on the clock (Foster et al. 1991). These studies demonstrated that the processing of light for circadian and visual responses must be different and hinted at the fact that there may be another class of ocular photoreceptor. Such suggestions were met with considerable scepticism and the favoured explanation was that as approximately 5 per cent of the cones survive in the retina of rd/rd mice (Carter-Dawson et al. 1978), it is probable that only a small number of photoreceptors are necessary for photoentrainment. Action spectrum studies on the spectral sensitivity of phase-shifting responses in rd/rd mice were subsequently conducted, suggesting a maximum sensitivity at either 511 or 480 nm, but these studies again failed to exclude the possibility of a residual cone contribution to these responses (Provencio & Foster 1995; Yoshimura & Ebihara 1996). The development of a mouse model lacking all rods and cones, the rd/rd cl, finally resolved these issues, and demonstrated that both phase-shifting responses and pineal melatonin suppression in response to light were apparently normal even when rod or cone opsins were undetectable (Freedman et al. 1999; Lucas et al. 1999).
The results from rd/rd cl mice provided the conclusive evidence that an additional photoreceptor exists within the mammalian eye and the conceptual framework for a host of further studies, including the finding that non-rod, non-cone photoreceptors do more than regulate the circadian system. Two examples are listed here: (i) In mammals, light-induced pupil constriction is regulated by both rods and cones, but still occurs in animals showing profound damage to these photoreceptors. Not unreasonably, it was assumed that the residual pupil light response was owing to the survival of a few rod and/or cone photoreceptors. Pupil measurements were undertaken in rd/rd cl mice and showed that these animals maintained a pupillary light response. Although less sensitive than congenic wild-type animals, rd/rd cl mice retained the ability to fully constrict their pupils (Lucas et al. 2001). (ii) Nocturnal rodents will inhibit their general activity when exposed to light during the night. This response, called masking, is thought to complement circadian entrainment by ensuring that activity is restricted to the hours of darkness or near darkness. Masking may be particularly important in environments where day length changes rapidly, and circadian behaviour may have difficulty keeping up with the expanding photoperiod (Mrosovsky 1999). Masking experiments were undertaken in rd/rd cl mice and demonstrated that there is marked inhibition of activity upon exposure to light presented two hours after normal lights off (Mrosovsky et al. 2001). Thus phase shifting, melatonin suppression, pupil constriction, masking and a number of other responses to light, such as sleep regulation (Lupi et al. 2008) are either intact or retained at some degree in mice lacking all their rods and cones.
The first action spectrum to be published on rd/rd cl mice was for pupil constriction, and the results described an opsin/vitamin A-based photopigment with a λmax in the blue part of the spectrum near 480 nm (opsin photopigment/OP480). The known visual pigments of the mouse have λmax values of 360, 508 and 498 nm for the ultraviolet-sensitive cone, long-wavelength sensitive cone and rod pigments, respectively. None of these classical photoreceptors could account for the pupillary responses to light (Lucas et al. 2001). Since 2001, a plethora of action spectra from mice to man have been deduced for a range of irradiance responses to light. These include the light responses of pRGCs in mice (Hattar et al. 2003), rats (Berson et al. 2002) and primates (Dacey et al. 2005) spanning pupil constriction, phase shifting circadian rhythms, plasma melatonin suppression, together with irradiance dependent regulation of human retinal cone function (Hankins & Lucas 2002). All these action spectra point to the existence of a single novel opsin photopigment with a λmax of around 480 nm. A single invariant spectral sensitivity for the pRGCs is in marked contrast to the cone pigments, which are highly divergent and appear spectrally tuned in a species-specific manner. It remains unclear what ecological advantage this wavelength might confer on such diverse species. One possibility is that the pRGCs are tuned to the dominant wavelength of light at twilight. When the sun is close to the horizon there is relative enrichment of ‘blue’ light in the dome of the sky because of the preferential scattering of short wavelengths of light passing obliquely through the atmosphere.
(b). Photosensitive retinal ganglion cells
The identification of the cells mediating non-rod, non-cone responses to light was provided by two sets of experiments. Studies on the rat used retrograde tracers injected into the SCN coupled with single cell recordings on isolated retina in which rod and cone responses were pharmacologically blocked (Berson et al. 2002). Parallel studies were undertaken using calcium imaging on the rd/rd cl retina (Sekaran et al. 2003). Both approaches identified a population of RGCs which responded directly to light. Significantly these pRGCs expressed the photopigment melanopsin (Hattar et al. 2002).
(c). Melanopsin knockout studies
The essential data that melanopsin plays a critical role in the transduction of light information in pRGCs came from gene ablation studies. Melanopsin knockout mice (Opn4−/−) exhibited attenuated phase-shifting and pupillary responses to light, as well as reduced period lengthening in constant light (LL) (Panda et al. 2002; Ruby et al. 2002; Lucas et al. 2003). However, the critical involvement of melanopsin in photoreception came from triple-knockout studies, lacking rods, cones and melanopsin. These animals were totally unresponsive to light, demonstrating that melanopsin is in some way essential for pRGC photosensitivity (Hattar et al. 2003), but precisely what function melanopsin was playing was only finally resolved by using functional expression studies.
(d). Melanopsin expression studies
The first investigation of the biochemistry of melanopsin involved expression of melanopsin in COS cells and reconstitution with 11-cis-retinal, an approach which has been particularly successful with visual pigments. This study produced a functional photopigment that was capable of activating transducin with a λmax between 420 and 440 nm, an absorption maxima considerably shifted away from OP480 (Newman et al. 2003). The discrepancy in λmax between spectroscopy and action spectra, coupled with low pigment yields, prompted other researchers to investigate whether expression of melanopsin alone was enough to confer photosensitivity. Quite independently, three groups combined the expression of melanopsin protein with physiological assays of cellular photosensitivity. All three studies showed that melanopsin transfection can confer photosensitivity to non-photosensitive cell types (Neuro-2a; HEK293-TRPC3; Xenopus oocyte) (Melyan et al. 2005; Panda et al. 2005; Qiu et al. 2005). In addition, these groups were able to show that specific forms of retinal (especially 11-cis-retinal) are needed for these responses to light, that light will ultimately trigger the release of intracellular calcium, and that this may involve a Gq-type G-protein rather than transducin. Furthermore, melanopsin acts as a bistable pigment able to regenerate (recycle) its chromophore (11-cis-retinal) using all-trans-retinal and long-wavelength light in a manner reminiscent of the invertebrate photopigments (Melyan et al. 2005). In this regard melanopsin may be unique among mammalian photopigments in forming a stable association with all-trans-retinal.
Expression studies on human melanopsin suggest that the λmax of light responses is close to 420–430 nm, and in this regard the findings were similar to those obtained by Newman and colleagues (Melyan et al. 2005). The studies on murine melanopsin, however, showed an action spectrum for light responses that exhibited a λmax very close to 480 nm (Qiu et al. 2005). The current consensus from the various groups is that something about the local environment in which melanopsin is reconstituted is important in determining its λmax.
(e). Melanopsin phototransduction
Most recently, research has turned to the phototransduction cascade used by melanopsin. Rod and cone opsins mediate a phototransduction cascade that involves the activation of transducin (a member of the Gi/G0 class of G-proteins), phosphodiesterase and closure of cyclic nucleotide gated channels and a hyperpolarizing membrane potential. By contrast, invertebrate phototransduction, most extensively characterized in Drosophila, involves activation of a Gq/G11-type G-protein, activation of phospholipase C (PLC), gating of transient receptor potential (TRP) channels and the depolarization of membrane potential (Hardie & Raghu 2001). Interestingly, the melanopsins appear to share some of the key characteristics of an invertebrate-like signal transduction pathway. Both pRGCs and cells transfected with melanopsin show depolarizing responses to light and, as discussed in §4d, melanopsin displays chromophore bistability, another feature of the invertebrate photopigments. Largely by analogy, it was proposed that melanopsin could be coupled to a G-protein of the Gq/G11 class (for review see Peirson & Foster 2006). While not conclusive, there is support for this from the expression studies. For example, melanopsin responses are greatly attenuated (although not blocked) by antibodies against Gq/G11 G-proteins (but not by antibodies to Gi/G0) (Panda et al. 2003). In Neuro-2a cells, the use of Gi/G0 blockers fails to inhibit melanopsin-dependent light responses (Melyan et al. 2005), while Gq/G11 agonists fully blocked the melanopsin-dependent light responses in HEK293-TRPC3 cells (Qiu et al. 2005). Collectively these initial results suggest that the Gq/G11 G-proteins could be activated by melanopsin-dependent phototransduction. It is important to stress, however, that the coupling potential in non-native host environments might not reflect the native pRGCs. Downstream of the G-protein, melanopsin-dependent light responses are greatly attenuated or blocked in Xenopus oocytes and HEK293-TRPC3 cells by PLC inhibitors (Panda et al. 2005; Qiu et al. 2005). Furthermore, co-expression of melanopsin with TRPC3 in Xenopus oocytes (similar to the Drosophila TRP channels) shows that TRPC3 channels can generate a light-activated photocurrent in the presence of melanopsin (Panda et al. 2005; Qiu et al. 2005). Collectively, a partial model of the phototransduction cascade has emerged, suggesting that light activated melanopsin may interact with Gq/G11 that in turn activates a PLC-β. PLC-β generates inositol triphosphate (IP3) and diacylglycerol (DAG), which may ultimately modulate a TRPC channel, possibly via a protein kinase C (PKC). Most recently combined pharmacolgical and anatomical approaches have suggested TRPC7 as the channel (Sekaran et al. 2007). In addition, a microarray-based approach has been used to investigate the transcriptional realignment that occurs in the rd/rd cl mouse eye following a light pulse. This approach identified a number of candidate genes/proteins that might be associated with the melanopsin cascade. Among these was the atypical protein kinase C zeta (Prkcz). Remarkably the genetic ablation of Prkcz mimics precisely the melanopsin knock-out phenotype in a battery of behavioural and pupillometric tests (Peirson et al. 2007). Why an ‘invertebrate-like’ signalling pathway, rather than a more conventional vertebrate phototransduction pathway, is employed by the pRGCs remains an intriguing sensory question and may be relevant to understanding the evolutionary origins of the melanopsin/pRGCs photoreceptor system (Arendt 2003).
5. Evolutionary considerations of non-image-forming photoreception
Given the multiplicity of photoreceptive tissues in the non-mammalian vertebrates, why have these been lost in the mammalian lineage? One possible explanation may be related to the early evolutionary history of the mammals and their passage through a ‘nocturnal bottleneck’. Modern mammals seem to have been derived from nocturnal insectivorous or omnivorous animals about 100 million years ago (Young 1962). Pineal and deep-brain photoreceptors would have been perfectly adequate for monitoring changes in diurnal light conditions but may not have been sufficiently sensitive to discriminate twilight changes in mammals living in burrows or otherwise concealed during the day. The occupation of the nocturnal realm may have led to the loss of the extraocular photoreceptors and the exclusive reliance on irradiance detection by the pRGCs (Foster & Menaker 1993; Menaker et al. 1997). But of course this explanation does not explain why the vertebrates evolved so many photosensitive tissues in the first place. In this context it is worth emphasizing that the sensory task of reliable irradiance detection is not trivial, and extracting time-of-day information from environmental irradiance is even more complex. For example, during twilight, the quality of light changes in three important respects: (i) the amount of light; (ii) the spectral composition of light; (iii) and the source of light (i.e. the position of the sun). These parameters all change in a systematic way and could be used by the circadian system to detect the phase of twilight and hence time of day (Roenneberg & Foster 1997). However, each of these parameters is subject to considerable sensory ‘noise’. The sources of this noise are summarized in table 2. Clearly, the impact of this noise will depend upon the organism and the environment it inhabits. Integrating the information from a multiplicity of photoreceptors, which collect light from different regions of the environment, with differing integration times, and tuned to different spectral channels will act to reduce signal noise and hence provide a more reliable measure of environmental irradiance. The non-mammalian vertebrates might integrate light information from the pineal, deep brain and eyes for reliable time-of-day detection, and there is good evidence for this in birds (Menaker & Underwood 1976). In mammals, twilight detection is either less precise, because of the reliance on a single photoreceptor type, or the pRGCs themselves show heterogeneity in their responses to light, for which there is also good evidence (Sekaran et al. 2005).
Table 2.
The major sources of noise associated with the detection of environmental irradiance. The main sources of signal noise for irradiance detection are listed with examples. In each case the impact of this noise will depend upon the organism, its developmental state and the environment that it inhabits. Integrating the information from multiple photoreceptors, which collect light from different regions of the environment, having differing integration times, and tuned to different spectral channels will act to reduce signal noise.
| source of signal noise | examples |
|---|---|
| fluctuation in the light signal | cloud cover, day-length |
| extraneous light signals | starlight, moonlight, lightning |
| receptor noise | variation in external temperature |
| sensory adaptation | receptor habituation |
| behavioural noise | emergence from burrow, place of rest, feeding etc. |
Opsins in general have evolved to mediate specific photoreceptive tasks in different light environments (Lythgoe 1979). For example, in environments where the spectral composition of the light is restricted, such as in deep water, the λmax of photopigments is spectrally tuned to match the maximum available photon flux around 480 nm (Douglas & Partridge 1997; Hope et al. 1997; Hunt et al. 2001). Whether similar spectral tuning arguments can be used to understand the λmax of the non-image-forming photopigments remains an intriguing question. Many photoreceptors involved in non-image-forming tasks appear to peak close to 480 nm, with a spread ranging from 460 to 530 nm (Shand & Foster 1999). In pineal and deep-brain photoreceptors the light available will be dominated by the transmission of the overlying tissues. This is primarily influenced by two factors. Firstly, short wavelength light is scattered more than longer wavelength light, resulting in relatively more light of long wavelengths penetrating to reach intracranial photoreceptors. Secondly, light will be modified by light-absorbing pigments before reaching these photoreceptors. The most important such pigment is haemoglobin. Haemoglobin has a transmission window between 460 and 540 nm, peaking around 490 nm (Hartwig & van Veen 1979; Foster & Follett 1985). This transmission window may have exerted a strong selection pressure on the spectral tuning of deep brain and pineal photoreceptors. But this cannot be the entire explanation as many non-image-forming photoreceptors are directly exposed to environmental light, such as the pRGCs in the eye or dermal photoreceptors, and these have λmax around 480 nm.
Changes in the amount and spectral composition of environmental irradiance occur throughout the diurnal cycle. As well as the obvious gross changes in irradiance at twilight (approx. 6 log units), changes in the spectral composition of light also occur and are known as the Chappuis effect (Lythgoe 1979). As the sun's rays must pass through a thicker layer of the atmosphere when the sun is lower in the sky, the absorption of light by ozone (500–650 nm) results in a relative enrichment of shorter wavelength light (<500 nm) at twilight (Munz & McFarland 1977). As changes in the light environment at twilight are critical for photoentrainment (Roenneberg & Foster 1997), ‘twilight detectors’ spectrally tuned to the blue part of the spectrum could allow increased photon capture and hence an increase in signal-to-noise detection. Perhaps, however, it is not simply the amount of light that is being detected at twilight but rather its change in spectral quality. Evidence for spectral discrimination, a chromatic response, was first shown in the pineal organ of fish (Meissl & Yanez 1994) and more recently in the parietal eye of lizards (Su et al. 2006). These chromatic responses could arise from an interaction between different photopigments with differing λmax or a single bistable photopigment. Significantly, melanopsin appears to act as a bi-stable pigment, able to regenerate its chromophore using all-trans-retinal and long-wavelength light (Melyan et al. 2005). This photoreversal capacity of melanopsin has also been observed with spectroscopic approaches in the case of Amphioxus melanopsin (Koyanagi et al. 2004). If the two stable states of melanopsin are capable of interacting with different downstream signalling transduction pathways, this may provide an alternative means of attaining spectral discrimination.
The spectral tuning of vertebrate opsins will also be influenced by their evolutionary history (Goldsmith 1990). For example, key amino acid residues influencing spectral tuning sites may provide structural or functional properties, such that any mutation of these residues will be deleterious to protein function. Additionally, there will be trade-offs between structure and function that will influence spectral tuning. Scotopic vision is limited by dark noise produced by spontaneous thermal isomerizations of the retinal chromophore (Barlow et al. 1993). Long wavelength sensitive photopigments have been suggested to be more prone to dark noise owing to their lower excitation energy (Barlow 1957). Thus the spectral tuning of the non-visual opsins, like the visual opsins, will always be a compromise between functional constraints and the photon flux of the light environment (Lythgoe 1984; Goldsmith 1990; Barlow et al. 1993).
6. Conclusions
Considerable progress has been made in the last decade in characterizing the photoreceptors and photopigments mediating non-image-forming responses to light, such as photoentrainment. While over a dozen different opsin photopigments have been identified in recent years (table 1), we are only just beginning to understand what roles these proteins play in the signalling of light information. Perhaps the greatest single advance has been the identification of a third photoreceptive system in the vertebrate eye, the melanopsin-expressing pRGCs, which mediate a range of irradiance detection tasks ranging from photoentrainment, pineal melatonin suppression, pupil constriction and the modulation of arousal states and sleep induction (Altimus et al. 2008; Lupi et al. 2008). By contrast, the photopigments responsible for non-image-forming responses to light in many non-mammalian species remain poorly characterized. Molecular studies are required to determine whether these opsins can form photopigments or whether they act as photoisomerases or retinal carrier proteins (Foster & Bellingham 2002). However a functional analysis of these opsins requires more than biochemistry. If we are to place these remarkable photoreceptors into any sort of evolutionary context we will need a much better understanding of their sensory ecology. We now appreciate that these photoreceptors do more than act as simple photon counters—but beyond this—any detailed understanding is lacking.
Footnotes
One contribution of 13 to a Theme Issue ‘The evolution of phototransduction and eyes’.
References
- Altimus C. M., Guler A. D., Villa K. L., McNeill D. S., Legates T. A., Hattar S.2008Rods–cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl Acad. Sci. USA 105, 19 998–20 003 (doi:10.1073/pnas.0808312105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J.1998Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 3, 13–22 (doi:10.1530/ror.0.0030013) [DOI] [PubMed] [Google Scholar]
- Arendt D.2003Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 47, 563–571 [PubMed] [Google Scholar]
- Aschoff J.1984Circadian timing. Ann. N. Y. Acad. Sci. 423, 442–468 (doi:10.1111/j.1749-6632.1984.tb23452.x) [DOI] [PubMed] [Google Scholar]
- Barlow H. B.1957Purkinje shift and retinal noise. Nature 179, 255–256 (doi:10.1038/179255b0) [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Birge R. R., Kaplan E., Tallent J. R.1993On the molecular origin of photoreceptor noise. Nature 366, 64–66 (doi:10.1038/366064a0) [DOI] [PubMed] [Google Scholar]
- Barr L., Alpern M.1963Photosensitivity of the frog iris. J. Gen. Physiol. 46, 1249–1265 (doi:10.1085/jgp.46.6.1249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham J., Foster R. G.2002Opsins and mammalian photoentrainment. Cell Tissue Res. 309, 57–71 (doi:10.1007/s00441-002-0573-4) [DOI] [PubMed] [Google Scholar]
- Bellingham J., Whitmore D., Philp A. R., Wells D. J., Foster R. G.2002Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position. Brain Res. Mol. Brain Res. 107, 128–136 (doi:10.1016/S0169-328X(02)00454-0) [DOI] [PubMed] [Google Scholar]
- Bellingham J., Wells D. J., Foster R. G.2003In silico characterisation and chromosomal localisation of human RRH (peropsin)—implications for opsin evolution. BMC Genomics 4, 3 (doi:10.1186/1471-2164-4-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham J., et al. 2006Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4, e254 (doi:10.1371/journal.pbio.0040254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J.1964The role of the eyes and of the hypothalamus in the photostimulation of gonads in the duck. Ann. N. Y. Acad. Sci. 117, 204–215 (doi:10.1111/j.1749-6632.1964.tb48175.x) [DOI] [PubMed] [Google Scholar]
- Berson D. M., Dunn F. A., Takao M.2002Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (doi:10.1126/science.1067262) [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Turansky D. G.1975Photoactivation of pupillary constriction in the isolated in vitro iris of mammal (Mesocricetus auratus). Comp. Biochem. Physiol. A 50, 407–413 (doi:10.1016/0300-9629(75)90034-1) [DOI] [PubMed] [Google Scholar]
- Blackshaw S., Snyder S. H.1997Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family. J. Neurosci. 17, 8083–8092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Snyder S. H.1999Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 19, 3681–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J., Hunt D. M.1999Molecular biology of photoreceptor spectral sensitivity. In Adaptive mechanisms in the ecology of vision (eds Archer S. N., Djamgoz M. B. A., Loew E. R., Partridge J. C., Vallerga S.), pp. 439–462 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- Carter-Dawson L. D., LaVail M. M., Sidman R. L.1978Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol. Vis. Sci. 17, 489–498 [PubMed] [Google Scholar]
- Chaurasia S. S., et al. 2005Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem. 92, 158–170 (doi:10.1111/j.1471-4159.2004.02874.x) [DOI] [PubMed] [Google Scholar]
- Dacey D. M., Liao H. W., Peterson B. B., Robinson F. R., Smith V. C., Pokorny J., Yau K. W., Gamlin P. D.2005Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (doi:10.1038/nature03387) [DOI] [PubMed] [Google Scholar]
- Dartnall H.1953The interpretation of spectral sensitivity curves. Br. Med. Bull. 9, 24–30 [DOI] [PubMed] [Google Scholar]
- Dodt E., Meissl H.1982The pineal and parietal organs of lower vertebrates. Experientia 38, 996–1000 (doi:10.1007/BF01955342) [DOI] [PubMed] [Google Scholar]
- Douglas R. H., Partridge J. C.1997On the visual pigments of deep-sea fish. J. Fish Biol. 50, 68–85 (doi:10.1111/j.1095-8649.1997.tb01340.x) [Google Scholar]
- Ebihara S., Tsuji K.1980Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a Zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol. Behav. 24, 523–527 (doi:10.1016/0031-9384(80)90246-2) [DOI] [PubMed] [Google Scholar]
- Foster R., Bellingham J.2002Opsins and melanopsins. Curr. Biol. 12, R543–R544 (doi:10.1016/S0960-9822(02)01047-3) [DOI] [PubMed] [Google Scholar]
- Foster R. G., Follett B. K.1985The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J. Comp. Physiol 157A, 519–528 (doi:10.1007/BF00615153) [Google Scholar]
- Foster R. G., Helfrich-Forster C.2001The regulation of circadian clocks by light in fruitflies and mice. Phil. Trans. R. Soc. B 356, 1779–1789 (doi:10.1098/rstb.2001.0962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. G., Menaker M.1993Circadian photoreception in mammals and other vertebrates. In Light and biological rhythms in man (ed. Wetterberg L.), pp. 73–91 Oxford, UK and New York: Pergamon [Google Scholar]
- Foster R. G., Timmers A. M., Schalken J. J., De Grip W. J.1989A comparison of some photoreceptor characteristics in the pineal and retina. II. The Djungarian hamster (Phodopus sungorus). J. Comp. Physiol 165A, 565–572 (doi:10.1007/BF00611242) [DOI] [PubMed] [Google Scholar]
- Foster R. G., Provencio I., Hudson D., Fiske S., De Grip W., Menaker M.1991Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol 169A, 39–50 (doi:10.1007/BF00198171) [DOI] [PubMed] [Google Scholar]
- Foster R. G., Provencio I., Bovee-Geurts P. H., DeGrip W. J.2003The photoreceptive capacity of the developing pineal gland and eye of the golden hamster (Mesocricetus auratus). J. Neuroendocrinol. 15, 355–363 (doi:10.1046/j.1365-2826.2003.01004.x) [DOI] [PubMed] [Google Scholar]
- Freedman M. S., Lucas R. J., Soni B., von Schantz M., Munoz M., David-Gray Z., Foster R.1999Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (doi:10.1126/science.284.5413.502) [DOI] [PubMed] [Google Scholar]
- Frigato E., Vallone D., Bertolucci C., Foulkes N. S.2006Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles. Naturwissenschaften 93, 379–385 (doi:10.1007/s00114-006-0119-9) [DOI] [PubMed] [Google Scholar]
- Goldsmith T. H.1990Optimization, constraint, and history in the evolution of eyes. Q. Rev. Biol. 65, 281–322 (doi:10.1086/416840) [DOI] [PubMed] [Google Scholar]
- Halford S., Freedman M., Bellingham J., Inglis S., Poopalasundaram S., Soni B., Foster R., Hunt D.2001Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43. Genomics 72, 203–208 (doi:10.1006/geno.2001.6469) [DOI] [PubMed] [Google Scholar]
- Hankins M. W., Lucas R. J.2002The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr. Biol. 12, 191–198 (doi:10.1016/S0960-9822(02)00659-0) [DOI] [PubMed] [Google Scholar]
- Hao W., Fong H. K.1996Blue and ultraviolet light-absorbing opsin from the retinal pigment epithelium. Biochemistry 35, 6251–6256 (doi:10.1021/bi952420k) [DOI] [PubMed] [Google Scholar]
- Hao W., Fong H. K.1999The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J. Biol. Chem. 274, 6085–6090 (doi:10.1074/jbc.274.10.6085) [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Raghu P.2001Visual transduction in Drosophila. Nature 413, 186–193 (doi:10.1038/35093002) [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H.1992Rhodopsin and phototransduction: a model system for G protein-linked receptors. FASEB J. 6, 2323–2331 [DOI] [PubMed] [Google Scholar]
- Hartwig H.-G., van Veen T.1979Spectral characteristics of visible radiations penetrating into the brain and stimulating extra-retinal photoreceptors. J. Comp. Physiol. A 120, 277–282 (doi:10.1007/BF00614615) [Google Scholar]
- Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W.2002Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (doi:10.1126/science.1069609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S., et al. 2003Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 75–81 (doi:10.1038/nature01761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Thompson J. D., Gibson T. J.1996Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266, 383–402 (doi:10.1016/S0076-6879(96)66024-8) [DOI] [PubMed] [Google Scholar]
- Hope A. J., Partridge J. C., Dulai K. S., Hunt D. M.1997Mechanisms of wavelength tuning in the rod opsins of deep-sea fishes. Proc. R. Soc. Lond. B 264, 155–163 (doi:10.1098/rspb.1997.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Dulai K. S., Partridge J. C., Cottrill P., Bowmaker J. K.2001The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J. Exp. Biol. 204, 3333–3344 [DOI] [PubMed] [Google Scholar]
- Isoldi M. C., Rollag M. D., Castrucci A. M., Provencio I.2005Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl Acad. Sci. USA 102, 1217–1221 (doi:10.1073/pnas.0409252102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A., Munoz M., Tarttelin E. E., Bellingham J., Foster R. G., Hankins M. W.2003VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr. Biol. 13, 1269–1278 (doi:10.1016/S0960-9822(03)00509-8) [DOI] [PubMed] [Google Scholar]
- Jiang M., Pandey S., Fong H. K.1993An opsin homologue in the retina and pigment epithelium. Invest. Ophthalmol. Vis. Sci. 34, 3669–3679 [PubMed] [Google Scholar]
- Kasper G., et al. 2002Different structural organization of the encephalopsin gene in man and mouse. Gene 295, 27–32 (doi:10.1016/S0378-1119(02)00799-0) [DOI] [PubMed] [Google Scholar]
- Kawamura S., Yokoyama S.1997Expression of visual and nonvisual opsins in American chameleon. Vision Res. 37, 1867–1871 (doi:10.1016/S0042-6989(96)00309-4) [DOI] [PubMed] [Google Scholar]
- Knowles A., Dartnall H.1977. In The photobiology of vision New York, NY: Academic Press [Google Scholar]
- Kojima D., Mano H., Fukada Y.2000Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J. Neurosci. 20, 2845–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D., Torii M., Fukada Y., Dowling J. E.2008Differential expression of duplicated VAL-opsin genes in the developing zebrafish. J. Neurochem. 104, 1364–1371 (doi:10.1111/j.1471-4159.2007.05093.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf H.-W., Moller M.1984The innervation of the mammalian pineal gland with special reference to central pinealopetal projections. Pineal Res. Rev. 2, 41–86 [Google Scholar]
- Korf H. W., Foster R. G., Ekstrom P., Schalken J. J.1985aOpsin-like immunoreaction in the retinae and pineal organs of four mammalian species. Cell Tissue Res. 242, 645–648 (doi:10.1007/BF00225432) [DOI] [PubMed] [Google Scholar]
- Korf H. W., Moller M., Gery I., Zigler J. S., Klein D. C.1985bImmunocytochemical demonstration of retinal S-antigen in the pineal organ of four mammalian species. Cell Tissue Res. 239, 81–85 (doi:10.1007/BF00214906) [DOI] [PubMed] [Google Scholar]
- Korf H. W., Schomerus C., Stehle J. H.1998The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv. Anat. Embryol. Cell Biol. 146, 1–100 [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., Terakita A.2004Bistable UV pigment in the lamprey pineal. Proc. Natl Acad. Sci. USA 101, 6687–6691 (doi:10.1073/pnas.0400819101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. C., So K. F., Campbell G., Lieberman A. R.1992Pupillary constriction in response to light in rodents, which does not depend on central neural pathways. J. Neurol. Sci. 113, 70–79 (doi:10.1016/0022-510X(92)90267-O) [DOI] [PubMed] [Google Scholar]
- Lucas R. J., Freedman M. S., Munoz M., Garcia-Fernandez J. M., Foster R. G.1999Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507 (doi:10.1126/science.284.5413.505) [DOI] [PubMed] [Google Scholar]
- Lucas R. J., Douglas R. H., Foster R. G.2001Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626 (doi:10.1038/88443) [DOI] [PubMed] [Google Scholar]
- Lucas R. J., Hattar S., Takao M., Berson D. M., Foster R. G., Yau K. W.2003Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247 (doi:10.1126/science.1077293) [DOI] [PubMed] [Google Scholar]
- Lupi D., Oster H., Thompson S., Foster R. G.2008The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat. Neurosci. 11, 1068–1073 [DOI] [PubMed] [Google Scholar]
- Lythgoe J.1979The ecology of vision Oxford, UK: Oxford University Press [Google Scholar]
- Lythgoe J. N.1984Visual pigments and environmental light. Vision Res. 24, 1539–1550 (doi:10.1016/S0042-6989(84)80003-6) [DOI] [PubMed] [Google Scholar]
- Mano H., Kojima D., Fukada Y.1999Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland. Brain Res. Mol. Brain Res. 73, 110–118 (doi:10.1016/S0169-328X(99)00242-9) [DOI] [PubMed] [Google Scholar]
- Max M., McKinnon P. J., Seidenman K. J., Barrett R. K., Applebury M. L., Takahashi J. S., Margolskee R. F.1995Pineal opsin: a nonvisual opsin expressed in chick pineal. Science 267, 1502–1506 (doi:10.1126/science.7878470) [DOI] [PubMed] [Google Scholar]
- Max M., Surya A., Takahashi J. S., Margolskee R. F., Knox B. E.1998Light-dependent activation of rod transducin by pineal opsin. J. Biol. Chem. 273, 26 820–26 826 (doi:10.1074/jbc.273.41.26820) [DOI] [PubMed] [Google Scholar]
- Meissl H.1997Photic regulation of pineal function. Analogies between retinal and pineal photoreception. Biol. Cell 89, 549–554 (doi:10.1016/S0248-4900(98)80158-5) [PubMed] [Google Scholar]
- Meissl H., Yanez J.1994Pineal photosensitivity: a comparison with retinal photoreception. Acta Neurobiol. Exp. 54, 19–29 [PubMed] [Google Scholar]
- Melyan Z., Tarttelin E. E., Bellingham J., Lucas R. J., Hankins M. W.2005Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745 (doi:10.1038/nature03344) [DOI] [PubMed] [Google Scholar]
- Menaker M., Underwood H.1976Extraretinal photoreception in birds. Photochem. Photobiol. 23, 299–306 (doi:10.1111/j.1751-1097.1976.tb07251.x) [DOI] [PubMed] [Google Scholar]
- Menaker M., Moreira L. F., Tosini G.1997Evolution of circadian organization in vertebrates. Braz. J. Med. Biol. Res. 30, 305–313 (doi./10.1590/S0100-879X1997000300003) [DOI] [PubMed] [Google Scholar]
- Minamoto T., Shimizu I.2002A novel isoform of vertebrate ancient opsin in a smelt fish Plecoglossus altivelis. Biochem. Biophys. Res. Commun. 290, 280–286 (doi:10.1006/bbrc.2001.6186) [DOI] [PubMed] [Google Scholar]
- Moutsaki P., Bellingham J., Soni B. G., David-Gray Z. K., Foster R. G.2000Sequence, genomic structure and tissue expression of carp (Cyprinus carpio L.) vertebrate ancient (VA) opsin. FEBS Lett. 473, 316–322 (doi:10.1016/S0014-5793(00)01550-7) [DOI] [PubMed] [Google Scholar]
- Moutsaki P., Whitmore D., Bellingham J., Sakamoto K., David-Gray Z. K., Foster R. G.2003Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res. Mol. Brain Res. 112, 135–145 (doi:10.1016/S0169-328X(03)00059-7) [DOI] [PubMed] [Google Scholar]
- Mrosovsky N.1999Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429 (doi:10.3109/07420529908998717) [DOI] [PubMed] [Google Scholar]
- Mrosovsky N., Lucas R., Foster R.2001Persistence of masking responses to light in mice lacking rods and cones. J. Biol. Rhythms 16, 585–587 (doi:10.1177/074873001129002277) [DOI] [PubMed] [Google Scholar]
- Munz F. W., McFarland W. N.1977Evolutionary adaptations of fishes to the photic environment. In Handbook of sensory physiology (ed. Crescitelli F.), pp. 193–274 Berlin, Germany: Springer [Google Scholar]
- Nakamura A., Kojima D., Imai H., Terakita A., Okano T., Shichida Y., Fukada Y.1999Chimeric nature of pinopsin between rod and cone visual pigments. Biochemistry 38, 14 738–14 745 (doi:10.1021/bi9913496) [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S.1983Isolation, sequence analysis, and intron–exon arrangement of the gene encoding bovine rhodopsin. Cell 34, 807–814 (doi:10.1016/0092-8674(83)90537-8) [DOI] [PubMed] [Google Scholar]
- Nelson R. J., Zucker I.1981Absence of extra-ocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp. Biochem. Physiol. 69A, 145–148 (doi:10.1016/0300-9629(81)90651-4) [Google Scholar]
- Newman L. A., Walker M. T., Brown R. L., Cronin T. W., Robinson P. R.2003Melanopsin forms a functional short-wavelength photopigment. Biochemistry 42, 12 734–12 738 (doi:10.1021/bi035418z) [DOI] [PubMed] [Google Scholar]
- Okada T., Ernst O. P., Palczewski K., Hofmann K. P.2001Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem. Sci. 26, 318–324 (doi:10.1016/S0968-0004(01)01799-6) [DOI] [PubMed] [Google Scholar]
- Okano T., Yoshizawa T., Fukada Y.1994Pinopsin is a chicken pineal photoreceptive molecule. Nature 372, 94–97 (doi:10.1038/372094a0) [DOI] [PubMed] [Google Scholar]
- Palczewski K., et al. 2000Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (doi:10.1126/science.289.5480.739) [DOI] [PubMed] [Google Scholar]
- Panda S., Sato T. K., Castrucci A. M., Rollag M. D., DeGrip W. J., Hogenesch J. B., Provencio I., Kay S. A.2002Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 (doi:10.1126/science.1076848) [DOI] [PubMed] [Google Scholar]
- Panda S., et al. 2003Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 (doi:10.1126/science.1086179) [DOI] [PubMed] [Google Scholar]
- Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T.2005Illumination of the melanopsin signaling pathway. Science 307, 600–604 (doi:10.1126/science.1105121) [DOI] [PubMed] [Google Scholar]
- Pandey S., Blanks J. C., Spee C., Jiang M., Fong H. K.1994Cytoplasmic retinal localization of an evolutionary homolog of the visual pigments. Exp. Eye Res. 58, 605–613 (doi:10.1006/exer.1994.1055) [DOI] [PubMed] [Google Scholar]
- Peirson S., Foster R. G.2006Melanopsin: another way of signaling light. Neuron 49, 331–339 (doi:10.1016/j.neuron.2006.01.006) [DOI] [PubMed] [Google Scholar]
- Peirson S. N., Bovee-Geurts P. H., Lupi D., Jeffery G., DeGrip W. J., Foster R. G.2004Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res. Mol. Brain Res. 123, 132–135 (doi:10.1016/j.molbrainres.2004.01.007) [DOI] [PubMed] [Google Scholar]
- Peirson S. N., Oster H., Jones S. L., Leitges M., Hankins M. W., Foster R. G.2007Microarray analysis and functional genomics identify novel components of melanopsin signaling. Curr. Biol. 17, 1363–1372 (doi:10.1016/j.cub.2007.07.045) [DOI] [PubMed] [Google Scholar]
- Pepe I. M.2001Recent advances in our understanding of rhodopsin and phototransduction. Prog. Retin Eye Res. 20, 733–759 (doi:10.1016/S1350-9462(01)00013-1) [DOI] [PubMed] [Google Scholar]
- Philp A. R., Bellingham J., Garcia-Fernandez J., Foster R. G.2000aA novel rod-like opsin isolated from the extra-retinal photoreceptors of teleost fish. FEBS Lett. 468, 181–188 (doi:10.1016/S0014-5793(00)01217-5) [DOI] [PubMed] [Google Scholar]
- Philp A. R., Garcia-Fernandez J. M., Soni B. G., Lucas R. J., Bellingham J., Foster R. G.2000bVertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar). J. Exp. Biol. 203, 1925–1936 [DOI] [PubMed] [Google Scholar]
- Pires S. S., Shand J., Bellingham J., Arrese C., Turton M., Peirson S., Foster R. G., Halford S.2007Isolation and characterization of melanopsin (Opn4) from the Australian marsupial Sminthopsis crassicaudata (fat-tailed dunnart). Proc. Biol. Sci. 274, 2791–2799 (doi:10.1098/rspb.2007.0976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. S.1993Temporal organisation: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55, 17–54 (doi:10.1146/annurev.ph.55.030193.000313) [DOI] [PubMed] [Google Scholar]
- Provencio I., Foster R. G.1995Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 694, 183–190 (doi:10.1016/0006-8993(95)00694-L) [DOI] [PubMed] [Google Scholar]
- Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D.1998Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345 (doi:10.1073/pnas.95.1.340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D.2000A novel human opsin in the inner retina. J. Neurosci. 20, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M.2005Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749 (doi:10.1038/nature03345) [DOI] [PubMed] [Google Scholar]
- Rodieck R. W.1998. In The first steps in seeing Sunderland, MA: Sinauer Associates [Google Scholar]
- Roenneberg T., Foster R. G.1997Twilight times: light and the circadian system. Photochem. Photobiol. 66, 549–561 (doi:10.1111/j.1751-1097.1997.tb03188.x) [DOI] [PubMed] [Google Scholar]
- Ruby N. F., Brennan T. J., Xie X., Cao V., Franken P., Heller H. C., O'Hara B. F.2002Role of melanopsin in circadian responses to light. Science 298, 2211–2213 (doi:10.1126/science.1076701) [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M.1987The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 [DOI] [PubMed] [Google Scholar]
- Sekaran S., Foster R. G., Lucas R. J., Hankins M. W.2003Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 13, 1290–1298 (doi:10.1016/S0960-9822(03)00510-4) [DOI] [PubMed] [Google Scholar]
- Sekaran S., Lupi D., Jones S. L., Sheely C. J., Hattar S., Yau K. W., Lucas R. J., Foster R. G., Hankins M. W.2005Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr. Biol. 15, 1099–1107 (doi:10.1016/j.cub.2005.05.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S., Lall G. S., Ralphs K. L., Wolstenholme A. J., Lucas R. J., Foster R. G., Hankins M. W.20072-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J. Neurosci. 27, 3981–3986 (doi:10.1523/JNEUROSCI.4716-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger H. H.1962Direct action of light in naturally pigmented muscle fibres. I. Action spectrum for contraction in eel iris sphincter. J. Gen. Physiol. 46, 277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand J., Foster R. G.1999The extraretinal photoreceptors of non-mammalian vertebrates. In Adaptive Mechanisms in the Ecology of Vision (eds Archer S. N., Djamgoz M. B. A., Loew E. R., Partridge J. C., Vallerga S.), pp. 197–222 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- Shichida Y., Matsuyama T.2009Evolution of opsins and phototransduction. Phil. Trans. R. Soc. B 364, 2881–2895 (doi:10.1098/rstb.2009.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R., Witkovsky P., Horvath P., Alones V., Barnstable C. J., Lehman M. N.1988Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue. Res. 253, 189–198 (doi:10.1007/BF00221754) [DOI] [PubMed] [Google Scholar]
- Solessio E., Engbretson G. A.1993Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature 364, 442–445 (doi:10.1038/364442a0) [DOI] [PubMed] [Google Scholar]
- Soni B. G., Foster R. G.1997A novel and ancient vertebrate opsin. FEBS Lett. 406, 279–283 (doi:10.1016/S0014-5793(97)00287-1) [DOI] [PubMed] [Google Scholar]
- Soni B., Philp A., Knox B., Foster R.1998Novel retinal photoreceptors. Nature 394, 27–28 (doi:10.1038/27794) [DOI] [PubMed] [Google Scholar]
- Su C. Y., Luo D. G., Terakita A., Shichida Y., Liao H. W., Kazmi M. A., Sakmar T. P., Yau K. W.2006Parietal-eye phototransduction components and their potential evolutionary implications. Science 311, 1617–1621 (doi:10.1126/science.1123802) [DOI] [PubMed] [Google Scholar]
- Sun H., Gilbert D. J., Copeland N. G., Jenkins N. A., Nathans J.1997Peropsin, a novel visual pigment-like protein located in the apical microvilli of the retinal pigment epithelium. Proc. Natl Acad. Sci. USA 94, 9893–9898 (doi:10.1073/pnas.94.18.9893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S.2007MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (doi:10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Hisatomi O., Yoshida M., Tokunaga F.2001Pinopsin expressed in the retinal photoreceptors of a diurnal gecko. FEBS Lett. 496, 69–74 (doi:10.1016/S0014-5793(01)02395-X) [DOI] [PubMed] [Google Scholar]
- Tarttelin E. E., Bellingham J., Hankins M. W., Foster R. G., Lucas R. J.2003Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 554, 410–416 (doi:10.1016/S0014-5793(03)01212-2) [DOI] [PubMed] [Google Scholar]
- Tu D. C., Batten M. L., Palczewski K., Van Gelder R. N.2004Nonvisual photoreception in the chick iris. Science 306, 129–131 (doi:10.1126/science.1101484) [DOI] [PubMed] [Google Scholar]
- van Veen T., Hartwig H. G., Müller K.1976Light dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L.). J. Comp. Physiol. A 111, 209–219 (doi:10.1007/BF00605532) [Google Scholar]
- Vigh-Teichmann I., Korf H. W., Oksche A., Vigh B.1982Opsin-immunoreactive outer segments and acetylcholinesterase-positive neurons in the pineal complex of Phoxinus phoxinus (Teleostei, Cyprinidae). Cell Tissue Res. 227, 351–369 (doi:10.1007/BF00210891) [DOI] [PubMed] [Google Scholar]
- Vigh-Teichmann I., Korf H. W., Nurnberger F., Oksche A., Vigh B., Olsson R.1983Opsin-immunoreactive outer segments in the pineal and parapineal organs of the lamprey (Lampetra fluviatilis), the eel (Anguilla anguilla), and the rainbow trout (Salmo gairdneri). Cell Tissue Res. 230, 289–307 [DOI] [PubMed] [Google Scholar]
- Vollrath L.1981. In The pineal organ Heidelberg, Germany: Springer [Google Scholar]
- von Frisch K.1911Beitrage zur Physiologie der Pigmentzellen in der Fischhaut. Pfluger's Archv. Gesamte Physiol. Menschen Tiere 138, 319–387 (doi:10.1007/BF01680752) [Google Scholar]
- Weber W.1983Photosensitivity of chromatophores. Am. Zool. 23, 495–506 [Google Scholar]
- White J. H., et al. 2008Identification of a novel asthma susceptibility gene on chromosome 1qter and its functional evaluation. Hum. Mol. Genet. 17, 1890–1903 (doi:10.1093/hmg/ddn087) [DOI] [PubMed] [Google Scholar]
- Whitmore D., Foulkes N. S., Sassone-Corsi P.2000Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 404, 87–91 (doi:10.1038/35003589) [DOI] [PubMed] [Google Scholar]
- Wolken J. J., Mogus M. A.1979Extra-ocular photo-sensitivity. Photochem. Photobiol. 29, 189–196 (doi:10.1111/j.1751-1097.1979.tb09281.x) [Google Scholar]
- Yokoyama S., Zhang H.1997Cloning and characterization of the pineal gland-specific opsin gene of marine lamprey (Petromyzon marinus). Gene 202, 89–93 (doi:10.1016/S0378-1119(97)00458-7) [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Okano T., Oishi T., Fukada Y.1998A deep brain photoreceptive molecule in the toad hypothalamus. FEBS Lett. 424, 69–72 (doi:10.1016/S0014-5793(98)00139-2) [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Ebihara S.1996Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J. Comp. Physiol 178A, 797–802 (doi:10.1007/BF00225828) [DOI] [PubMed] [Google Scholar]
- Young J. Z.1962. In The life of the vertebrates Oxford, UK: The Clarendon Press [Google Scholar]