Abstract
All evidence currently available indicates that obligatory sterile eusocial castes only arose via the association of lifetime monogamous parents and offspring. This is consistent with Hamilton's rule (brs > roc), but implies that relatedness cancels out of the equation because average relatedness to siblings (rs) and offspring (ro) are both predictably 0.5. This equality implies that any infinitesimally small benefit of helping at the maternal nest (b), relative to the cost in personal reproduction (c) that persists throughout the lifespan of entire cohorts of helpers suffices to establish permanent eusociality, so that group benefits can increase gradually during, but mostly after the transition. The monogamy window can be conceptualized as a singularity comparable with the single zygote commitment of gametes in eukaryotes. The increase of colony size in ants, bees, wasps and termites is thus analogous to the evolution of multicellularity. Focusing on lifetime monogamy as a universal precondition for the evolution of obligate eusociality simplifies the theory and may help to resolve controversies about levels of selection and targets of adaptation. The monogamy window underlines that cooperative breeding and eusociality are different domains of social evolution, characterized by different sectors of parameter space for Hamilton's rule.
Keywords: Hamilton's rule, insect societies, kin selection, levels of selection, germ line
‘Hence I can see no real difficulty in any character having become correlated with the sterile condition of certain members of insect-communities: the difficulty lies in understanding how such correlated modifications of structure could have been slowly accumulated by natural selection’.
(Darwin 1859, p. 258)
‘I here suggest that the burden of proof may be upon the investigator who argues that sterile castes have evolved other than within broods of single mothers’.
(Alexander 1974, p. 359)
‘Monogamy and especially monogamy outside the breeding season, is the rare exception’ … . ‘In the animal world, fidelity is a special condition that evolves when the Darwinian advantage of cooperation in rearing offspring outweighs the advantage of either partner of seeking extra mates’.
(Wilson 1975, pp. 315, 330)
1. Introduction
Extensive clades characterized by societies with obligatorily sterile members evolved in the ants, bees, wasps and termites (Wilson 1971, 1975). These eusocial forms of life have been associated with a major transition in organic evolution (Maynard Smith & Szathmáry 1995), and some of them have been singled out as spectacularly sophisticated superorganisms (Hölldobler & Wilson 1990, 2008; Moritz & Southwick 1992; Seeley 1995), but the fundamental nature of their evolutionary origins remains the subject of considerable debate (for recent contributions see Crozier 2008; Wilson 2008). This is remarkable, as Darwin had already provided the outline of an answer by suggesting that selection at the family level could explain why workers gave up personal reproduction and came to express different traits than queens and males. As the first quote above illustrates, a major issue for Darwin was to explain the evolution of worker sterility syndromes as a gradual directional process without any sudden leaps. As he writes, ‘Natura non facit saltum’ is an old canon in natural history that every experienced naturalist of his days adhered to. Re-reading the seventh ‘Instinct’ chapter in ‘The origin’ makes it clear that Darwin's understanding of the problem was straightforward: insect workers lose their reproductive totipotency because of selection at the level of the close relatives around them and not merely any randomly formed group. William Morton Wheeler (1928) echoes Darwin's insight by considering the transition to full sociality as a mere final step of increased family coherence in which ‘The progeny are not only protected and fed by the mother, but eventually cooperate with her in rearing additional broods of young, so that parent and offspring live together in an annual or perennial society’.
A more pluralistic spectrum of hypothetical origins of eusociality arose in the second half of the twentieth century. The Darwin–Wheeler scenario was questioned because some eusocial bees mass-provision their cells before egg-laying, which precludes direct interaction between mother and offspring during the larval stage. Because of this apparent difficulty, Michener (1958) proposed that there might have been two precursor states for eusociality: The association between parents and offspring (the subsocial route) and the association between same generation breeders (the parasocial or semisocial route; see also Lin & Michener 1972; West Eberhard 1975). Although direct positive evidence for the parasocial route towards obligate eusociality has not been obtained (even 50 years after this hypothesis was conceived Bourke & Franks 1995; Boomsma 2007), this alternative paradigm was provisionally accepted by many (e.g. West Eberhard 1975; Wilson 1975) and appears to have retained some prevalence until the present day. This may be partly due to Hamilton's (1964, 1972) inclusive fitness concept leaving open the possibility of multiple pathways towards eusociality, as his inequality condition for the evolution of altruism (Hamilton's rule) can be fulfilled by a range of relatedness values. However, he also stressed that it is difficult if not impossible to conceptualize how sufficiently high relatedness in groups of same-generation females can be maintained across enough generations to make subordinates irreversibly lose their reproductive totipotency (Hamilton 1964, 1972; Wilson 1971), points that were reinforced by Alexander (1974) (see quote above) and Alexander et al. (1991).
The main theme of the first part of the present review will be to refute the parasocial route towards eusociality more firmly, as being both conceptually untenable and inconsistent with empirical evidence, and to reinforce the subsocial scenario by explicitly connecting it to lifetime parental monogamy. I will argue that parasocial arrangements apply only to cooperative breeders and to those facultatively eusocial groups that are, in reality, cooperative breeders because they never realized the transition to having obligately eusocial helper castes. Ambiguity about the selection forces that ultimately caused individuals to irreversibly lose most or all of their reproductive potential has recently expanded into an extensive debate on the relative importance of kin selection and group selection (Wilson & Hölldobler 2005; Fletcher et al. 2006; Foster et al. 2006a; Helanterä & Bargum 2007; West et al. 2007, 2008; Wilson & Wilson 2007; Gardner & Grafen 2009), and on the necessity of high relatedness to pass the eusociality threshold (Wilson 2005, 2008; Foster et al. 2006b; Crozier 2008). I hope to contribute to the resolution of the eusociality part of this debate by proposing a relatively simple and parsimonious scenario based on the notion that sexual partners commit for life in all presently known obligately eusocial ants, bees, wasps and termites. I will use the term ‘obligate eusociality’ to indicate situations where caste is irreversibly determined early in development (before pupation in the Hymenoptera), and to such extent that no individuals of predestined worker cohorts retain the behavioural, and often also physiological, option to disperse and found their own colonies (Crespi & Yanega 1995). Rather than focusing on Hamilton's rule, I will concentrate on the lifetime monogamous mating system conditions that must have characterized lineages at the very origin of these eusocial clades. It was these conditions of ‘dying with the only sexual partner you ever have’ that gave Hamilton's rule the most optimal conditions for forging the sweep towards eusociality without any leaps, or ‘salta’, because they implied that relatedness to siblings was no longer a variable, but a predictable equivalent of relatedness towards own offspring (Charnov 1978). When that is so, the relatedness terms cancel out of Hamilton's rule when the actual transition towards obligate eusociality takes place.
In the later sections of this review, I will briefly explore some of the implications, novel predictions and perspectives that this approach to the evolution of eusociality allows. I will argue that lifetime monogamy makes the evolution of obligate eusociality analogous to the evolution of multicellularity and that both types of development happened at roughly equal frequencies over evolutionary time. I will outline the kind of phenotypic and genetic predictions that can be derived from the lifetime monogamy idea and conclude that the obligatorily eusocial lineages are best considered as a separate domain of social evolution relative to the solitary and cooperative breeders. Finally, I will return to the analogy with multicellularity and briefly explore how the conceptualization of colony-level analogues of germ line and soma may further enhance our understanding of collective adaptations of eusocial colonies.
2. Lifetime sexual commitment of parents
The parents of most eusocial insects (queens and males, the latter are sometimes referred to as drones or kings) produce only full sibling offspring throughout their lives (Boomsma & Ratnieks 1996; Strassmann 2001). They have a single brief period of irreversible mate choice as newly emerged adults and the ensuing monogamous relationship persists until they die (Boomsma et al. 2005). Physical lifetime monogamy is the default in termites, but queens of ants, bees and wasps have a functional equivalent of this in that their mates die without ever participating in colony founding, but have their sperm stored (Wilson 1971). These hymenopteran queens never re-mate even though they may survive and reproduce for years or decades (Hölldobler & Bartz 1985; Boomsma et al. 2005; Kronauer & Boomsma 2007). The complete absence of re-mating promiscuity (Boomsma 2007) not only imposes extraordinary selection for maintaining viability of stored sperm (Hölldobler & Bartz 1985; Baer et al. 2006; Den Boer et al. 2008), but also implies that altruism (as soldiers or workers) benefits siblings with an average relatedness (r) of 0.5 when queens are singly inseminated and there is equal Fisherian sex allocation. For haplodiploid Hymenoptera this average is between 0.75 relatedness towards sisters and 0.25 relatedness towards brothers, whereas the diploid termites are related to siblings of both genders by 0.5 (see also Queller 2000). Multiple queen-mating arose in many clades of eusocial Hymenoptera (Boomsma & Ratnieks 1996; Boomsma et al. 2009) but, as predicted by Hamilton (1964): ‘ … if the trend to multiple insemination occurs after the firm establishment of the worker caste, its threat to colonial discipline is a rather remote one’. This was recently confirmed by a formal comparative analysis (Hughes et al. 2008), which showed that all presently known clades of eusocial ants, bees and wasps with multiple queen-mating are derived from ancestors with single queen-mating. Multiple mating therefore neither complicates the early evolution of eusociality nor its later elaborations.
As worked out in more detail in a previous review (Boomsma 2007), every ancestor of an extant independent eusocial lineage can be predicted to have passed through a monogamy window. This prediction has been—and will be below—primarily elaborated for the four classical examples of advanced eusociality (ants, most corbiculate bees, vespine wasps, higher termites), but should equally apply to thrips, bark beetles, sphecid wasps, shrimps, naked mole-rats (Heterocephalus glaber) and aphids (with due consideration of clonality), if they were to be considered as eusocial lineages or advanced cooperative breeders on the brink towards making the transition (Crespi 1996). The rationale of this prediction is that only the lifetime exact equivalence (in average relatedness terms) between offspring and sibling production is a parsimonious universal condition to start and maintain consistent directional selection for the loss of reproductive totipotency of entire cohorts of offspring. Once this average r = 0.5 condition is fulfilled, there may be (but often will not be) cost–benefit factors that push a species into the eusocial state (Bourke & Franks 1995; Crespi 1996; Crozier & Pamilo 1996; Gadagkar 1996; Queller 1996) in the gradual accumulative way envisaged by Darwin (1859) and with the necessary genetic changes as hypothesized by West Eberhard (1996) and Linksvayer & Wade (2005).
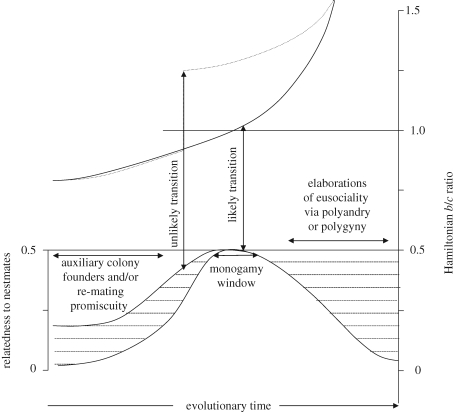
When parents commit their lifetime reproductive success to a single sexual partner, any infinitesimal cost–benefit advantage (c/b sensu Hamilton's rule) would suffice to make the irreversible transition towards obligate eusociality. Lifetime monogamy would make such advantage last a helper's lifetime, where it would not in cooperatively breeders where sexual partners do get exchanged. Thus, entire cohorts of offspring would be selected to give up the ability to mate and reproduce in the former, but not in the latter social setting. Any minute degree of parental coercion (Charnov 1978) would suffice to achieve the same result, and could easily trigger an increased dependence on indirect fitness benefits in offspring (cf. Linksvayer & Wade 2005), because the transition towards eusociality is neutral in terms of offspring inclusive fitness and unambiguously favourable for direct parental fitness (Bourke & Franks 1995; Crozier & Pamilo 1996). To see this, it is important to realize that for the evolution of eusociality, Hamilton's rule is not written as: br > c, but as br > 0.5c, because the cost is paid as a reduction in offspring to which the actor is related by 0.5, rather than in the survival probability of self to whom the actor is related by 1 (as is, for example, the case for vertebrate alarm calls). Lifetime monogamy thus implies that the relatedness term cancels out of Hamilton's rule when the average relatedness to siblings is predictably 0.5, so that becoming a sterile helper should merely be a matter of time when b>c is fulfilled throughout the lifetime of cohorts of offspring. Any other mating system that would not necessitate that you die with the single mate that you found early in life would produce a less favourable scenario for the evolution of obligate reproductive altruism as it would probably require leaps in the Hamiltonian b/c ratio for making the transition (figure 1; see also Boomsma 2007, fig. 2).
Figure 1.
Evolving obligate eusociality via a monogamy window, with nestmate relatedness to the left and the per capita Hamiltonian b/c ratio to the right (both lifetime averages as in Boomsma 2007, fig. 3). Given that promiscuity and some degree of multiple breeder aggregation are the default settings of most breeding systems, nestmate relatedness (lower curves) is typically low but positive in distant ancestors and has to increase to 0.5 (either shallowly via a cooperative breeding system, or steeply from a polygamous solitary ancestor—the hatched area towards the left illustrates the likely ranges of relatedness). However, when lifetime monogamy has been established (i.e. the monogamy window has been reached), infinitesimally small but consistent group benefits (b/c > 1) will be sufficient to make the transition towards eusociality (short vertical arrow). Once obligate non-matedness (complete or partial sterility) of helper cohorts has been established, polyandry (multiple queen-mating) or (secondary) polygyny may re-evolve (but would not necessarily do so) and will reduce nestmate relatedness (hatched area towards the right). With the possible exception of inquiline social parasites, and the poneroid ants where adult workers may later become mated to assume dominant breeder roles, this has never led to the abandonment of obligately eusocial phenotypes. This must have been because the group-size benefit curve b/c increases more sharply than the relatedness curve decreases. Inbreeding is not considered here because there seem to be no examples where inbreeding has been associated with the evolution of eusociality without parents also being lifetime monogamous (Pamilo 1991). Any transition that could conceivably take place at, say, r = 0.4, would require a per capita group size (b/c) benefit >1.25 to be consistent with Hamilton's rule. Given that b/c cannot be expected to exceed 1 before group-living is established, this would require a step-wise transition in the b/c curve, which makes this scenario (long vertical arrow) unlikely.
3. Evidence for ancestral lifetime monogamy in eusocial lineages
Termite queens normally produce full sibling offspring throughout their lives, because they commit to a single male when founding a colony. The only difference with the ants, bees and wasps is that males have similarly long lifespans to queens and that mating continues throughout life. Exceptions to this rule may occur in evolutionarily derived termite lineages where multiple breeders are sometimes found (Thorne 1983, 1985; Roisin 1987; Darlington 1988; Atkinson & Adams 1997; Thompson & Hebert 1998; Brandl et al. 2001; Hacker et al. 2005; Atkinson et al. 2008), but no cases of effective re-mating promiscuity followed by successful colony continuation appear to have been documented with genetic markers. Issues of mate-choice and sexual selection during swarming (e.g. courtship, sex pheromone communication, display) and society building therefore appear to be as fully separated in the termites as they are in the eusocial Hymenoptera (Boomsma et al. 2005). The crucial point is that, as a rule, no ‘new blood’ ever seems to enter an existing termite colony (Boomsma 2007; see below for an evaluation of apparent exceptions).
In spite of lifetime parental monogamy at colony founding, the lower termites remained cooperative breeders in a functional sense (Korb 2008; Lo et al. 2009). This may be related to most of them having life histories of ‘one piece’ (‘single-site’) nesting, which implies that they gradually excavate their nest in the log that they feed on. Larger and older colonies thus become more likely to lose their local nesting and feeding monopoly, as the probability of being confronted with neighbouring colonies in the same log increases when less of the food and nest substrate remains. This maintains selection for reproductive totipotency in offspring, as dispersal will remain the ultimately superior solution when nestmate relatedness stands to become diluted by joining non-relative breeders, re-assortment of parentage and finally, starvation. This is consistent with the first eusocial castes in termites arising as soldiers rather than workers, as the former are more effective in maintaining the integrity of a monogamous family against assaults of neighbouring conspecifics (Shellman-Reeve 1997; Roisin 1999; Korb 2008).
Reproductively altruistic workers apparently only evolved after termites had reached the derived state of having both a nest and an external foraging range (multiple site nesters and central place foragers), as envisaged by Abe (1991) and Higashi et al. (1991). It is still unclear how often these nesting habits and worker castes evolved (Thompson et al. 2000; Inward et al. 2007), but the correlation between the presence of true workers and foraging beyond the boundaries of the nest is apparently a perfect one (Inward et al. 2007). Both the cost of foraging (Korb 2008) and disease pressure (Thorne & Traniello 2003) probably increased significantly when colonies came to extend beyond the confinements of a single log, which may have gradually increased the group-wise benefits from task partitioning and mutual grooming, so that obligate altruism evolved in combination with increased rates of senescence of the now more exposed helpers (Alexander et al. 1991; Bourke 1999, 2007; Crespi 2007). However, the decisive selection force for evolving lifetime sterile worker phenotypes may well have been that inescapable mergers of mature colonies no longer occurred so that the risk of sudden drops in relatedness towards nestmates due to remaining parents finding new mates had disappeared. The fact that colony boundaries became defined by foraging ranges rather than nest space thus implied that the inclusive fitness benefits owing to parental monogamy became guaranteed across the lifetime of any entire cohort of helpers.
All free-living ants have (had ancestors with) an obligatorily eusocial worker caste, whereas rather few derived lineages with large colonies also have soldiers (Wilson 1971; Hölldobler & Wilson 1990). This is consistent with the early evolution of the ants being characterized by foraging beyond the nests boundaries, which was unavoidable as primitive ants were predators, so they could not live within their food as the ancestral termites could. The subsocial origin of the ants appears to be generally accepted as the most likely scenario from Wheeler (1928) onwards, and lifetime monogamy of the ancestral ant is consistent with the comparative data available (Boomsma & Ratnieks 1996; Boomsma 2007; Hughes et al. 2008). A unique feature of the ants is that they have a large basal branch, the poneroid complex, that retained an exclusively predatory lifestyle and realized relatively little further elaboration of eusociality compared with the formicoid ants (Brady et al. 2006; Moreau et al. 2006; Hölldobler & Wilson 2008; Rabeling et al. 2008). Some of these ants have workers that may become mated after having gained single breeder status, whereas others have lost the queen caste, either in part of the colony or altogether (Peeters 1997; Hölldobler & Wilson 2008). The latest phylogenetic reconstructions seem to imply that the poneroid ants and a few other lineages might have lost a number of key eusocial traits that probably characterized the ancestor of all ants, e.g. queen castes were lost in some clades, whereas others have many workers that mate and compete with queens for full reproduction. These ants have thus reverted to advanced forms of cooperative breeding comparable to, for example, naked mole-rats (Peeters 1997; Hart & Ratnieks 2005; Hölldobler & Wilson 2008). The crucial trait that makes them cooperative breeders is that females with a morphologically distinct worker phenotype can mate later in life to become the dominant breeder in the same colony in which they hatched (Hölldobler & Wilson 2008).
The recent discovery of the sister group of all previously known ants (Rabeling et al. 2008) suggests that many early ants lived as hidden soil-dwellers (also the next branch, the Leptanillinae, have such a lifestyle). As Hamilton (1978) argued, life under the bark of dead trees (or its equivalent in the soil under decaying logs) may have imposed consistent selection for wing polymorphism and facultative non-dispersal of offspring. At the same time, the spaced-out and hidden nest cavities that he describes may have provided many of the conditions favouring lifetime parental monogamy. Likewise, nesting in or under decaying logs may have selected for a long lifespan because of low extrinsic mortality after colony establishment (Keller & Genoud 1997), which would explain that all ants (and termites to which the same selection forces must have applied) have perennial colonies in contrast to all but the most evolutionary derived eusocial wasps and bees. This inference is not necessarily in conflict with the oldest known fossil ant having large eyes (Wilson 1971; Grimaldi & Engel 2005), as many ants combine deep soil nesting with diurnal surface or arboreal foraging. However, clades combining these traits may have been more prone to extinction than those specialized for a completely underground lifestyle, so that only the latter are extant.
The early social evolution pathways in the vespid wasps were characterized by cooperative breeding rather than eusocial commitment and it seems that open nesting may have prevented single females from creating full sibling colonies. Whether related or not, if females compete for nests or nestsites, full sibling families will arise only if one female can exclude all nest-founding competitors until the first offspring cohort hatches. The prevalence of primary polygyny (following pleometrosis) in the tropical stenogastrine and polistine wasps is therefore consistent with the maintenance of individual totipotency, as options for direct fitness benefits either in a co-founded nest or elsewhere remain a realistic option. The stenogastrine clade never evolved obligate eusociality, whereas the sister clade consisting of the polistine and vespine wasps has a single transition towards obligate eusociality in the ancestor of the vespines that adopted single queen breeding (Hines et al. 2007). This scenario was already conceptualized by Wheeler (1928), and by Wilson (1971) who wrote: ‘The life cycle of the vespines is basically similar to that of Polistes, except that the queen is not joined by auxiliaries during nest founding in spring’. Thus, although single mating as a precondition for eusociality was fulfilled in all basal wasp lineages (Hughes et al. 2008) it was only after obligate monogyny arose in the ancestor of the vespine wasps that the transition to obligate eusociality happened, as predicted by the monogamy window hypothesis.
The arguments above illustrate that social systems like Polistes, and their close relatives such as Ropalidia, are best considered as cooperative breeders, because they have no permanent castes as defined in the introduction of this review (Gadagkar 1994, 1996), as broods tend to have at least some individuals that become early diapausing queens rather than helpers at the nest (Reeve et al. 1998). Just like many poneroid ants, these social systems are characterized by most individuals ‘queuing’ for possible future reproductive dominance (i.e. direct fitness benefits). Similar to vertebrate cooperative breeders, relatedness-based inclusive fitness benefits may or may not be found, as both the ability to recognize kin and the (in)direct benefits from helping vary across species so that each of these parameters needs to be explicitly considered (Griffin & West 2003). The data are noisy, but Polistes gynes in spring tend to voluntarily associate only with those natal nestmates of the previous season that are relatives. Later in the season interactions between unrelated females increase in frequency, but females that join at this stage behave quite differently than related cofoundresses. They are highly likely to have lost their own nests to predators and usurp nests for direct fitness benefits rather than indirect ones (Strassmann 1996). In addition, colonies that suffer a sudden reduction in relatedness due to usurpation events will tend to have more female larvae developing into dispersing gynes (aiming for direct future fitness benefits) than into staying workers (continuing to rely on indirect fitness benefits) (Strassmann 1996).
The epiponine (polybiine) wasps, which puzzled Hamilton (1964, 1972) as odd enigmas for inclusive fitness theory, have since been shown to produce males when colony relatedness is low, but gynes later in the colony cycle when relatedness is high because the number of egg-layers has been reduced to one (Queller et al. 1993). This implies that largely totipotent helpers (Strassmann et al. 2002) reap considerable indirect fitness benefits through sex ratio biasing (Boomsma & Grafen 1991) in a social system that is cyclically monogamous (Queller et al. 1993; Hastings et al. 1998). This highly successful clade of wasps with perennial nests even managed to decouple swarm production from queen production (Strassmann et al. 1998). Yet, although the collective worker interests are largely met—in contrast to what is generally found in Polistes (Hastings et al. 1998)—it seems doubtful whether the epiponine wasps crossed the threshold towards obligate eusociality in the sense of evolving a worker caste that is uniformly determined before larval pupation (Strassmann et al. 2002). This is consistent with founding new colonies by multiple females (swarm founding), which precludes lifetime monogamous parenting (Boomsma 2007).
Interpreting the early evolution of eusociality in bees as a straightforward subsocial transition has been hampered by the apparent absence of progressive larval provisioning (adults continuing to actively feed larvae until pupation), which is one of the crucial brood-care traits of eusociality, in the stingless bees. This seemed to imply that the ancestor of the corbiculate bees, which also include the largely solitary euglossine bees and the eusocial bumble-bees and honeybees that have progressive provisioning, might have had a different family structure (Michener 1958; Lin & Michener 1972). Also the origins of incipient, facultative forms of eusociality in the halictid bees did not seem to depend on progressive provisioning. However, recent work has indicated that all eusocial halictid bees that have been studied do in fact have regular brood inspection by a single mother bee, which is likely to be adaptive for reasons of sanitation and adjustment of the quality and quantity of the pollen provision masses (Plateaux-Quénu 2008). The most parsimonious explanation for the three emergences of facultative eusociality (in the sense that worker broods contain at least some early diapausing individuals) in halictid bees (Danforth 2002) would therefore now appear to be the subsocial route. As all corbiculate bees are monogynous (Wheeler 1928) and have a singly mated ancestor (Hughes et al. 2008), this must also apply to the origin of obligate eusociality in this clade. A universal explanation of eusocial evolution via the monogamy window hypothesis would therefore imply that the extant practice of mass provisioning (adults completing provisioning of brood cells before egg-laying and capping cells shortly afterwards) in stingless bees is a secondary development that arose after the ancestor of the corbiculate bees had become obligately eusocial. It is tempting to speculate that increased disease pressure on perennial colonies of these tropical bees, relative to their annual temperate zone bumble-bee sister clade (Kawakita et al. 2008), may have selected for capping cells immediately after provisioning, and that this was not required in honeybees because they evolved genetically more diverse colonies via multiple queen-mating (Boomsma & Ratnieks 1996).
As in the polistine and stenogastrine wasps, it is essential for comparative evaluations of sociality in bees to be precise on whether eusocial helping is obligate, i.e. whether individual caste fate is irreversibly determined before pupation. Even though females often remain wholly or largely sterile, this is not a universal trait for entire cohorts of same-age offspring in clades such as the halictids and allodapines that have been called ‘eusocial’ (cf. Crespi & Yanega 1995). Workers have maintained their spermathecae and many are mated and have the option to express full breeding potential elsewhere, either alone or with other females. The halictid and allodapine bees are therefore best considered to be cooperative breeders, where individuals can facultatively adjust their helping and dominance behaviour to the particular mixture of direct and indirect fitness opportunities that they encounter. Even a very low frequency of early diapausing individuals in worker cohorts implies that the social system has not passed the point of no return towards obligate eusociality. Deviations from lifetime monogamy in lineages that are likely to still have such early diapausers (Soro et al. 2009) therefore do not refute the monogamy window hypothesis, but rather assert that such a lineage will not make the transition in the future either. Not having passed the threshold towards obligate eusociality does not imply that worker roles do not allow considerable indirect fitness benefits to be obtained. Similar to the epiponine wasps, Augochlorella bees have been shown to capitalize on relative relatedness asymmetries by producing adaptive split sex ratios based on colony-level variation in relatedness asymmetry (Mueller 1991).
It thus appears that the monogamy window hypothesis is consistent with most if not all evidence available, which is satisfying as it lends credit to the most general and parsimonious explanation for the convergent origins of eusociality, without any of them requiring sudden step-wise leaps (figure 1) in the b/c ratio of Hamilton's rule (see Darwin's quote at the start of this essay). The seeming absence of countervailing evidence is also somewhat surprising, as it might be argued that the monogamy window hypothesis may be a rather crude oversimplification. It has, for example, been shown that a fraction of unmated, male producing foundresses and partial bivoltinism may both select for female biased sex ratios so that Hamilton's rule is fulfilled at sibling relatednesses that are somewhat below 0.5 on average (Seger 1983; Godfray & Grafen 1988). A similar effect has been shown to apply when a newly evolved worker caste produces some of the males (Pamilo 1991). This implies that low frequencies of double-mating or foundress association would theoretically be compatible with the gradual evolution of obligate eusociality. Yet, there is nothing in the available data that suggests that scenarios like this were likely to have applied. Clarifying why this is so is beyond the scope of this paper and would require formal modelling. I assume though that such models will vindicate the monogamy window hypothesis, when they make reasonable assumptions on the additional costs of cofoundress-conflict, sexual selection and ejaculate competition, when they assume that there is a cost of discriminating between the haploid eggs to be replaced versus the diploid eggs to be left alone, when they consider geometric mean fitness rather than arithmetic mean fitness, and when they allow for realistic amounts of stochasticity. Inbreeding might be included as a factor in such models, although it seems unlikely that this would have a significant effect (Pamilo 1991).
4. Implications
One could argue that the monogamy hypothesis makes the evolution of eusociality too easy. However, where previous authors (Stubblefield & Charnov 1986; Maynard Smith & Szathmáry 1995) used this argument when discussing a rather unspecified form of monogamy, it does make a difference that the lifetime type of monogamy considered here is a very rare condition (e.g. E. O. Wilson quote at the start of this review), particularly when it would have to be maintained over thousands of generations to reshape entire gene expression networks, as would be required for the evolution of permanent helper castes (cf. Linksvayer & Wade 2005). At least two further factors would also hamper the evolution of obligate eusociality. First, the monogamy window hypothesis requires that the ‘point of no return’ transition towards eusociality (Wilson & Hölldobler 2005; Wilson 2008) can only be passed when the b/c ratio remains >1 (if only just) throughout the life of entire helper cohorts. When this is not completely met, social evolution will remain stalled in an advanced form of cooperative breeding where at least some helpers can move on to breed independently as, for example, in halictid and allodapine bees, stenogastrine and polistine wasps, lower termites, social spiders, and naked and Damaraland mole-rats (Cryptomys damarensis) (cf. Hart & Ratnieks 2005). Second, there may be many factors that enhance the Hamiltonian benefits of group living (b), but there are also many (e.g. temporal variation in food availability) that constrain such benefits relative to the direct fitness gains of personal reproduction (c), thus effectively precluding anything other than solitary breeding (e.g. Bourke & Franks 1995; Crespi 1996; Crozier & Pamilo 1996; Gadagkar 1996; Queller 1996). This may explain why lineages may be life-time monogamous for a long time before eusociality evolves.
A striking benefit of group living is improved nest defence (fortress defence sensu Strassmann & Queller 2007), but the other side of this coin is that nest predation has probably been a major general factor that precluded eusocial breeding, as high family-level mortality will maintain selection for dispersal and solitary breeding. Closed and easily defendable nests, often with a single entrance, may thus have provided both protection from conspecific female auxiliaries and usurpers (preventing dilution of sibling relatedness) and protection against nest predators (providing consistent b/c benefits) when lineages were passing through the monogamy window towards eusociality.
When the 0.5 relatedness term cancels out of Hamilton's rule, the conditions for the evolution of eusociality become equivalent to those that apply for the evolution of clonal multicellularity (Queller 2000). This is because the relatedness ratio of siblings versus offspring is equal to the relatedness ratio of adhering cell copies versus dispersing ones, in spite of the twofold difference in absolute values of relatedness (0.5 versus 1.0). Similar to lifetime monogamy not always leading to eusociality (e.g. the lower termites and many solitary wasps and bees), clonal kinship is an essential condition for making the transition to multicellularity, but there are many clonal eukaryotes that never achieved this. The respective statistics between origins of eusociality and multicellularity are remarkably similar: There have been at least 25 independent transitions towards multicellularity (plus a number of secondary reversals), but only approximately three to five of these concerned eukaryotes and produced extensive radiations of complex organisms (Grosberg & Strathmann 2007). These figures are unlikely to be significantly different from the still increasing number of shallow origins of facultative eusociality relative to the four ‘classic’ deep origins of obligate eusociality (Crespi 1996, 2007). Thus, if there is a problem in obligate eusociality having evolved rather rarely (Stubblefield & Charnov 1986), there is an analogous problem in the scattered evolution of multicellularity. In this perspective, it is not surprising that three of these four deep evolutionary origins occurred in the haplodiploid Hymenoptera, as the ancestors of the ants, the vespine wasps and the corbiculate bees must have had lifetime sperm storage by females, which makes it easier to maintain lifetime monogamy. The selective advantages that drove the evolution of multicellularity were size-related returns to scale and benefits from functional specialization and division of labour (Grosberg & Strathmann 2007), analogous to the advantages that must have accompanied the origins and early elaborations of eusociality (Pamilo 1991; Bourke & Franks 1995; Crespi 1996; Crozier & Pamilo 1996; Queller 1996) (cf. the accelerating b/c curve in figure 1).
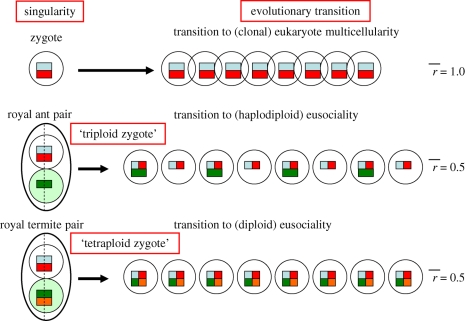
When partners commit for life, their ‘triploid’ (Hymenoptera) or ‘tetraploid’ (termites) union is analogous to the diploid zygote that initiates every individual of a multicellular sexually reproducing species (figure 2). The origin of the zygote is generally considered to have been a crucial bottlenecking singularity that reduced conflict by starting each individual as a merger of the minimal number of independent nuclear genomes to allow recombination and a single clone of uniparentally transmitted cytoplasmic symbionts that became organelles while contributing, and ultimately retaining some of their own genomes (Buss 1987; Maynard Smith & Szathmáry 1995; Queller 2000; Grosberg & Strathmann 2007; Michod 2007). Just like life-time monogamous pairs, the sexual zygote allowed transitions towards lifetime-committed group-living based on the predictable production equivalence of vertical (adhering) versus horizontal (dispersing) gene copies in the next generation (see also Queller 2000). It is useful, therefore, to distinguish them as each having initiated their own domains of social evolution, the zygote by establishing the individual as unit of selection and target of adaptation and the lifetime monogamous parents of insect societies by offering the same potential to the eusocial colony (table 1). However, while the clonal nature of multicellular bodies allowed them to become inclusive fitness maximizing vehicles for their gene replicators (Dawkins 1982), the evolution of explicitly eusocial colony-level adaptations was constrained—in spite of the importance of colony level selection—because internal conflict repression is difficult in non-clonal groups (Wenseleers et al. 2004; Ratnieks et al. 2006) and a higher degree of such repression appears needed for the evolution of superorganismality (Gardner & Grafen 2009) than previously thought (e.g. Reeve & Hölldobler 2007). The evolution of anisogamous sex itself that preceded the origins of multicellularity in eukaryotes could only happen after a twofold disadvantage was overcome (e.g. Williams 1985; Maynard Smith & Szathmáry 1995; Cavalier-Smith 2006), so it is not surprising that this only happened once at the base of the eukaryote tree.
Figure 2.
Schematic comparison of the evolutionary transition from unicellularity to multicellularity and the evolution of eusociality in the haplodiploid ants (the same applies to bees and wasps) and diploid termites. The diploid zygote that originates when syngamous haploid gametes commit for life is an analogous singularity to the permanent ‘triploid’ or ‘tetraploid’ unit that is created by lifetime monogamous mates when they co-found a eusocial colony. Zygotes create multiple, genetically identical (r = 1) copies when making multicellular bodies, whereas lifetime monogamous mating pairs create genetically variable offspring that are on average 50 per cent (r = 0.5) identical. All three examples are fully equivalent for the transmission of maternally inherited cytoplasmatic organelles. When multiple queen-mating evolves secondarily in the eusocial Hymenoptera, the ‘ploidy’ of the founding unit may increase considerably (up to approx. 50 haplotypes in army ants and honeybees). This implies that relatedness asymptotically drops to 0.25, but it does not change the principle of lifetime commitment. Parental (chromosomal) haplotype contributions are marked with different colours; the female cytoplasmatic background is kept in white, whereas the non-transmitted male one is marked in light green. For the eusocial colonies, a sample of their sexual production is plotted, assuming Fisherian sex allocation with 50 per cent haploid males in the Hymenoptera. The workers of such colonies are all females (diploid). No such asymmetries apply in the diploid termites.
Table 1.
A partial reappraisal of the major eukaryote transitions in evolutionary complexity (cf. Maynard Smith & Szathmáry 1995), emphasizing the singularities that initiated them, the main selection drivers that pushed ancestors through these singularities, and the major threats that might have prevented the transition and that needed further evolutionary elaboration to be sufficiently controlled for the higher level of selection to prevail. The three classes represent different domains of social evolution, characterized (roughly) by outbred sex as the only cooperative social interaction (1); a combination of (usually outbred) sex and (possibly) social interactions between relatives (the latter in case of cooperative breeding) that normally overlap in space and time (2); a strict separation between solitary sexual behaviour and family-based social interactions in time, and usually also in space (3). Because of these fundamental differences and the presence/absence of a committed worker caste, secondary developments towards cooperative breeding in the eusocial domain 3 (e.g. poneroid ants; secondary polygynous formicoid ants) are often not directly comparable with non-eusocial cooperative-breeding systems that belong (together with all solitary breeding) to domain 2. The integrity of the domains is threatened by genetically distinct elements that themselves represent different levels of organization. Those relevant for domain 1 are reviewed in Burt & Trivers (2006) and those relevant for domain 2 in Buss (1987) and Michod (2005). Threats of domain 3 may include workers that reproduce in the presence of the queen and socially parasitic additional queens that may ultimately give rise to inquiline species (Buschinger 1990) and selfish patrilines (Hughes & Boomsma 2008).
| singularity | transition | drivers | threats | prevailing level of selection |
|---|---|---|---|---|
| 1. haploid symbiotic cell | sexuality | recombination/repair | selfish genetic elements | cell |
| 2. life-time committed zygote | multicellularity | group selection | selfish cell lineages | individual |
| 3. life-time committed parents | eusociality | group selectiona | selfish individuals | colony |
aIn contrast to domain 2 where group selection leads to individual adaptation, group selection in domain 3 does not necessarily lead to group adaptation.
The monogamy window hypothesis makes a sharp distinction between cooperative breeding and eusociality, and thus explicitly sides with the restricted definition of eusociality formulated by Crespi & Yanega (1995). It makes their definition more precise by merging the facultative eusociality and cooperative breeding categories. This is based on the notion that expressing facultative, context-dependent caste phenotypes is something fundamentally different from expressing irreversible physical caste phenotypes. Linksvayer & Wade (2005) have outlined a three-step hypothetical scenario for the genetic mechanisms mediating transitions towards eusociality that is consistent with this distinction. First, they assume that maternal care genes start being pre-reproductively expressed for sibling-rearing functions (cf. West Eberhard 1996) in association with nutritional state or other environmental factors (e.g. Hunt 1994; Wheeler et al. 2006; Patel et al. 2007), which themselves may have been influenced by parental manipulation. Second, these phenotypically plastic reaction norms of optimal performance as a breeder or helper may then become associated with the expression of additional genes that specifically produce good queens or good workers. Third, the transition to obligate eusociality requires further evolution or elaboration of caste-specific gene expression, for example through gene duplications, to reduce the relative significance of the original pleiotropic genes that affect both helper and breeder performance. Whereas it is easy to see how the first two steps apply to cooperative breeders such as Polistes wasps, step 3 requires a long process of directional selection for decoupling the expression of genes coding for maternal and sibling care and for these alternative phenotypes to become associated with an early developmental bifurcation and correlated with the expression of novel mutations at other loci so that permanent morphological castes emerge (Hunt 1994; West Eberhard 1996; Abouheif & Wray 2002; Linksvayer & Wade 2005; Wilson 2008). Recent evidence has demonstrated the key significance of nutrition for caste determination (Hunt 2007), providing direct insights into the proximate factors that characterize transitions to obligate eusociality. However, it is important to separate this type of explanations from the ultimate causes, i.e. the notion that selection is only likely to work consistently and directionally on these mechanisms to forge transitions to obligate eusociality when lifetime parental monogamy is ensured (figure 1).
I conclude that all extant obligatorily eusocial clades appear to have in common that their distant ancestral mother became a lone nest founder and stopped mating with additional males, so that entire cohorts of her offspring could give up mating at all. This notion is consistent with a general trade-off between parental effort and mating effort (West Eberhard 1983; Boomsma 2007; Crespi 2007) and with Yanega's (1997) conclusion that (non-)mating is the main correlate across halictid bees of helping and dying in the same year versus early diapause and breeding the following year. The loss of a functional spermatheca in hymenopteran workers is a much later development and has only been documented for the honeybees and most of the ants (Gotoh et al. 2008). This implies that many groups that have passed the no-return threshold towards obligate eusociality have workers with spermathecae although these workers never mate (Gotoh et al. 2008). This would explain that some exceptions to this rule, for example in the poneroid ants, could resume worker mating even though they likely had ancestors with behaviourally sterile workers (Gobin et al. 2006; Rabeling et al. 2008). This underlines the notion already expressed by Wheeler (1928) that most traits that characterize extant crown groups of obligatorily eusocial insects are secondary elaborations that cannot shed light on the early evolution of eusociality.
5. Predictions of the monogamy window hypothesis
The lifetime monogamy hypothesis is a bold generalization that implies strong inferences about the parasocial route towards obligate eusociality being incorrect and Hamilton's rule being applicable in a general, but uniquely restricted manner. Neither of these restrictions should apply to cooperative breeding, including many facultatively eusocial forms, where associations between same-generation females are often relevant and where relatedness towards nestmates or siblings may vary freely without jeopardizing the evolutionary stability of these breeding systems (e.g. Griffin & West 2003; see also Hamilton 1964, 1972; Alexander 1974; Alexander et al. 1991).
Although the monogamy window hypothesis at present appears to be compatible with the available data (see above and also Boomsma 2007; Hughes et al. 2008), its predictions need to be made more quantitative by explicit modelling and be tested by further empirical work. A general qualitative prediction is that the secondary evolution of polygyny and polyandry in the eusocial higher termites (Termitidae) should be constrained, because their worker and soldier caste determination systems are likely to have remained more reversible than in the ants (e.g. Roisin & Pasteels 1987). Parental promiscuity would introduce sexual conflict into existing societies and instigate selection on helper castes to express selfish rather than altruistic traits, developments that would tend to destabilize the eusocial breeding system. Such constraints would not apply to any of the eusocial Hymenoptera, because sexually conflictual re-mating promiscuity is precluded by early male death and life-time sperm storage by females. This appears consistent with the data as multiple breeders, although reported from tens of termite species, are almost always a rare and facultative phenomenon at the population level (Thorne 1985; Roisin 1987). Given these interesting differences between the ants and the termites, it would be of paramount importance to critically evaluate the sparse records on multiple breeders in colonies of the higher termites (Thorne 1983, 1985; Roisin 1987; Darlington 1988; Atkinson & Adams 1997; Thompson & Hebert 1998; Brandl et al. 2001; Hacker et al. 2005; Atkinson et al. 2008) to ascertain that: 1. They are derived from unrelated co-founders for each of the sexes, rather than being secondary reproductives produced by a single founding pair; 2. The combination of breeders does indeed allow re-mating promiscuity, which would require that there are both multiple unrelated kings and queens in a single colony. If only one of the sexes is found as multiple breeders, the principle of life-time commitment would probably be upheld, so that the breeding system is analogous with multiple queen mating in the eusocial Hymenoptera (e.g. records of multiple kings per colony are rare and these colonies might be monogynous); and 3. All multiple reproductives do indeed contribute to the offspring of the colony. The overall expectation would be that occasional cases of sexual partner shift can occur in the lower termites where helper castes have in any case maintained the possibility to develop into staying or dispersing (winged) breeder phenotypes, although documentation of the reproductive fitness of such novel parental combinations is needed. However, in the higher termites early caste determination should have evolved a high degree of irreversibility for remating promiscuity to be evolutionarily stable.
Somewhat less precise qualitative tests would be possible in the advanced cooperative breeders for which developments towards eusociality have been documented: haplodiploid thrips, bark beetles and sphecid wasps, the clonal aphids and the diploid social Crustacea and naked and Damaraland mole-rats. All of these have well-defendable nests, galls or sponges and overlapping generations that extend tenure of the colonies (Crespi 1996) and all of them should be expected to have very low promiscuity. However, it is important to realize that many of them are lineages of recent origin with slight radiations at best and with close relatives that have lost or never gained eusocial traits (e.g. Stern & Foster 1997; Duffy 2003; Chapman et al. 2002), so that they will not fit the strict obligate eusociality definition of Crespi & Yanega (1995) that I adhered to in Boomsma (2007) and the present review. I expect that even the naked mole-rat, with its social system based on sterile foragers and nurses rather than soldiers, will turn out not to be obligatorily eusocial, because its helpers are not sufficiently differentiated in lifespan (in captivity, Sherman & Jarvis 2002) and at least some of them can shift to a breeder phenotype when the dominant of the same gender disappears. This underlines another prediction that has already been hinted at. As long as obligate lifetime non-matedness of helper cohorts has not been established, it cannot be inferred that the threshold towards obligate eusociality has been passed and that the species in question should thus necessarily be lifetime monogamous (e.g. Soro et al. 2009).
The rapidly increasing availability of genomic databases will provide a good test bed for the lifetime monogamy hypothesis. When every extant eusocial lineage has a series of lifetime monogamous ancestors, antagonistic genes involved in interlocus sexual conflicts inherited from earlier promiscuous ancestors are expected to have been lost or become dysfunctional. This implies that such genes had to re-evolve in lineages of ants, bees and wasps that later evolved multiple queen-mating to regulate novel types of male–female conflict over sperm survival or sperm storage. Extant gene networks of the latter kind are therefore expected to be convergent and lineage-specific. The same prediction would apply for genes that are expressed to mediate issues of dominance and reproductive skew (Reeve & Keller 2001). A parasocial route towards eusociality would predict that genetic mechanisms have remained similar and homologous, so that for example polistine wasps and poneroid ants should share some of them. However, punctuation by a long-lasting monogamy singularity in the common ancestor of the ants should imply that novel gene expression networks had to evolve to regulate novel conflicts when polygyny re-emerged in the poneroid ants.
Finally, I would expect that—as far as they are genetic—the kin-recognition systems of clades that represent independent evolutionary contrasts of cooperative breeding versus eusociality (e.g. the polistine and vespine wasps, and the halictid and corbiculate bees) may well be based on non-homologous genes, as only nestmate versus non-nestmate recognition was required in the full sibling colonies that characterized the monogamy window. When eusocial lineages secondarily evolved genetically more variable colonies, owing to multiple queen-mating or polygyny, the (re)establishment of any nepotistic recognition cues via random mutation was highly constrained, because of increased group selection for colony-level productivity and significant erosion of informative genetically determined cues (Crozier 1987). This inference matches an emerging consensus that nepotistic recognition cues are absent in the multiply mated ants, bees and wasps, and rare in the polygynous ants (Keller 1997; Boomsma et al. 2003; Gardner & West 2007) and seems to provide an interesting contrast with at least a few documented cases of recognition of degree of kin in non-eusocial insects (Greenberg 1979; Lihoreau & Rivault 2009). This is consistent with Wilson & Hölldobler's (2005) view that this form of nepotistic kin selection is a disruptive force in obligatorily eusocial systems, but a potentially binding force in cooperative breeders. As long as a species breeds cooperatively, it may pay (but not necessarily always; cf. Griffin & West 2003) to be able to estimate the degree of relatedness of co-breeders because focal individuals are likely to have retained alternative, dispersal-based reproductive options. However, obligatorily eusocial systems are mostly characterized by unconditional rather than conditional altruism and by the rejection of individuals that deviate from a colony Gestalt, rather than acceptance or preferential treatment of individuals according to their degree of similarity with such a recognition template (Guerrieri et al. 2009).
6. Perspectives
Looking back, the history of explaining the evolution of eusociality has been confusing. Although the simplest (r = 0.75) predictions of the haplodiploidy hypothesis were quickly corrected (Trivers & Hare 1976), the search for relatednesses higher than 0.5 continued focusing, among others, on mechanisms associated with partial bivoltinism, partial unmatedness, inbreeding and chromosomal idiosyncrasies (Bourke & Franks 1995; Crozier & Pamilo 1996; Shellman-Reeve 1997; Crozier 2008). At the same time, the bees seemed to require a separate explanation (Michener 1958), multiple queen-mating was considered a problem because early origins and later evolutionary elaborations of mating systems were insufficiently distinguished (Boomsma & Ratnieks 1996), and a number of new instances of phylogenetically shallow and facultative eusocial helping were discovered in both diploid and haplodiploid taxa (Crespi 1996) and given similar status to the four classic eusocial lineages. Ambiguity was further enhanced by controversies over the definitions of eusociality (e.g. Gadagkar 1994; Crespi & Yanega 1995; Sherman et al. 1995; Keller & Perrin 1995; Costa & Fitzgerald 1996) and finally led to challenges of the merits of kin-selection theory (Wilson 2005, 2008; Wilson & Hölldobler 2005; Fletcher et al. 2006; Wilson & Wilson 2007) that had insufficient connection with the insights that had already gained unambiguous mathematical support in the early days of sociobiology (Foster et al. 2006a,b; Helanterä & Bargum 2007; West et al. 2007, 2008; Crozier 2008; Gardner & Grafen 2009).
During the almost five decades that discussions about the origin of eusociality have been ongoing, William D. Hamilton, Richard D. Alexander, Eric L. Charnov, Richard Dawkins, David Queller, Mary Jane West Eberhard, Edward O. Wilson and many others have realized that monogamy provided very special conditions for the evolution of reproductive altruism, but the crucial significance of lifetime monogamous parental commitment and complete absence of re-mating promiscuity failed to surface as possibly the most fundamental principle of all. The theory has therefore remained unnecessarily complex and has precluded seeing the wood for the trees. The present review aims to rectify this situation and outlines the contours of a research agenda that: (i) Removes some of the obstacles that appear to prevent some ‘advocates’ of group selection and kin selection language to understand each other's agenda. (ii) Emphasizes the need to recognize different domains of social evolution that are separated by singularities such as the monogamy window. In the paragraphs below, I will outline some further perspectives of this approach, which will hopefully stimulate more unified directions in future research.
As Queller (2000) noticed, a single cell or singly mated queen bottleneck in each generation prevents the expression of most selfish genetic traits that could burden a new organism or colony. This notion is consistent with, and becomes more precise when applying the ‘triploid’ or ‘tetraploid’ zygote analogies (figure 2), as this demonstrates that transitions towards eusociality require kin selection (precisely r = 0.5 to siblings on average) to be achieved, but are ultimately driven by benefits obtained from group(colony)-level selection (table 1). This illustrates that the largely semantic debate on the relative merits of kin selection and group selection for the evolution of eusociality had best be abandoned. Both approaches were shown to be mathematically equivalent by Hamilton (1975), when he reformulated his ‘rule’ in the more general notation allowed by the Price equation (see also Wade 1980; Queller 1992; West et al. 2007, 2008; Wilson & Wilson 2007). Group-selection approaches are a shortcut for levels-of-selection models on processes of genetic change (Reeve & Keller 1998; Linksvayer & Wade 2005), whereas kin-selection models address the adaptive evolutionary endpoints of such processes. This complementarity implies that levels-of-selection models by themselves cannot decide whether superorganismal properties of colonies reflect colony-level adaptation or inclusive fitness maximization of the individuals within such colonies (Gardner & Grafen 2009). Rather, it appears that complete resolution of internal conflict is required before colony processes can become colony adaptations (Ratnieks & Reeve 1992; Gardner & Grafen 2009). In this perspective, non-conflict behaviours and communication processes that relate to resource acquisition can easily become supercolonial, whereas it is almost impossible to achieve this for traits involved in reproductive resource allocation (Boomsma & Franks 2006).
As long as a social system is defined as cooperative breeding, group selection is likely to be of variable significance as it will be over-ruled by individual selection for anything between 1 and 80 per cent of the subordinate individuals who end-up reproducing in each generation (Brockmann 1997). After the transition towards obligate eusociality has been made, not a single helper will realize full reproductive potential, so that colony-level selection has become the leading determinant of inclusive fitness. The instalment of eusociality thus implies that a new level of organization has become decisive for both parental and offspring fitness, but also that new conflicts of interest come to challenge the arrangement as the interests of the generations are only partly aligned. For example, parent–offspring conflict over who reproduces is replaced by parent–offspring conflict over who to invest in (Alexander 1974). As illustrated in figure 2, the reproductive conflict load of newly emerged obligate eusociality is relatively severe in the haplodiploid Hymenoptera, because parents contribute unequally to the triploid zygote analogue setting the stage for the classic worker–queen conflicts over sex allocation and worker reproduction (Trivers & Hare 1976) and the interaction between these conflicts (Foster & Ratnieks 2001; Reuter & Keller 2001). Termite societies lack this fundamental parental asymmetry, so that only conflict over caste fate can be expressed, a conflict that they share with the eusocial Hymenoptera (Bourke & Ratnieks 1999). What characterizes any current supercolonial endpoints of evolutionary developments that started with passing through a monogamy window is that virtually all potential conflicts have been resolved or carefully regulated to ensure minimal damage to society (Bourke 1999; Ratnieks et al. 2006). However, these conflict regulations can normally be explained as having evolved to maximize inclusive fitness of individuals and not as a colony-level adaptation (Gardner & Grafen 2009).
The most fruitful way to progress in understanding the evolution of eusociality would seem to concentrate research efforts on a further conceptual unification with already developed theory on the origin of multicellularity (Korb & Heinze 2004). Models have shown that the shape of a crucial trade-off between survival and fecundity changes when cell number increases, so that the cost of unicellular reproduction gradually increases with the benefits of joint reproduction (Michod 2005, 2006). This process, which is reminiscent of the synergistic benefits of increasing colony size in insect societies (Bourke 1999; see also figure 1) results in a significant increase in the heritability of fitness at the collective level (Michod & Roze 1997) and is connected to the emergence of a totipotent germ line and a majority of cells that have been terminally determined to serve somatic functions. The emergence of individual germ lines has been hypothesized to be either parentally enforced or voluntarily altruistic (Queller 2000). Also, this is similar to the concepts of parental manipulation and offspring choice that dominated discussions on the origin of eusociality, until both were shown to be consistent with the same force of kin-selection (Craig 1979; Bourke & Franks 1995, but see Linksvayer & Wade 2005 for differences when taking a level of selection approach). Comparisons of this kind show that extant multicellular organisms differ 13 orders of magnitude in cell number, but only two orders of magnitude in the number of cell types, whereas insect societies vary five orders of magnitude in the number of individuals and less than one order of magnitude in the number of castes (Strassmann & Queller 2007). Both relationships show similar positive correlations but there are roughly an order of magnitude fewer castes than cell types throughout the ranges of cell numbers and colony nestmates (Strassmann & Queller 2007).
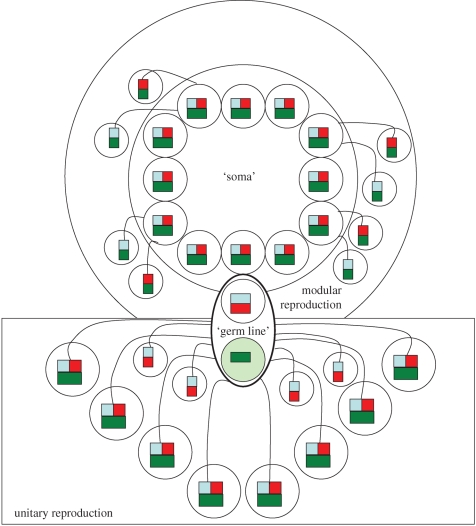
Comparative explorations of this kind ought to include explicit considerations on the analogues of the multicellular germ line and soma that characterize eusociality. Bourke & Franks (1995) established that the growth of insect societies is modular in the sense that a colony can remain viable even after half of the workers are removed. However, it is also clear that a colony of Atta leafcutter ants with five million sterile workers has all but completed the unitary superorganism analogy of having a fully separated ‘germ line’ for reproductive purposes, except perhaps for the final step of raising new cohorts of dispersing queens from genetically predisposed eggs of superior quality (Dijkstra & Boomsma 2006). However, as illustrated in figure 3, there are many stages in between the early monogamy window origin of obligate eusociality and this advanced superorganism state where the ‘germ line’ is only partly sequestered and where a significant part of the colony's total reproductive effort is based on an equivalent of modular ‘somatic’ reproduction. As long as workers still have functional ovaries, hymenopteran colonies partly reproduce like plants rather than animals, in particular when they become queenless so that male production by workers has become the only option for future inclusive fitness. It is this modular form of reproduction that is institutionalized in ants that evolved secondary polygyny, as re-adopted newly inseminated daughter-queens facilitate unconstrained ‘somatic’ reproduction, relative to unmated workers that can only produce males. When such adoption cycles are repeated within the same long-lived nest, colonies may lose their founding ‘germ line’ entirely and become modular chimaeras that mostly reproduce by vegetative budding (Keller 1993; Bourke & Franks 1995; Crozier & Pamilo 1996). Termite societies can also be interpreted in this manner, although some of the details differs, as replacement reproductives in termites are merely extensions of the colony's germ line when their partners are full siblings (e.g. Thorne 1985).
Figure 3.
The ‘soma’ and ‘germ line’ analogues of a eusocial colony of ants, bees or wasps. Symbols are the same as in figure 1. The small ellipse in the centre is the founding pair, which for simplicity has been depicted as a singly mated queen. The diagram would be similar for a multiply mated queen, in which case her multiple unrelated mates and their offspring could be depicted as having different shades of dark green. The box at the bottom represents the queen-produced gynes and males, i.e. the fraction of the colony's reproduction that is derived from the analogue of the unitary ‘germ line’ (assuming 50/50 Fisherian sex allocation in this example, but female biased sex ratios would not make a principal difference for the argument). The inner circle at the top represents the collective ‘soma’ of all the colony's workers and the larger circle the fraction of modular (‘germ line’ independent) reproduction that the ‘soma’ pursues in the form of worker-produced haploid males. Active coercion via policing (Ratnieks et al. 2006) and self restraint due to decreasing pay-offs of ‘somatic’ reproduction when colony size increases (Bourke 1999; Wenseleers et al. 2004) tend to remove most of the modular outer circle in the more advanced societies. Superorganismic societies such as colonies of Atta leafcutter ants or honeybees have lost the outer circle of modular worker reproduction completely (except when queenless in the case of honeybees), but most eusocial Hymenoptera have retained some of this modular reproduction mode over which the queen ‘germ line’ and the worker ‘soma’ are in conflict as long as the queen is alive. Termite colonies have a modular reproduction ellipse when replacement reproductives become established in the existing colonies, although they are in reality an extension of the existing germ line when they mate with full siblings.
As noted above, the monogamy window separates eusociality, which evolves only when Hamilton's rule is fulfilled throughout the lives of entire helper cohorts, from cooperative breeding (including facultative eusociality), which is maintained when Hamilton's rule applies during some period of life. During the transition towards obligate eusociality, within-colony selection proceeds from being a major force of gradually waning significance (when cooperative breeders converge on monogamous mating systems; cf. figure 1) to being a subordinate force that has been surpassed by colony-level selection but keeps threatening colony productivity (table 1). Re-mating promiscuity is compatible with cooperative breeding and solitary breeding, but not with becoming eusocial (Boomsma 2007) and most likely not with remaining eusocial either, unless secondary partner shifts become documented in some higher termites. This is analogous to promiscuous exchange of genetic elements being compatible with prokaryote reproduction, but not with eukaryote reproduction based on life-time commitment of gametes to a single zygote (figure 2 and table 1). Cooperative breeding is not separated from solitary breeding by a transition singularity comparable to the monogamy window, consistent with cooperative breeding often being as optional as facultative eusociality. The major transition between facultative and obligate eusociality rather than between cooperative breeding and facultative eusociality has been noted by many but has, paradoxically, resulted in arguments in favour of lumping social categories (e.g. Gadagkar 1994; Sherman et al. 1995) to stress that the same Hamiltonian principles apply throughout. The overview provided here maintains this commonality of principle, but highlights the necessity of recognizing obligate eusociality as a separate domain of social evolution (table 1). This logic implies that it was not the origins of social groups per se that triggered major transitions in evolution (Maynard Smith & Szathmáry 1995), but rather the multiple passings through monogamy windows. The latter allowed entries into the novel domain of permanent eusociality, whereas the former were less fundamental extensions of solitary life.
The evolutionary ecology of cooperative breeding and facultative eusociality is often richer and more complicated than the study of obligate eusociality, because all three parameters in Hamilton's rule are continuous variables, whereas relatedness tends to be a class variable (e.g. in haplodiploidy 0.75 to full sisters, 0.25 to half sisters, etc.) in obligatorily eusocial systems. In addition, sexual behaviour or the consequences of matedness always interact with other social behaviours in cooperative breeders, whereas these fundamental activities are completely separated in time (and often also space) in eusocial breeders (Boomsma 2007; see also table 1). This implies that biological idiosyncrasy and ecological contingency, although important, are less overwhelming across the obligatorily eusocial clades than across the cooperatively breeding clades, so that an overall synthetic theory for the evolution and maintenance of stable cooperation and altruism may be reached earlier for the eusociality domain than for cooperative breeding.
Acknowledgements
I thank Tim Clutton-Brock, Francis Ratnieks and Stuart West for the opportunity to present a summary of this review at the Royal Society discussion meeting on the Evolution of Society in January 2009, the Danish National Research Foundation for funding, and Joao Alpedrinha, Trine Bilde, Ross Crozier, Raghavendra Gadagkar, Tamara Hartke, Bert Hölldobler, Luke Holman, Daniel Kronauer, Tim Linksvayer, Steve Stearns and Stuart West for comments on an earlier version of the manuscript.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘The evolution of society’.
References
- Abe T.1991Ecological factors associated with the evolution of worker and soldier castes in termites. Ann. Entomol. 9, 101–107 [Google Scholar]
- Abouheif E., Wray G. A.2002Evolution of the gene network underlying wing polyphenism in ants. Science 297, 249–252 (doi:10.1126/science.1071468) [DOI] [PubMed] [Google Scholar]
- Alexander R. D.1974The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–383 (doi:10.1146/annurev.es.05.110174.001545) [Google Scholar]
- Alexander R. D., Noonan K. M., Crespi B. J.1991. In The biology of the naked mole rat (eds Sherman P. W., Jarvis J. U. M., Alexander R. D.), pp. 3–44 Princeton, NJ: Princeton University Press [Google Scholar]
- Atkinson L., Adams E. S.1997The origins and relatedness of multiple reproductives in colonies of the termite Nasutitermes corniger. Proc. R. Soc. Lond. B 264, 1131–1136 (doi:10.1098/rspb.1997.0156) [Google Scholar]
- Atkinson L., Teschendorf G., Adams E. S.2008Lack of evidence for nepotism by workers tending queens of the polygynous termite, Nasutitermes corniger. Behav. Ecol. Sociobiol. 62, 805–812 (doi:10.1007/S00265-007-0506-z) [Google Scholar]
- Baer B., Armitage S. A. O., Boomsma J. J.2006Sperm storage induces an immunity cost in ants. Nature 441, 872–875 (doi:10.1038/nature04698) [DOI] [PubMed] [Google Scholar]
- Boomsma J. J.2007Kin selection versus sexual selection: why the ends do not meet. Curr. Biol. 17, R673–R683 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- Boomsma J. J., Grafen A.1991Colony-level sex ratio selection in the eusocial Hymenoptera. J. Evol. Biol. 4, 383–407 (doi:10.1046/j.1420-9101.1991.4030383.x) [Google Scholar]
- Boomsma J. J., Ratnieks F. L. W.1996Paternity in eusocial Hymenoptera. Phil. Trans. R. Soc. Lond. B 351, 947–975 (doi:10.1098/rstb.1996.0087) [Google Scholar]
- Boomsma J. J., Nielsen J., Sundström L., Oldham N. J., Tentschert J., Petersen H. C., Morgan E. D.2003Informational constraints on optimal sex allocation in ants. Proc. Natl Acad. Sci. USA 100, 8799–8804 (doi:10.1073/pnas.1430283100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma J. J., Baer B., Heinze J.2005The evolution of male traits in social insects. Ann. Rev. Entomol. 50, 395–420 (doi:10.1146/annurev.ento.50.071803.130416) [DOI] [PubMed] [Google Scholar]
- Boomsma J. J., Kronauer D. J. C., Pedersen J. S.2009The evolution of social insect mating systems. In Organization of insect societies—from genomes to sociocomplexity (eds Gadau J., Fewell J.), pp. 3–25 Cambridge, MA: Harvard University Press [Google Scholar]
- Bourke A. F. G.1999Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 12, 245–257 (doi:10.1046/j.1420-9101.1999.00028.x) [Google Scholar]
- Bourke A. F. G.2007Kin selection and the evolutionary theory of aging. Annu. Rev. Ecol. Evol. Syst. 38, 103–128 (doi:10.1146/annurev.ecolsys.38.091206.095528) [Google Scholar]
- Bourke A. F. G., Franks N. R.1995Social evolution in ants Princeton, NJ: Princeton University Press [Google Scholar]
- Bourke A. F. G., Ratnieks F. L. W.1999Kin conflict over caste determination in social Hymenoptera. Behav. Ecol. Sociobiol. 46, 287–297 (doi:10.1007/s002650050622) [Google Scholar]
- Brady S., Schultz T., Fisher B., Ward P.2006Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl Acad. Sci. USA 103, 18172–18177 (doi:10.1073/pnas.0605858103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl R., Hacker M., Bagine R. K. N., Kaib M.2001Geographic variation of polygyny in the termite Macrotermes michaelseni (Sjostedt). Insectes Soc. 48, 134–137 (doi:10.1007/PL00001755) [Google Scholar]
- Brockmann H. J.1997Cooperative breeding in wasps and vertebrates: the role of ecological constrains. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 347–371 Cambridge, UK: Cambridge University Press [Google Scholar]
- Burt A., Trivers R.2006Genes in conflict Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- Buschinger A.1990Sympatric speciation and radiative evolution of socially parasitic ants—heretic hypotheses and their factual background. Zeitschr. Zool. Syst. Evolutionsforsch. 28, 241–260 [Google Scholar]
- Buss L. W.1987The evolution of individuality Princeton, NJ: Princeton University Press [Google Scholar]
- Cavalier-Smith T.2006Cell evolution and Earth history: stasis and revolution. Phil. Trans. R. Soc. B 361, 969–1006 (doi:10.1098/rstb.2006.1842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. W., Kranz B. D., Bejah K. L., Morris D. C., Schwarz M. P., Crespi B. J.2002The evolution of soldier reproduction in social thrips. Behav. Ecol. 13, 519–525 (doi:10.1093/beheco/13.4.519) [Google Scholar]
- Charnov E. L.1978Evolution of eusocial behavior: offspring choice or parental parasitism? J. Theor. Biol. 75, 451–465 (doi:10.1016/0022-5193(78)90356-9) [DOI] [PubMed] [Google Scholar]
- Costa J. T., Fitzgerald T. D.1996Developments in social terminology: semantic battles in a conceptual war. Trends Ecol. Evol. 11, 285–289 (doi:10.1016/0169-5347(96)10035-5) [DOI] [PubMed] [Google Scholar]
- Craig R.1979Parental manipulation, kin selection, and the evolution of altruism. Evolution 33, 319–334 (doi:10.2307/2407622) [DOI] [PubMed] [Google Scholar]
- Crespi B. J.1996Comparative analysis of the origins and losses of eusociality: causal mosaics and historical uniqueness. In Phylogenetics and the comparative method in animal behavior (ed. Martins E. P.), pp. 253–287 New York, NY: Oxford University Press [Google Scholar]
- Crespi B. J.2007Comparative evolutionary ecology of social and sexual systems: water-breathing insects come of age. In Evolutionary ecology of social and sexual systems: crustaceans as model organisms (eds Duffy J. E., Thiel M.), pp. 442–460 Oxford, UK: Oxford University Press [Google Scholar]
- Crespi B. J., Yanega D.1995The definition of eusociality. Behav. Ecol. 6, 109–115 (doi:10.1093/beheco/6.1.109) [Google Scholar]
- Crozier R. H.1987Genetic aspects of kin recognition: concepts, models, and synthesis. In Kin recognition in animals (eds Fletcher D. J. C., Michener C. D.), pp. 55–73 New York, NY: John Wiley and Sons [Google Scholar]
- Crozier R. H.2008Advanced eusociality, kin selection and male haploidy. Aust. J. Entomol. 47, 2–8 (doi:10.1111/j.1440-6055.2007.00621.x) [Google Scholar]
- Crozier R. H., Pamilo P.1996Evolution of social insect colonies. Oxford, UK: Oxford University Press [Google Scholar]
- Danforth B. N.2002Evolution of sociality in a primitively eusocial lineage of bees. Proc. Natl Acad. Sci. USA 99, 286–290 (doi:10.1073/pnas.012387999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington J.1988Multiple reproductives in nests of Macrotermes herus (Isoptera: Termitidae). Sociobiology 14, 347–351 [Google Scholar]
- Darwin C.1859On the origin of species by means of natural selection, 1st edn London, UK: John Murray; (Published by Penguin Books in 1968.) [Google Scholar]
- Dawkins R.1982The extended phenotype Oxford, UK: Oxford University Press [Google Scholar]
- Den Boer S. P. A., Boomsma J. J., Baer B. C.2008Seminal fluid ensures high sperm viability in the leafcutter ant Atta colombica. Behav. Ecol. Sociobiol. 62, 1843–1849 (doi:10.1007/s00265-008-0613-5) [Google Scholar]
- Dijkstra M. B., Boomsma J. J.2006Are workers of Atta leafcutter ants capable of reproduction? Insectes Soc. 53, 136–140 (doi:10.1007/s00040-005-0848-3) [Google Scholar]
- Duffy J. E.2003The ecology and evolution of eusociality in sponge-dwelling shrimp. In Genes, behavior and evolution in social insects (eds Kikuchi T., Azuma N., Higashi S.), pp. 217–252 Sapporo, Japan: Hokkaido University Press [Google Scholar]
- Fletcher J. A., Zwick M., Doebeli M., Wilson D. S.2006What's wrong with inclusive fitness? Trends Ecol. Evol. 21, 597–598 (doi:10.1016/j.tree.2006.08.008) [DOI] [PubMed] [Google Scholar]
- Foster K. R., Ratnieks F. L. W.2001The effect of sex allocation biasing on the evolution of worker policing in hymenopteran societies. Am. Nat. 158, 615–623 (doi:10.1086/323588) [DOI] [PubMed] [Google Scholar]
- Foster K. R., Wenseleers T., Ratnieks F. L. W.2006aKin selection is the key to altruism. Trends Ecol. Evol. 21, 57–60 (doi:10.1016/j.tree.2005.11.020) [DOI] [PubMed] [Google Scholar]
- Foster K. R., Wenseleers T., Ratnieks F. L. W., Queller D. C.2006bThere is nothing wrong with inclusive fitness. Trends Ecol. Evol. 21, 599–600 (doi:10.1016/j.tree.2006.08.010) [Google Scholar]
- Gadagkar R.1994Why the definition of eusociality is not helpful to understand its evolution and what should we do about it. Oikos 70, 485–488 (doi:10.2307/3545789) [Google Scholar]
- Gadagkar R.1996The evolution of eusociality, including a review of the social status of Ropalidia marginata. In Natural history and evolution of paper-wasps (eds Turillazzi S., West Eberhard M. J.), pp. 248–271 Oxford, UK: Oxford University Press [Google Scholar]
- Gardner A., Grafen A.2009Capturing the superorganism: a formal theory of group adaptation. J. Evol. Biol. 22, 659–667 [DOI] [PubMed] [Google Scholar]
- Gardner A., West S. A.2007Social evolution: The decline and fall of genetic kin recognition. Curr. Biol. 17, R810–R812 (doi:10.1016/j.cub.2007.07.030) [DOI] [PubMed] [Google Scholar]
- Gobin B., Ito F., Peeters C., Billen J.2006Queen–worker differences in spermatheca reservoir of phylogenetically basal ants. Cell Tissue Res. 326, 169–178 (doi:10.1007/s00441-006-0232-2) [DOI] [PubMed] [Google Scholar]
- Godfray H. C. J., Grafen A.1988Unmatedness and the evolution of eusociality. Am. Nat. 131, 303–305 (doi:10.1086/284791) [Google Scholar]
- Gotoh A., Billen J., Hashim R., Ito F.2008Comparison of spermatheca morphology between reproductive and non-reproductive females in social wasps. Arthropod Struct. Dev. 37, 199–209 (doi:10.1016/j.asd.2007.11.001) [DOI] [PubMed] [Google Scholar]
- Greenberg L.1979Genetic component of bee odor in kin recognition. Science 206, 1095–1097 (doi:10.1126/science.206.4422.1095) [DOI] [PubMed] [Google Scholar]
- Griffin A. S., West S. A.2003Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- Grimaldi D., Engel M. S.2005Evolution of the insects Cambridge, UK: Cambridge University Press [Google Scholar]
- Grosberg R. K., Strathmann R. R.2007The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654 (doi:10.1146/annurev.ecolsys.36.102403.114735) [Google Scholar]
- Guerrieri F. J., Nehring V., Jørgensen C. G., Nielsen J., Galizia C. G., d'Ettorre P.2009Ants recognize foes and not friends. Proc. R. Soc. B 276, 2461–2468 (doi:10.1098/rspb.2008.1860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker M., Kaib M., Bagine R. K. N., Epplen J. T., Brandl R.2005Unrelated queens coexist in colonies of the termite Macrotermes michaelseni. Mol. Ecol. 14, 1527–1532 (doi:10.1111/j.1365-294X.2005.02507.x) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour, I & II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1972Altruism and related phenomena, mainly in social insects. Annu. Rev. Ecol. Syst. 3, 193–232 (doi:10.1146/annurev.es.03.110172.001205) [Google Scholar]
- Hamilton W. D.1975Innate social aptitudes of man: an approach from evolutionary genetics. In Biosocial anthropology (ed. Fox R.), pp. 133–155 London, UK: Malaby Press [Google Scholar]
- Hamilton W. D.1978Evolution and diversity under bark. In Diversity of insect faunas, vol. 9 (eds Mound L. A., Waloff N.), pp. 154–175 Oxford, UK: Blackwell; Symposia of the Royal Entomological Society of London [Google Scholar]
- Hart A. G., Ratnieks F. L. W.2005Crossing the taxonomic divide: conflict and its resolution in societies of reproductively totipotent individuals. J. Evol. Biol. 18, 383–395 (doi:10.1111/j.1420-9101.2004.00832.x) [DOI] [PubMed] [Google Scholar]
- Hastings M. D., Queller D. C., Eischen F., Strassmann J. E.1998Kin selection, relatedness, and worker control of reproduction in a large-colony epiponine wasp, Brachygastra mellifica. Behav. Ecol. 9, 573–581 (doi:10.1093/beheco/9.6.573) [Google Scholar]
- Helanterä H., Bargum K.2007Pedigree relatedness, not greenbeard genes, explains eusociality. Oikos 116, 217–220 (doi:10.1111/j.0030-1299.2007.15411.x) [Google Scholar]
- Higashi M., Yamamura N., Abe T., Burns T. P.1991Why don't all termite species have a sterile worker caste? Proc. R. Soc. Lond. B 246, 25–30 (doi:10.1098/rspb.1991.0120) [DOI] [PubMed] [Google Scholar]
- Hines H. M., Hunt J. H., O'Connor T. K., Gillespie J. J., Cameron S. A.2007Multigene phylogeny reveals eusociality evolved twice in vespid wasps. Proc. Natl Acad. Sci. USA 104, 3295–3299 (doi:10.1073/pnas.0610140104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B., Bartz S. H.1985Sociobiology of reproduction in ants. In Experimental behavioral ecology and sociobiology (eds Hölldobler B., Lindauer M.), pp. 237–257 Stuttgart, Germany; New York, NY: Gustav Fischer [Google Scholar]
- Hölldobler B., Wilson E. O. The ants. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Hölldobler B., Wilson E. O.2008The super-organism New York, NY: Norton [Google Scholar]
- Hughes W. O. H., Boomsma J. J.2008Genetic royal cheats in leaf-cutting ant societies. Proc. Natl Acad. Sci. USA 105, 5150–5153 (doi:10.1073/pnas.0710262105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. O. H., Oldroyd B. P., Beekman M., Ratnieks F. L. W.2008Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216 (doi:10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- Hunt J. H.1994Nourishment and evolution in wasps sensu lato. In Nourishment and evolution in insect societies (eds Hunt J. H., Nalepa C. A.), pp. 211–244 Boulder, CO: Westview Press [Google Scholar]
- Hunt J. H.2007The evolution of social wasps New York, NY: Oxford University Press [Google Scholar]
- Inward D., Beccaloni G., Eggleton P.2007Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol. Lett. 3, 331–335 (doi:10.1098/rsbl.2007.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita A., Ascher J. S., Sota T., Kato M., Roubik D. W.2008Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae). Apidologie 39, 163–175 (doi:10.1051/apido:2007046) [Google Scholar]
- Keller L. (ed.) 1993Queen number and sociality in insects. Oxford, UK: Oxford University Press [Google Scholar]
- Keller L.1997Indiscriminate altruism: unduly nice parents and siblings. Trends Ecol. Evol. 12, 99–103 (doi:10.1016/S0169-5347(96)10065-3) [DOI] [PubMed] [Google Scholar]
- Keller L., Genoud M.1997Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature 389, 958–960 (doi:10.1038/40130) [Google Scholar]
- Keller L., Perrin N.1995Quantifying the level of eusociality. Proc. R. Soc. Lond. B 260, 311–315 (doi:10.1098/rspb.1995.0097) [Google Scholar]
- Korb J.2008The ecology of social evolution in termites. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 151–174 Berlin, Germany: Springer [Google Scholar]
- Korb J., Heinze J.2004Multilevel selection and social evolution of insect societies. Naturwissenschaften 91, 291–304 [DOI] [PubMed] [Google Scholar]
- Kronauer D. J. C., Boomsma J. J.2007Do army ant queens re-mate later in life? Insectes Soc. 54, 20–28 (doi:10.1007/s00040-007-0904-2) [Google Scholar]
- Lihoreau M., Rivault C.2009Kin recognition via cuticular hydrocarbons shapes cockroach social life. Behav. Ecol. 20, 46–53 (doi:10.1093/beheco/arn113) [Google Scholar]
- Linksvayer T. A., Wade M. J.2005The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 80, 317–336 (doi:10.1086/432266) [DOI] [PubMed] [Google Scholar]
- Lin N., Michener C. D.1972Evolution of sociality in insects. Q. Rev. Biol. 47, 131–159 (doi:10.1086/407216) [Google Scholar]
- Lo N., Hayashi Y., Kitade O.2009Should environmental caste determination be assumed for termites? Am. Nat 173, 848–853 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J., Szathmáry E.1995The major transitions in evolution Oxford, UK: Freeman [Google Scholar]
- Michener C. D.1958The evolution of social behavior in bees. Proc. Tenth Int. Cong. Entomol. 2, 441–447 [Google Scholar]
- Michod R. E.2005On the transfer of fitness from the cell to the multicellular organism. Biol. Philos. 20, 967–987 [Google Scholar]
- Michod R. E.2006The group covariance effect and fitness trade-offs during evolutionary transitions in individuality. Proc. Natl Acad. Sci. USA 103, 9113–9117 (doi:10.1073/pnas.0601080103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod R. E.2007Evolution of individuality during the transition from unicellular to multicellular life. Proc. Natl Acad. Sci. USA 104, 8613–8618 (doi:10.1073/pnas.0701489104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod R. E., Roze D.1997Transitions in individuality. Proc. R. Soc. Lond. B 264, 853–857 (doi:10.1098/rspb.1997.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C., Bell C., Vila R., Archibald S., Pierce N.2006Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101–104 (doi:10.1126/science.1124891) [DOI] [PubMed] [Google Scholar]
- Moritz R. F. A., Southwick E. E.1992Bees as superorganisms: an evolutionary reality Berlin, Germany: Springer [Google Scholar]
- Mueller U. G.1991Haplodiploidy and the evolution of facultative sex ratios in a primitively eusocial bee. Science 254, 442–444 (doi:10.1126/science.254.5030.442) [DOI] [PubMed] [Google Scholar]
- Pamilo P.1991Evolution of the sterile caste. J. Theor. Biol. 149, 75–95 (doi:10.1016/S0022-5193(05)80073-6) [DOI] [PubMed] [Google Scholar]
- Patel A., Fondrk M. K., Kaftanoglu O., Emore C., Hunt G., Frederick K., Amdam G. V.2007The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2, e509 (doi:10.1371/journal.pone.0000509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters C.1997Morphologically ‘primitive’ ants: comparative review of social characters, and the importance of queen–worker dimorphism. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 372–391 Cambridge, UK: Cambridge University Press [Google Scholar]
- Plateaux-Quénu C.2008Subsociality in halictine bees. Insectes Soc. 55, 335–346 (doi:10.1007/s00040-008-1028-z) [Google Scholar]
- Queller D. C.1989The evolution of eusociality: reproductive head starts of workers. Proc. Natl Acad. Sci. USA 86, 3224–3226 (doi:10.1073/pnas.86.9.3224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller D. C.1992Quantitative genetics, inclusive fitness, and group selection. Am. Nat. 139, 540–558 (doi:10.1086/285343) [Google Scholar]
- Queller D. C.1996The origin and maintenance of eusociality: the advantage of extended parental care. In Natural history and evolution of paper-wasps (eds Turillazzi S., West Eberhard M. J.), pp. 218–234 Oxford, UK: Oxford University Press [Google Scholar]
- Queller D. C.2000Relatedness and the fraternal major transitions. Proc. R. Soc. Lond. B 355, 1647–1655 (doi:10.1098/rstb.2000.0727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller D. C., Strassmann J. E., Solis C. R., Hughes C. R., DeLoach D. M.1993A selfish strategy of social insect workers that promotes social cohesion. Nature 365, 639–641 (doi:10.1038/365639a0) [Google Scholar]
- Rabeling C., Brown J. M., Verhaagh M.2008Newly discovered sister lineage sheds light on early ant evolution. Proc. Natl Acad. Sci. USA 105, 14913–14917 (doi:10.1073/pnas.0806187105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnieks F. L. W., Reeve H. K.1992Conflict in single-queen hymenopteran societies: the structure of conflict and processes that reduce conflict in advanced eusocial species. J. Theor. Biol. 158, 33–65 (doi:10.1016/S0022-5193(05)80647-2) [Google Scholar]
- Ratnieks F. L. W., Foster K. R., Wenseleers T.2006Conflict resolution in insect societies. Ann. Rev. Entomol. 51, 581–608 (doi:10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- Reeve H. K., Keller L.1998Levels of selection: burying the units-of-selection debate and unearthing the crucial new issues. In Levels of selection in evolution (ed. Keller L.), pp. 3–14 Princeton, NJ: Princeton University Press [Google Scholar]
- Reeve H. K., Keller L.2001Tests of reproductive-skew models in social insects. Annu. Rev. Entomol. 46, 347–385 (doi:10.1146/annurev.ento.46.1.347) [DOI] [PubMed] [Google Scholar]
- Reeve H. K., Peters J. M., Nonacs P., Starks P. T.1998Dispersal of first ‘workers’ in social wasps: causes and implications of an alternative reproductive strategy. Proc. Natl Acad. Sci. USA 95, 13737–13742 (doi:10.1073/pnas.95.23.13737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve H. K., Hölldobler B.2007The emergence of a superorganism through intergroup competition. Proc. Natl Acad. Sci. USA 104, 9736–9740 (doi:10.1073/pnas.0703466104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Keller L.2001Sex ratio conflict and worker production in eusocial Hymenoptera. Am. Nat. 158, 166–177 (doi:10.1086/321311) [DOI] [PubMed] [Google Scholar]
- Roisin Y.1987Polygyny in Nasutitermes species: field data and theoretical approaches. Experientia 54(Suppl.), 379–404 [Google Scholar]
- Roisin Y.1999Philopatric reproduction, a prime mover in the evolution of termite sociality? Insectes Soc. 46, 297–305 (doi:10.1007/s000400050149) [Google Scholar]
- Roisin Y., Pasteels J. M.1987Caste developmental potentialities in the termite Nasutitermes novarumhebridarum. Entomol. Exp. Appl. 44, 277–287 [Google Scholar]
- Seeley T. D.1995The wisdom of the hive Cambridge, MA: Harvard University Press [Google Scholar]
- Seger J.1983Partial bivoltinism may cause alternating sex ratio biases that favour eusociality. Nature 301, 59–62 (doi:10.1038/301059a0) [Google Scholar]
- Shellman-Reeve J. S.1997The spectrum of eusociality in termites. Social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 52–93 Cambridge, UK: Cambridge University Press [Google Scholar]
- Sherman P. W., Jarvis J. U. W.2002Extraordinary life spans of naked mole-rats (Heterocephalus glaber). J. Zool. Lond. 258, 307–311 (doi:10.1017/S0952836902001437) [Google Scholar]
- Sherman P. W., Lacey E. A., Reeve H. K., Keller L.1995The eusociality continuum. Behav. Ecol. 6, 102–108 (doi:10.1093/beheco/6.1.102) [Google Scholar]
- Soro A., Ayasse M., Zobel M. U., Paxton R. J.2009Complex sociogenetic organization and the origin of unrelated workers in a eusocial sweat bee Lasioglossum malachurum. Insectes Soc. 56, 55–63 (doi:10.1007/s00040-008-1037-y) [Google Scholar]
- Stern D. L., Foster W. A.1997The evolution of sociality in aphids: a clone's-eye view. In Social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 150–165 Cambridge, UK: Cambridge University Press [Google Scholar]
- Strassmann J. E.1996Selective altruism towards closer over more distant relatives in colonies of the primitively eusocial wasp, Polistes. In Natural history and evolution of paper-wasps (eds Turillazzi S., West Eberhard M. J.), pp. 190–201 Oxford, UK: Oxford University Press [Google Scholar]
- Strassmann J. E.2001The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc. 48, 1–13 (doi:10.1007/PL00001737) [Google Scholar]
- Strassmann J. E., Queller D. C.2007Insect societies as divided organisms: the complexities of purpose and cross-purpose. Proc. Natl Acad. Sci. USA 104, 8619–8626 (doi:10.1073/pnas.0701285104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann J. E., Goodnight K. F., Klingler C. J., Queller D. C.1998The genetic structure of swarms and the timing of their production in the queen cycles of neotropical wasps. Mol. Ecol. 7, 709–718 (doi:10.1046/j.1365-294x.1998.00381.x) [Google Scholar]
- Strassmann J. E., Sullender B. W., Queller D. C.2002Caste totipotency and conflict in a large-colony social insect. Proc. R. Soc. Lond. B 269, 263–270 (doi:10.1098/rspb.2001.1880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield J. W., Charnov E. L.1986Some conceptual issues in the origin of eusociality. Heredity 57, 181–187 (doi:10.1038/hdy.1986.108) [DOI] [PubMed] [Google Scholar]
- Thompson G. J., Herbert P. D. N.1998Population genetic structure of the Neotropical termite Nasutitermes nigriceps (Isoptera: Termitidae). Heredity 80, 48–55 (doi:10.1046/j.1365-2540.1998.00277.x) [Google Scholar]
- Thompson G. J., Kitate O., Lo N., Crozier R. H.2000Phylogenetic evidence for a single, ancestral origin of a ‘true’ worker caste in termites. J. Evol. Biol. 13, 869–881 (doi:10.1046/j.1420-9101.2000.00237.x) [Google Scholar]
- Thorne B. L.1983Alate production and sex ratio in colonies of the Neotropical termite Nasutitermes corniger (Isoptera; Termitidae). Oecologia 58, 103–109 (doi:10.1007/BF00384548) [DOI] [PubMed] [Google Scholar]
- Thorne B. L.1985Termite polygyny: the ecological dynamics of queen mutualism. In Experimental behavioural ecology and sociobiology (eds Hölldobler B., Lindauer M.), pp. 325–341 Stuttgart, Germany; New York, NY: Gustav Fischer [Google Scholar]
- Thorne B. L., Traniello J. F. A.2003Comparative social biology of basal taxa of ants and termites. Annu. Rev. Entomol. 48, 283–306 (doi:10.1146/annurev.ento.48.091801.112611) [DOI] [PubMed] [Google Scholar]
- Trivers R. L., Hare H.1976Haplodiploidy and the evolution of the social insects. Science 191, 249–263 (doi:10.1126/science.1108197) [DOI] [PubMed] [Google Scholar]
- Wade M. J.1980Kin selection: its components. Science 210, 665–667 (doi:10.1126/science.210.4470.665) [DOI] [PubMed] [Google Scholar]
- Wenseleers T., Hart A. G., Ratnieks F. L. W.2004When resistance is useless: policing and the evolution of reproductive acquiescence in insect societies. Am. Nat. 164, 154–167 [DOI] [PubMed] [Google Scholar]
- West S. A., Griffin A. S., Gardner A.2007Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432 (doi:10.1111/j.1420-9101.2006.01258.x) [DOI] [PubMed] [Google Scholar]
- West S. A., Griffin A. S., Gardner A.2008Social semantics: how useful has group selection been? J. Evol. Biol. 21, 374–385 [DOI] [PubMed] [Google Scholar]
- West Eberhard M. J.1975The evolution of social behavior by kin selection. Q. Rev. Biol. 50, 1–33 [Google Scholar]
- West Eberhard M. J.1983Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (doi:10.1086/413215) [Google Scholar]
- West Eberhard M. J.1996Wasp societies as microcosms for the study of development and evolution. In Natural history and evolution of paper-wasps (eds Turillazzi S., West Eberhard M. J.), pp. 290–317 Oxford, UK: Oxford University Press [Google Scholar]
- Wheeler D. E., Buck N., Evans J. D.2006Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 15, 597–602 (doi:10.1111/j.1365-2583.2006.00681.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler W. M.1928The social insects New York, UK: Harcourt, Brace & co [Google Scholar]
- Williams G. C.1985Sex and evolution Princeton, NJ: Princeton University Press [Google Scholar]
- Wilson E. O.1971The insect societies Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- Wilson E. O.1975Sociobiology, the new synthesis 2nd edn (2000) Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- Wilson E. O.2005Kin selection as the key to altruism: its rise and fall. Soc. Res. 72, 159–166 [Google Scholar]
- Wilson E. O.2008One giant leap: how insects achieved altruism and colonial life. BioScience 58, 17–25 (doi:10.1641/B580106) [Google Scholar]
- Wilson E. O., Hölldobler B.2005Eusociality: origin and consequences. Proc. Natl Acad. Sci. USA 102, 13367–13371 (doi:10.1073/pnas.0505858102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. S., Wilson E. O.2007Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 82, 327–348 (doi:10.1086/522809) [DOI] [PubMed] [Google Scholar]
- Yanega D.1997Demography and sociality in halictine bees. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 293–315 Cambridge, UK: Cambridge University Press [Google Scholar]