Abstract
The ‘division of labour’ model of eye evolution is elaborated here. We propose that the evolution of complex, multicellular animal eyes started from a single, multi-functional cell type that existed in metazoan ancestors. This ancient cell type had at least three functions: light detection via a photoreceptive organelle, light shading by means of pigment granules and steering through locomotor cilia. Located around the circumference of swimming ciliated zooplankton larvae, these ancient cells were able to mediate phototaxis in the absence of a nervous system. This precursor then diversified, by cell-type functional segregation, into sister cell types that specialized in different subfunctions, evolving into separate photoreceptor cells, shading pigment cells (SPCs) or ciliated locomotor cells. Photoreceptor sensory cells and ciliated locomotor cells remained interconnected by newly evolving axons, giving rise to an early axonal circuit. In some evolutionary lines, residual functions prevailed in the specialized cell types that mirror the ancient multi-functionality, for instance, SPCs expressing an opsin as well as possessing rhabdomer-like microvilli, vestigial cilia and an axon. Functional segregation of cell types in eye evolution also explains the emergence of more elaborate photosensory–motor axonal circuits, with interneurons relaying the visual information.
Keywords: division of labour model, eye evolution, proto-eye
1. Introduction
This review puts forward the notion that ‘cell-type functional segregation', the differential distribution of cellular functions between sister cell types (Arendt 2008), has played a major role in eye evolution. This is evident if one takes into account two simple observations: (i) that the number of cell types has gone up concomitantly with the increasing complexity of evolving eye types and (ii) the number of protein-coding genes representing cellular eye-related functions has not grown at a similar pace.
The evolution of animal eyes is a story of a stunning rise in complexity, from a very simple common evolutionary precursor, the hypothetical ‘proto-eye’ (Gehring & Ikeo 1999)—a simple organ composed of two functionally distinct cells, a light-sensitive photoreceptor cell (PRC) and a shading pigment cell (SPC)—to something as intricate as the human eye or the cephalopod eye. As one simple measure for complexity is the number of different parts of which a given structure is composed (McShea 2000; Oakley & Rivera 2008), the more complex organ should be the one harbouring more cell types (McShea 2000; Oakley & Rivera 2008). Therefore, as an organ, the human eye with seven major retinal cell types and the Drosophila eye with 10 different ommatidial cell types are considerably more complex than, for example, the Platynereis larval eye that is composed of two cell types only, a rhabdomeric PRC and a SPC (Rhode 1992), thus resembling the hypothesized proto-eye (Gehring & Ikeo 1999).
If there has been a gain of complexity at the organ level, how about complexity at the cellular level? Here, contrary to what one would expect, it appears that cell types have lost complexity during animal evolution, at least on average. If one assumes that the more complex cell type should be the one having more proteins at its disposal (representing a larger variety of cellular functions), ancient metazoan cell types must have been, at least on average, more complex than their modern descendants. This notion follows from the fact that the overall number of protein-coding genes of a human (with more than 400 cell types; Vickaryous & Hall 2006) is not much higher than that of a sea anemone (with maybe a dozen cell types; Carroll 2001), with two-thirds of the human genes descendent from eumetazoan progenitors (Technau et al. 2005; Putnam et al. 2007). Obviously, the increase in metazoan complexity, and, thus, cell-type number, was not coupled to a similar rise in the number of protein-coding genes. In other words, the number of proteins expressed per cell type necessarily decreased, at least on average, unless diversifying cell types acquired a disproportionately high number of new, cell-type specific genes encoding novel functions. This idea of ancestrally more complex cell types has been developed previously and has been referred to as ‘complexity drain on cells in the evolution of multicellularity’ (McShea 2002).
With regard to the so-called ‘eye genes’, many of these were already present in the last common ancestors of cnidarians and bilaterians (e.g. Koyanagi et al. 2008). This means that initially a surprisingly high number of conserved metazoan protein-coding genes involved in eye-related functions must have been active in only a few ancient cell types that accordingly expressed a substantial fraction of the ancestral protein inventory, which implies that they were ‘multi-functional’ (Arendt 2008). We can also infer that the differential distribution of various kinds of regulatory, structural and physiological effector proteins to sister cell types must have accompanied the subsequent cell-type diversification in eye evolution, resulting in cell-type-specific, differential gene expression and cell-type-specific functions, as manifest, for example, in the Drosophila eye. This principle of ‘division of labour’ between cell types has recently been referred to as cell-type functional segregation (Arendt 2008). It is fair to say that during eye evolution (and animal evolution in general), cell types became more specialized but not necessarily more complex.
Here, the ‘division of labour’ concept is elaborated for the evolution of rhabdomeric eyes and some of their constituent cell types, such as rhabdomeric PRCs and SPCs and, ultimately, visual interneurons. We reason that early metazoans had eyes with few—if not only one—multi-functional cell types, combining photoreceptor, shading pigment and locomotor effector functions. Several examples are presented here for highly complex multi-functional cell types in the eyes of animals of relatively low tissue complexity. We also discuss cases in which besides the major defining function of a cell type such as photosensitivity, vestiges of other functions remain present, for instance, a certain amount of shading pigment granules that may still contribute to the overall shielding of the photoreceptive organelle and that we interpret as a remnant of incomplete functional segregation during cell-type evolution. Apparently, in many cases, cell types retain functions at low levels, which have otherwise become characteristic of their functionally segregated sister cell type. The rhabdomeric PRCs are another example, exhibiting vestigial cilia reminiscent of the locomotor cilia of their presumed ancestor cell types (discussed subsequently).
Finally, the presence of functional ‘remnants’ in various extant cell types allows one to reconstruct the complement of functions that were present in the ancestral multi-functional cell types. This way, the multi-functional cell types constituting early metazoan eyes can be reconstructed in cellular detail.
2. Ancient multi-functional photoreceptor cells mediating phototaxis
We can envisage two fundamentally different functions of light sensitivity in early metazoans: (i) mediating photoperiodic activities such as diel vertical migration, feeding or reproduction and (ii) phototaxis, the movement towards or away from light. Very likely, these two functions were separated early in metazoan evolution and this might have triggered the diversification of PRC types among Metazoa, characterized by distinct morphologies and molecular fingerprints (Arendt & Wittbrodt 2001; Arendt 2003). Whereas the former function only requires a PRC to measure light intensity, the latter needs an eye, a system allowing tracking sources of light (Foster 2009). In unicellular eukaryotes and in the swimming planktonic larvae of invertebrate animals, phototaxis is driven by locomotor flagella or cilia (Jékely 2009). In these metazoan larvae, there are no muscular systems involved.
For phototactic steering to evolve, several basic functions have to be directly coupled: photoreception mediated by locally concentrated photopigment, a shading ‘eyespot’ for directional sensing of light and the local alteration of ciliary beating (Jékely 2009). This has happened several times independently in unicellular eukaryotes (Jékely 2009). For a new functional phototactic system to evolve in an early metazoan organism, it is plausible that these functions were also initially localized in one cell, because multi-functional PRCs with pigment granules and locomotor cilia can mediate phototaxis even in the absence of a nervous system or any other intercellular information exchange (Nordstrom et al. 2003). Given the absence of eyes and phototaxis in choanoflagellates, the sister group of animals (Jékely 2009), such multi-functional PRC, must have evolved at least once at the base of the Metazoa.
In the sponge larva, multi-functional photosensitive steering rudder cells have been described that must harbour some (unknown) photopigment and also contain shading pigment granules and employ a locomotor cilium as a steering rudder (figure 1a; Leys & Degnan 2001). Cnidarian cubozoan larvae have very similar multi-functional cells comprising photoreceptor, shading pigment and a locomotor cilium (Nordstrom et al. 2003). These cells employ a transmembrane photopigment, as extrapolated from the presence of a rhabdom, a strongly enlarged membranous surface ideally suited to harbour massive amounts of protein (figure 1b). This is most probably an animal opsin, because several opsins have been detected in cnidarian genomes, while bacteriorhodopsins (mediating phototaxis in Chlamydomonas, for example) have not yet been detected in Metazoa. A large variety of cnidarian opsins have meanwhile been identified with different affinities to bilaterian opsin subfamilies (Plachetzki et al. 2007; Koyanagi et al. 2008; Suga et al. 2008), but the identity of the opsin mediating larval phototaxis in the cubozoan Tripedalia remains unknown. Similar pigmented spots on the swimming planulae of the cubozoan Carybdea rastonii and Carybdea alata have been reported earlier (Okada 1927; Arneson & Cutress 1976), indicating that similar rhabdomeric photoreceptors are probably present in other cubozoans. However, they have not been reported for any other cnidarians, leaving it open whether they are homologous to bilaterian rhabdomeric photoreceptors. Molecular fingerprinting of the cubozoan rhabdomeric photoreceptors and a broader taxonomic search for possible counterparts in other cnidarian groups will be needed to settle this issue.
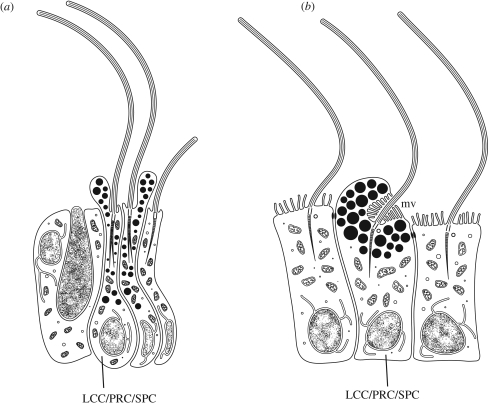
Figure 1.
Simple multi-functional PRCs (LCC/PRC/SPC) with motile cilia, sensory microvilli and shading pigment granules. (a) In the demosponge larva (Amphimedon), shading pigment prevents admittance of light to light-sensitive part of the cells from several directions. The cilia is active in phototactic steering. Note the absence of microvilli. After Leys & Degnan (2001) and Maldonado et al. (2003). (b) Rhabdomeric PRC in the planula larva of the cubozoan cnidarian Tripedalia; rhabdomeric microvilli (mv) shaded from various directions (Nordstrom et al. 2003).
3. Evolution of the first photosensory–motor axonal circuit
Eyespots mediating phototaxis have also been described in the zooplankton larvae of various bilaterian groups, annelids (Brandenburger & Eakin 1981; Verger-Bocquet 1983; Marsden & Hsieh 1987; Sensenbaugh & Franzén 1987; Bartolomaeus 1992a), molluscs (Chia & Koss 1978; Bartolomaeus 1992b; Blumer 1994, 1995, 1996, 1998), echinoderms and hemichordates (Brandenburger et al. 1973). These groups exhibit helical swimming in three dimensions, and thus the overall mechanism of phototaxis may be very similar to unicellular eukaryotes and to the sponge or cnidarian larvae that show a similar swimming pattern (Jékely et al. 2008; Jékely 2009). However, there is a major difference: these eyespots are composed of more than one cell and they are connected via axons to separate locomotor ciliated cells (LCCs). We have recently unravelled cellular composition, transmitter usage and axonal connectivity of a very simple nervous system mediating phototaxis in the marine annelid Platynereis dumerilii (Jékely et al. 2008). In this system, a simple two-celled eye is composed of one rhabdomeric PRC and one SPC. The rhabdomeric photoreceptor functions not only as a sensory neuron receiving light but also as a motor neuron directly innervating ciliated cells of the equatorial ciliary girdle by direct innervation (figure 2).
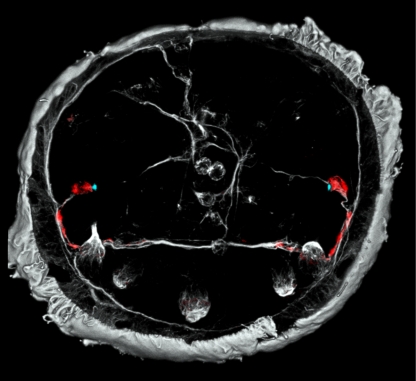
Figure 2.
The axonal scaffold of the larval brain of P. dumerilii at 48 hpf. Rhabdomeric PRCs (red) projecting to the ciliary girdle, where they locally alter the strength of ciliary beating (Jékely et al. 2008). Grey colour indicates axons and cilia labelled by an antibody directed against acetylated tubulin. Blue dots have been added following phalloidin stainings that specifically label the actin filaments of microvilli making up the rhabdomeres. Photograph courtesy of G. Jékely.
If the evolution of phototaxis started with a single cell system, as postulated earlier, how can we imagine the transition to a simple multicellular system comprising distinct rhabdomeric PRCs, SPCs and ciliary motor cells? This is not trivial because this process has to be highly coordinated with the evolution of one component crucially depending on the evolution of the other. The SPC and the rhabdomeric photoreceptor must coevolve because either of them is useless alone. Moreover, both cells can function properly only if the PRC connects to the ciliated locomotor cells from the very beginning.
Figure 3 depicts a possible scenario for the evolution of a simple photosensory–motor circuit interconnecting a two-celled eye and LCCs. As a starting point, this scenario envisages a small population of multi-functional PRCs located around the equator of the swimming larvae, conceptually similar to the situation in sponge or cubozoan larvae. It assumes that all cells are initially equal but mutually interconnected to locally spread and thus enhance the light signal (figure 3a). If we now assume that a subpopulation of these cells loses light sensitivity (e.g. by turning off opsin expression), these cells could still be functional as steering cells because they still receive the information on directional light detection from neighbouring cells (figure 3b). If, as the next step, the newly evolved sister cell types (locomotor steering cells versus rhabdomeric PRC/SPC) move away from each other but retain cellular contact, a simple axonal connection and thus nervous system would have evolved (figure 3c). Finally, one can envisage that the photoreceptor function and the shading pigment function equally segregate into two sister cell types, one specializing as rhabdomeric photoreceptor and the other as SPC (figure 3d).
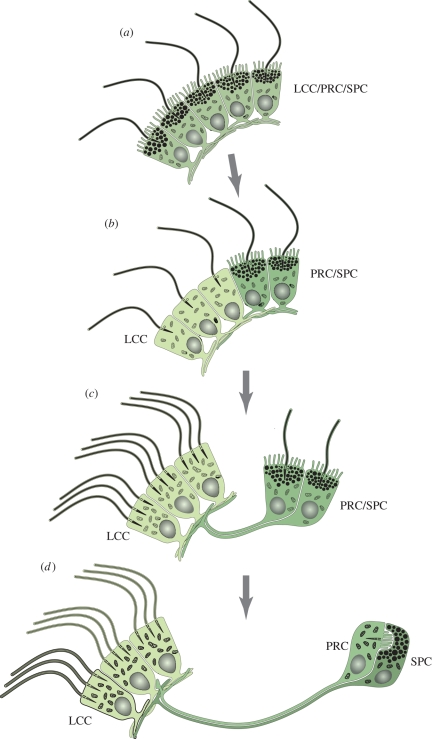
Figure 3.
Evolution of two-celled rhabdomeric larval eyes mediating phototaxis, according to the division of labour model. Multi-functional locomotor-photosensory precursor cells evolve into LCCs by cell-type functional segregation. (a) Precursor LCC/PRC/SPC combining a locomotor cilium, photosensory rhabdomeric microvilli and shading pigment granules. (b) A subset of cells loses photosensitivity and shading pigment to specialize on locomotion (LCC, light green). (c) The LCCs become multiciliated. A spatially separate ‘eye’ comprising two combined PRC/SPC remains connected to the multiciliated cells via gradually extending cellular processes that evolve into axons. (d) Functional segregation of PRCs (green) and SPCs (dark green). For further explanations, see text.
This scenario resembles other scenarios put forward for the evolution of the first mechanosensory–motor circuit in cnidarians (Seipel et al. 2004) and, more recently, for the evolution of the chemosensory–neurosecretory brain circuit in bilaterians (olfactory–hypothalamal–adenohypophyseal circuit; Arendt 2008). Initially, the functional segregation may be a neutral evolutionary event. Once functions are segregated, however, it is plausible that the division of labour reduces the pleiotropy of individual cell types and thus allows more efficient functional specialization (Arendt 2008).
In our evolutionary scenario, we envisage a monociliated cell as the ancestral multi-functional PRC (figure 3a). However, it cannot be determined with certainty whether this cell type was mono- or multiciliated. An accessory cilium or vestiges thereof are rather common in PRCs, at least in annelids (Purschke 2005; Purschke et al. 2006). Such vestigial cilia occur in larval eyes, e.g. Polygordius sp. (Brandenburger & Eakin 1981) as well as in multicellular adult eyes (Purschke et al. 2006; Suschenko & Purschke 2009). However, there are a few known examples of rhabdomeric PRCs in annelids possessing more than one cilium (e.g. in Polygordius appendiculatus and Scoloplos armiger; Wilkens & Purschke 2009, in press), which might be indicative of a multiciliary origin.
4. Evolutionary remnants of cellular multi-functionality
Our evolutionary model can be tested both morphologically and molecularly. It predicts that despite their different, segregated functions, the distinct cell types making up the multicellular metazoan phototaxis system should show morphological resemblances and vestiges reflecting their common evolutionary ancestry. We can envisage that in some cases, the functionally segregated sister cell types should show residual functions (which they had otherwise lost), reflecting the ancient multicellular state. A literature survey reveals that such cell types indeed exist in the eyespots of invertebrate larvae and adults, both in the form of PRCs retaining some shading pigment and SPCs retaining small sensory organelles.
In several species of the polychaete Saccocirrus, the SPC exhibits an apical extension with some rhabdom-like microvilli and vestigial cilia (figure 4a; Eakin et al. 1977; Purschke 1992, 2005; Purschke et al. 2006). In these species, both SPC and PRC are part of the epidermal layer and the eye cup opens to the exterior. Both cells form axon-like processes that are closely aligned and enter the neuropil of the brain. A similar observation has been made regarding the adult eyes of the annelid S. armiger, which possess an SPC with a dense array of microvilli presumed to be sensory (Wilkens & Purschke in press). Adding to this, the SPC of the larval eye in Platynereis and in the sedentary Cirratulidae, as well as of the bicellular ocelli of Saccocirrus, send out sensory axons at later developmental stages (G. Purschke, H. Hausen & D. Arendt 2009, unpublished data), corroborating the (hidden) sensory nature of these cells.
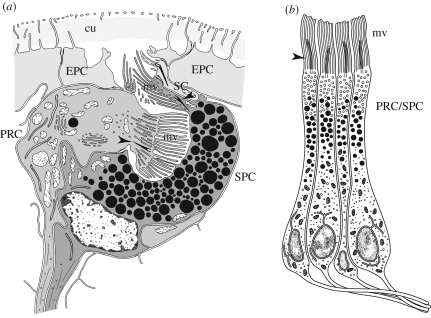
Figure 4.
(a) Two-celled eye of Saccocirrus papillocercus after transmission electron microscopy observations. PRC and SPC are embedded in the layer of epidermal cells (EPCs); both cells with microvilli (mv) in rhabdomeric arrangement, vestigial cilia (arrowheads) and axonal processes; a few scattered electron-dense vesicles in PRC may comprise shading pigment. Note additional sensory cell (SC) adjacent to pigment cell. cu, cuticle. (b) Adult eye retina cells of Patella exclusively composed of combined PRC/SPC. Note that each cell possesses a cilium (arrowhead) and sends out an axon. mv, microvilli (adapted from Marshall & Hodgson 1990).
It is well established that PRCs show characteristics of SPCs to various extents. For example, pigment vesicles have been found in cephalopod (Yamamoto et al. 1965; Budelmann et al. 1997) and gastropod eyes (Blumer 1999; Zhukov et al. 2006) and appear to be a common feature of annelids with multicellular adult eyes, as observed in the Platynereis adult eye PRCs (Rhode 1992); at least in Phyllodocida and Eunicida, this very likely belongs to the ground pattern of eyes, and in these taxa, the absence of pigment vesicles may be interpreted as a secondary character state (Suschenko & Purschke 2009). In line with a general affinity of PRC and SPC, the retina of the Patella eye is exclusively composed of pigmented PRCs (figure 4b).
Finally, the more or less reduced accessory cilium that is usually present in annelid rhabdomeric PRCs of adult eyes (see, for example, Purschke 2005) can be interpreted as the remnants of a former locomotor function. Unfortunately, up to now, nothing is known about the function of any of these accessory cilia.
Corroborating the affinity of PRCs and SPCs, we have determined the molecular fingerprint of cell types making up the larval and adult eyes of Platynereis (Guy et al. submitted) and found that the rhabdomeric PRC and the SPC of the larval eyes are more closely related to each other than they are to their functional counterparts in the adult eye or to any other cell of the developing larval episphere. Both cell types specifically express the combination of the transcription factors pax6, lhx2, sim and chx10, as their regulatory signature, and the acetylcholine receptor AChR7/8, which confer a unique molecular fingerprint to these cells (Arendt 2008). None of these genes is expressed in the PRCs or SPCs of the adult eyes. The latter, in turn, express the transcription factors six1/2, eyes absent, dachshund and Distalless, plus the sepiapterin synthase A, a key enzyme in the synthesis of the shading pigment. Again, none of these genes is active in the larval eyes. These data suggest that the PRCs and SPCs of the larval eyes and those of the adult eyes have evolved by cell-type functional segregation in two independent evolutionary events.
5. The evolution of visual eyes and bipolar interneurons
Another important transition in eye evolution has been the transition from simple eyes sensing the direction of light only, involved in phototaxis, to the more complex visual eyes. This transition appears to be paralleled by the change from using ciliary bands in locomotion such as those present in planktotrophic metazoan larvae to muscular systems. In such systems, integrative neuronal processes coordinate the activity of several longitudinal and circular or transverse muscle fibres (see, for example, Tzetlin & Filippova 2005; Purschke & Müller 2006). This larva-to-adult transition involves the development of two different sets of eyes in many invertebrates: larval eyes directly associated with the ciliated cells such as those recently described by Jékely et al. (2008) and adult eyes with neuronal projections into the central nervous system (as is present, for example, in Platynereis; see Jékely et al. 2008 and references therein).
This fundamental transition towards visual eyes requires the evolution of a more elaborate axonal circuit, with one or more serially connected interneurons, between the PRCs of the eye and the effector system (figure 5). Much of this circuit will eventually become part of a more and more elaborate brain.
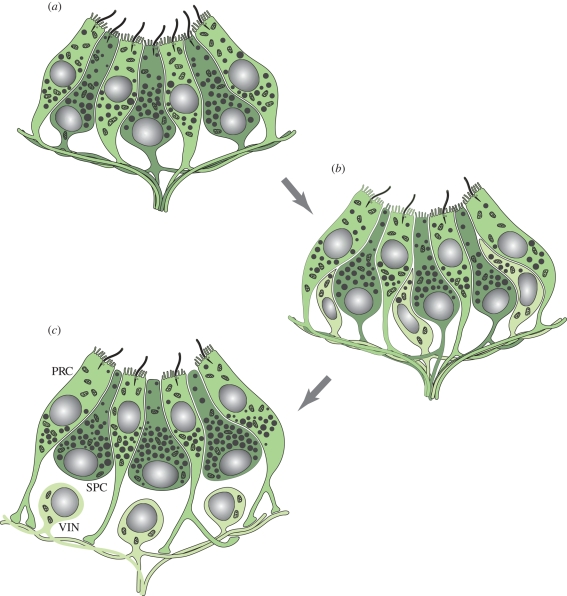
Figure 5.
Evolution of visual interneurons according to the division of labour model. (a) Pigment cup eye composed of partially segregated photoreceptor (green) and SPCs (dark green). Note that both cell types bear sensory microvilli and shading pigment granules, but in different quantity. Together, the pigment granules of both cell types form the eye cup. Both cell types also send out axons. (b) A subset of cells loses photosensitivity and shading pigment to specialize on information integration (light green). The pigment cells lose the cilia. (c) Evolution of fully developed unipolar visual interneurons (VIN) that retain axonal connections to multiple PRC. The SPCs (dark green) have specialized on their shading function and lost the sensory microvilli as well as basal axons. Note that PRCs axons connect to VIN exclusively. For further explanations, see text.
As a first step in the evolution of vision, we can envisage an increase in the number of PRCs. To perceive an image, these cells must communicate and integrate information on their individual state of illumination via axonal connections (figure 5a). We can then assume that some cells specialize in information processing and others in light perception, giving birth to first populations of interneurons that remain interconnected by axon collaterals (figure 5b). This way we can explain the evolution of first processing circuits to perform tasks such as lateral inhibition or integration across several photoreceptors (Nilsson & Arendt 2008). For further refinement of visual information processing, interneurons will specialize into first- and second-order interneurons and assemble into optic ganglia (figure 5c). While primitive vision with elaborate optics is not necessarily coupled to the existence of a brain, visual systems that allow detection of more complex stimuli such as visualization of form or movement require a central nervous system enabling the organisms to respond differentially.
The vertebrate retina represents a prominent example for the evolution of visual circuits by functional segregation of sister cell types, as recently hypothesized (Arendt 2008) based on resemblances of rods, cones and bipolar cells in molecular fingerprints (Lamb et al. 2007; Arendt 2008). Consistent with this scenario is the situation in the most primitive chordates, the hagfish, in which ciliary PRCs connect directly to ganglion cells, and bipolar cells appear to be absent (Holmberg 1977). The neural connectivity of pineal photoreceptors in non-mammalian vertebrates also suggests that bipolar cells are late additions specific to vertebrate lateral eyes (Lamb et al. 2007; Nilsson & Arendt 2008). A functional segregation scenario would also account for the molecular similarity of both Drosophila rhabdomeric PRCs and second-order interneurons that express the atonal gene (Jarman et al. 1994), indicative of a possible sister cell-type relationship.
Based on the common expression of Chx10/Vsx, Math5/ATO and Brn3b/ACJ6 in visual interneurons in mice and flies, it has recently been speculated that the evolution of interneurons might have predated urbilaterians (Erclik et al. 2008). Alternatively, visual interneurons might have evolved independently in insects and vertebrates, as would be suggested by the absence of bipolar cells in the hagfish (discussed earlier). Extensive expression profiling of the cell types will be required to elucidate cell-type interrelationships between and within species and will ultimately allow us to trace back the diversification of cell types during eye evolution from very primitive precursors to the complex visual eyes found in insects, vertebrates and chordates.
6. Conclusion
The evolution of highly complex metazoan eyes as found in vertebrates, insects and cephalopods is an excellent showcase for the evolution of metazoan complexity. We reason here that this process may have relied more on cell-type functional segregation and less on the acquisition of entirely new cellular functions than one might expect at first glance. We propose that one major evolutionary trend in Metazoa has been the specialization of cells into distinct and diverse cell types, each with a limited number or even with only one specific function rather than the evolution of new functions, especially with regard to the molecular toolbox of light perception.
Footnotes
One contribution of 13 to a Theme Issue ‘The evolution of phototransduction and eyes’.
References
- Arendt D.2003Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 47, 563–571 [PubMed] [Google Scholar]
- Arendt D.2008The evolution of cell types in animals: emerging principles from molecular studies. Nat. Rev. Genet. 9, 868–882 (doi:10.1038/nrg2416) [DOI] [PubMed] [Google Scholar]
- Arendt D., Wittbrodt J.2001Reconstructing the eyes of Urbilateria. Phil. Trans. R. Soc. Lond. B 356, 1545–1563 (doi:10.1098/rstb.2001.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneson A. C., Cutress C. E.1976Life history of Carybdea alata Reynaud, 1830 (Cubomedusae). In Coelenterate ecology and behavior (ed. Mackie G. O.), New York, NY: Plenum [Google Scholar]
- Bartolomaeus T.1992aUltrastructure of the photoreceptors in certain larvae of the Annelida. Microfauna Mar. 7, 191–214 [Google Scholar]
- Bartolomaeus T.1992bUltrastructure of the photoreceptors in the larvae of Lepidochiton cinereus (Mollusca, Polyplacophora) and Lacuna divaricata (Mollusca, Gastropoda). Microfauna Mar. 7, 215–236 [Google Scholar]
- Blumer M. J. F.1994The ultrastructure of the eyes in the veliger-larvae of Aporrhais sp. and Bittium reticulatum (Mollusca; Caenogastropoda). Zoomorphology 114, 149–159 (doi:10.1007/BF00403262) [Google Scholar]
- Blumer M. J. F.1995The ciliary photoreceptors in the teleplanic larvae of Smaragdia sp. and Strombus sp. (Mollusca, Gastropda). Zoomorphology 115, 73–81 (doi:10.1007/BF00403256) [Google Scholar]
- Blumer M. J. F.1996The ciliary photoreceptors in the teleplanic larvae of Smaragdia sp. and Strombus sp. (Mollusca, Gastropda). Zoomorphology 116, 123–131 (doi:10.1007/BF02526944) [Google Scholar]
- Blumer M. J. F.1998Alterations of the eyes of Carinaria lamarcki (Gastropoda, Heteropoda) during the long pelagic cycle. Zoomorphology 118, 183–194 (doi:10.1007/s004350050068) [Google Scholar]
- Blumer M. J. F.1999Development of a unique eye: photoreceptors of the pelagic predator Atlanta peroni (Gastropoda, Heteropoda). Zoomorphology 119, 81–91 (doi:10.1007/s004350050083) [Google Scholar]
- Brandenburger J. L., Eakin R. M.1981Fine structure of ocelli in larvae of an archiannelid, Polygordius cf. appendiculatus. Zoomorpology 99, 23–36 (doi:10.1007/BF00310352) [Google Scholar]
- Brandenburger J. L., Woollacott R. M., Eakin R. M.1973Fine structure of eyespots in tornarian larvae (phylum: Hemichordata). Z. Zellforsch Mikrosk Anat. 142, 89–102 (doi:10.1007/BF00306706) [DOI] [PubMed] [Google Scholar]
- Budelmann B. U., Schipp R., Boletzky S. V.1997Cephalopoda. Mollusca II. In Microscopic anatomy of invertebrates, vol. 6A (eds Harrison F. W., Kohn A. J.), pp. 119–414 New York, NY: Wiley Liss [Google Scholar]
- Carroll S. B.2001Chance and necessity: the evolution of morphological complexity and diversity. Nature 409, 1102–1109 (doi:10.1038/35059227) [DOI] [PubMed] [Google Scholar]
- Chia F. S., Koss R.1978Development and metamorphosis of the planktotrophic larvae Rostanga pulchra (Mollusca, Nudibranchia). Mar. Biol. 46, 109–119 (doi:10.1007/BF00391526) [Google Scholar]
- Eakin R. M., Martin G. G., Reed C. T.1977Evolutionary significance of fine structure of archiannelid eyes. Zoomorphologie 88, 1–18 (doi:10.1007/BF00993301) [Google Scholar]
- Erclik T., Hartenstein V., Howard D., Lipshitz H. D., McInnes R. R.2008Conserved role of the Vsx genes supports a monophyletic origin for bilaterian visual systems. Curr. Biol. 18, 1278–1287 (doi:10.1016/j.cub.2008.07.076) [DOI] [PubMed] [Google Scholar]
- Foster K. W.2009Eye evolution: two eyes can be better than one. Curr. Biol. 19, R208–R210 (doi:10.1016/j.cub.2009.01.019) [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Ikeo K.1999Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 15, 371–377 (doi:10.1016/S0168-9525(99)01776-X) [DOI] [PubMed] [Google Scholar]
- Guy K., Tomer R., Jekely G., Hausen H., Arendt D.Submitted Molecular fingerprinting of photoreceptor and pigment cell types indicates an early evolutionary divergence of annelid larval and adult eyes. [Google Scholar]
- Holmberg K.1977The cyclostome retina. In Handbook of sensory physiology, vol. VII/5 (ed. Crescitelli F.), pp. 47–66 Berlin, Germany: Springer [Google Scholar]
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y., Jan Y. N.1994Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398–400 (doi:10.1038/369398a0) [DOI] [PubMed] [Google Scholar]
- Jékely G.2009Evolution of phototaxis. Phil. Trans. R. Soc. B 364, 2795–2808 (doi:10.1098/rstb.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jékely G., Colombelli J., Hausen H., Guy K., Stelzer E., Nedelec F., Arendt D.2008Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399 (doi:10.1038/nature07590) [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Takano K., Tsukamoto H., Ohtsu K., Tokunaga F., Terakita A.2008Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc. Natl Acad. Sci. USA 105, 15 576–15 580 (doi:10.1073/pnas.0806215105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Collin S. P., Pugh E. N., Jr2007Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 8, 960–975 (doi:10.1038/nrn2283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys S. P., Degnan B. M.2001Cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338 (doi:10.2307/1543611) [DOI] [PubMed] [Google Scholar]
- Maldonado M., Durfort M., McCarthy D. A., Young C. M.2003The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Mar. Biol. 143, 427–441 (doi:10.1007/s00227-003-1100-1) [Google Scholar]
- Marsden J. R., Hsieh J.1987Ultrastructure of the eyespot in three polychaete trochophore larvae (Annelida). Zoomorphology 106, 361–368 (doi:10.1007/BF00312259) [Google Scholar]
- Marshall D. J., Hodgson A. N.1990Structure of the cephalic tentacles of some species of prosobranch limpet (Patellidae and Fissurellidae). J. Moll. Stud. 56, 415–424 (doi:10.1093/mollus/56.3.415) [Google Scholar]
- McShea D. W.2000Functional complexity in organisms: parts as proxies. Biol. Phil. 15, 641–668 (doi:10.1023/A:1006695908715) [Google Scholar]
- McShea D. W.2002A complexity drain on cells in the evolution of multicellularity. Evolution 56, 441–452 [DOI] [PubMed] [Google Scholar]
- Nilsson D.-E., Arendt D.2008Eye evolution: the blurry beginning. Curr. Biol. 18, R1096–R1098 (doi:10.1016/j.cub.2008.10.025) [DOI] [PubMed] [Google Scholar]
- Nordstrom K., Wallen R., Seymour J., Nilsson D.2003A simple visual system without neurons in jellyfish larvae. Proc. R. Soc. Lond. B 270, 2349–2354 (doi:10.1098/rspb.2003.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley T. H., Rivera A. S.2008Genomics and the evolutionary origins of nervous system complexity. Curr. Opin. Genet. Dev. 18, 479–492 (doi:10.1016/j.gde.2008.12.002) [DOI] [PubMed] [Google Scholar]
- Okada Y. K.1927Note sur l'ontogenie de Carybdea rastonii Haacke. Bull. Biol. Fr. 61, 241–249 [Google Scholar]
- Plachetzki D. C., Degnan B. M., Oakley T. H.2007The origins of novel protein interactions during animal opsin evolution. PLoS ONE 2, e1054 (doi:10.1371/journal.pone.0001054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purschke G.1992Ultrastructural investigations of presumed photoreceptive organs in two Saccocirrus species (Polychaeta, Saccocirridae). J. Morphol. 211, 7–21 (doi:10.1002/jmor.1052110103) [DOI] [PubMed] [Google Scholar]
- Purschke G.2005Sense organs in polychaetes (Annelida). Hydrobiologia 535/536, 53–78 (doi:10.1007/s10750-004-4358-5) [Google Scholar]
- Purschke G., Müller M. C. M.2006Evolution of body wall musculature. Integr. Comp. Biol. 46, 497–507 (doi:10.1093/icb/icj053) [DOI] [PubMed] [Google Scholar]
- Purschke G., Arendt D., Hausen H., Müller M. C. M.2006Photoreceptor cells and eyes in Annelida. Arthrop. Struct. Dev. 35, 211–230 (doi:10.1016/j.asd.2006.07.005) [DOI] [PubMed] [Google Scholar]
- Putnam N. H., et al. 2007Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (doi:10.1126/science.1139158) [DOI] [PubMed] [Google Scholar]
- Rhode B.1992Development and differentiation of the eye in Platynereis dumerilii (Annelida, Polychaeta). J Morphol 212, 71–85 (doi:10.1002/jmor.1052120108) [DOI] [PubMed] [Google Scholar]
- Seipel K., Yanze N., Schmid V.2004Developmental and evolutionary aspects of the basic helix–loop–helix transcription factors Atonal-like 1 and Achaetescute homolog 2 in the jellyfish. Dev. Biol. 269, 331–345 (doi:10.1016/j.ydbio.2004.01.035) [DOI] [PubMed] [Google Scholar]
- Sensenbaugh T., Franzén Å.1987Fine structural observations of the apical organ in the larva of Polygordius (Annelida: Polychaeta). Scan Microsc. 1, 181–189 [Google Scholar]
- Suga H., Schmid V., Gehring W. J.2008Evolution and functional diversity of jellyfish opsins. Curr. Biol. 18, 51–55 (doi:10.1016/j.cub.2007.11.059) [DOI] [PubMed] [Google Scholar]
- Suschenko D., Purschke G.2009Ultrastructure of pigmented adult eyes in errant polychaetes (Annelida: implications for annelid evolution. Zoomorphology 128, 75–96 (doi:10.1007/s00435-008-0075-3) [Google Scholar]
- Technau U., et al. 2005Maintenance of ancestral complexity and non-metazoan genes in two basal cnidarians. Trends Genet. 21, 633–639 (doi:10.1016/j.tig.2005.09.007) [DOI] [PubMed] [Google Scholar]
- Tzetlin A. B., Filippova A.2005Muscular system in polychaetes (Annelida). Hydrobiologia 535/536, 113–126 (doi:10.1007/s10750-004-1409-x) [Google Scholar]
- Verger-Bocquet M.1983Etude infrastructurale des organs photorécepteurs chez les larves de deux Syllidiens (Annélides, Polychètes). J. Ultrastruct. Res. 84, 67–72 [DOI] [PubMed] [Google Scholar]
- Vickaryous M. K., Hall B. K.2006Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol. Rev. Camb. Phil. Soc. 81, 425–455 (doi:10.1017/S1464793106007068) [DOI] [PubMed] [Google Scholar]
- Wilkens V., Purschke G.2009Central nervous system and sense organs with special reference to photoreceptor-like sensory elements in Polygordius appendiculatus (Annelida), an interstitial polychaete with uncertain phylogenetic affinities. Invert. Biol. 128, 46–64 (doi:10.1111/j.1744-7410.2008.00145.x) [Google Scholar]
- Wilkens V., Purschke G.In press Pigmented eyes, photoreceptor-like sense organs and central nervous system in the polychaete Scoloplos armiger (Orbiniidae, Annelida) and their phylogenetic importance. J. Morphol (doi:10.1002/jmor.10758) [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tasaki K., Sugawara Y., Tonosaki A.1965The fine structure of the octopus retina. J. Cell Biol. 25, 345–359 (doi:10.1083/jcb.25.2.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukov V. V., Borissenko S. L., Zieger M. V., Vakoliuk I. A., Meyer-Rochow V. B.2006The eye of the freshwater prosobranch gastropod Viviparus viviparus: ultrastructure, electrophysiology and behaviour. Acta Zool. (Stockh.) 87, 13–24 (doi:10.1111/j.1463-6395.2006.00216.x) [Google Scholar]