Abstract
In primitively eusocial societies, all individuals can potentially reproduce independently. The key fact that we focus on in this paper is that individuals in such societies instead often queue to inherit breeding positions. Queuing leads to systematic differences in expected future fitness. We first discuss the implications this has for variation in behaviour. For example, because helpers nearer to the front of the queue have more to lose, they should work less hard to rear the dominant's offspring. However, higher rankers may be more aggressive than low rankers, even if they risk injury in the process, if aggression functions to maintain or enhance queue position. Second, we discuss how queuing rules may be enforced through hidden threats that rarely have to be carried out. In fishes, rule breakers face the threat of eviction from the group. In contrast, subordinate paper wasps are not injured or evicted during escalated challenges against the dominant, perhaps because they are more valuable to the dominant. We discuss evidence that paper-wasp dominants avoid escalated conflicts by ceding reproduction to subordinates. Queuing rules appear usually to be enforced by individuals adjacent in the queue rather than by dominants. Further manipulative studies are required to reveal mechanisms underlying queue stability and to elucidate what determines queue position in the first place.
Keywords: social queues, social aggression, helping, group stability, Polistes, reproductive skew
1. Introduction
In primitively eusocial insect societies, some individuals, known as subordinates or helpers, sacrifice their own reproduction and help to rear the offspring of other individuals known as the queen or dominant. The defining feature of primitively eusocial societies, however, is that all individuals, including the helpers, are potentially capable of mating and independent reproduction. The key fact that we focus on in this paper is that the individuals in such societies are often in a queue to inherit breeding positions. The individuals in the queue inherit breeding positions in a predictable order. This leads to systematic differences in waiting times, which means that individuals differ in their future prospects. The differences are most marked in the short queues typical of the taxa we discuss. We first summarize the consequences of queuing for behavioural variation between individuals in the society. We then discuss evidence for behavioural mechanisms that might stabilize the queue. The organisms we focus on are primitively eusocial wasps in two subfamilies, Stenogastrinae (hover wasps) and Polistinae (paper wasps), but where relevant we draw comparisons with social queues in other Hymenoptera and vertebrates.
(a). Natural history of primitively eusocial wasps
Paper wasps and hover wasps probably represent independent origins of eusociality (Hines et al. 2007). In temperate populations of Polistes, females, known as foundresses, start building their characteristic open paper nests in spring after overwintering as mated adults. In some species, each nest has only a single foundress, but founding by multiple females is common in other species. In this paper, we discuss species in which some nests have multiple foundresses. On such nests, one of the foundresses, known as the dominant or rank 1, lays most of the eggs. The other foundresses, which are often, but not always, relatives of the dominant, act as helpers and carry out tasks such as foraging to feed the larvae. When the larvae reach adulthood, many of the newly matured females stay and become helpers on their natal nests, but here we will discuss studies of populations during the pre-worker phase, when only foundresses are present. The most recent general review of Polistes nesting biology is by Reeve (1991).
Whereas Polistes has an almost cosmopolitan distribution, hover wasps are restricted to the tropics of southeast Asia and New Guinea. We discuss studies of the hairy-faced hover wasp Liostenogaster flavolineata in which, unlike temperate Polistes, brood rearing continues all year. Nests are usually initiated by a single female, and multiple-female nests arise mainly through some adult offspring remaining on their natal nests as helpers. Other offspring leave to follow alternative strategies such as founding nests of their own. Groups never become very large, in this respect resembling the pre-worker nests of Polistes, with group sizes of typically one to four females, very rarely more than 10. Turillazzi (1991) and Field (2008) are two recent reviews of hover wasp nesting biology.
Reproductive skew is generally high at any one time in primitively eusocial wasps (Field & Cant 2009). However, when the dominant female dies, one of the other females in the group inherits the egg-laying position, so that skew is lower when viewed across the group's entire lifespan.
2. Consequences of queuing: individual variation in behaviour
One of the most noticeable features of primitively eusocial societies is behavioural variation between the different individuals in a group. Some individuals are more aggressive than others, some work harder than others, and some are more likely than others to defend the group against outside threats (e.g. Clutton-Brock et al. 2000; Cant & Field 2001; Field et al. 2006; Cant et al. 2006a,b; Cronin & Field 2007b). Little of this variation is correlated with within-group variation in genetic relatedness, although further work, particularly manipulative experiments, is needed to confirm this (e.g. Queller et al. 2000; Griffin & West 2003; Cant et al. 2006b; Field et al. 2006). There is, however, good evidence that much of the variation in behaviour is caused by variation in expected future fitness. Life-history theory suggests that helpers in primitively eusocial societies face a fundamental trade-off between helping effort and future fitness (Cant & Field 2001, 2005). By working harder to rear the offspring of their dominant relative, they can increase the indirect component of their fitness. But this comes only at a cost of reduced personal survival, and reduced fecundity if they survive to inherit the dominant position themselves. A major prediction from this life-history framework is that because individuals with greater expected future fitness have more to lose, they should invest less in working to rear the dominant's offspring.
In primitively eusocial insects, foraging is probably the costliest activity performed by helpers because it involves energy-expensive flight and an increased risk of predation away from the safety of the nest (Cant & Field 2001). We can therefore make two clear predictions. First, helpers nearer to the front of the queue should forage less because they have more to lose. Second, helpers at a given position in the queue should forage less if they are part of a larger group. This second prediction relies on the fact that in most primitively eusocial societies, the reproductive payoff from inheriting the dominant position is greater in larger groups because there are more helpers available to rear the dominant's offspring in such groups. Thus, a helper has more to lose if the group she stands to inherit is larger, assuming that her chance of inheritance from a given rank is independent of group size (Field & Cant 2006).
Consistent with these predictions, the expected correlations between foraging effort and both group size and queue position are found in Polistes cofoundress associations and in the hairy-faced hover wasp (Cant & Field 2001; Field et al. 2006). Note that the correlation with group size is unlikely to result simply from the larger number of helpers available to feed offspring in larger groups, as may be the case in some cooperatively breeding vertebrates (Field & Cant 2006). In primitively eusocial insects, clutch size is typically not fixed, so that the number of dependent offspring per helper is roughly constant across group sizes (e.g. Field et al. 2000; reviewed in Shreeves & Field 2002). In addition to these correlations, manipulation of expected future fitness has been carried out in the hairy-faced hover wasp (Field et al. 2006). These manipulations took advantage of the fact that queueing is strictly age-based in this species, so that by knowing the relative ages of the individuals in the group, we know the order in which they will inherit the dominant position. It is therefore possible to experimentally promote focal individuals up the queue by removing higher ranking nest-mates. The group size that a focal subordinate stands to inherit can also be reduced by removing lower ranking nest-mates. The results of these manipulations were that focal individuals worked less hard than unmanipulated controls when they were promoted, but harder than controls when their group size was reduced (Field & Cant 2006; Field et al. 2006). Both results supported the theoretical predictions.
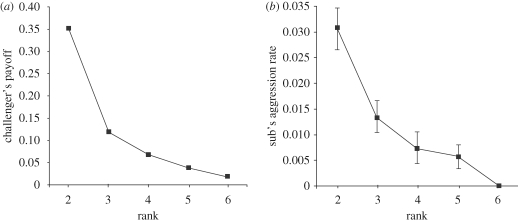
The life-history perspective outlined above does not mean that high-ranked individuals should necessarily always be the ones that take the fewest risks. For example, Cant et al. (2006a) developed a kin selection model of aggression in social queues. It turns out that if aggression functions to challenge the status of individuals further up the queue, high-ranking individuals should be the most aggressive, even though they risk injury in the process (figure 1a). This is because high rankers have the most to gain by jumping the queue, in terms of increasing their probability of inheriting the rank 1 position. Probability of inheritance declines exponentially with decreasing rank, so that the effect of moving one place up the queue is larger for an individual nearer the front (Field et al. 1999). Thus, the predicted correlation with rank will differ for different behaviours, depending on the pattern of costs and benefits. High rankers should take the fewest risks with foraging, but may be more likely to risk injury via aggression (figure 1b). The important general point, however, is that variation in future fitness may be the hidden factor that explains much of the previously unexplained variation in behaviour within social queues.
Figure 1.
(a) Theoretical payoff to a subordinate of challenging the individual ahead of it in the queue to inherit dominant status, versus inheritance rank. In this model (model 1 of Cant et al. 2006a), a successful challenge leads to a reversal in dominance rank, but incurs a cost to group productivity. (b) Observed rates of aggression by subordinate Polistes dominulus to their immediate dominant as a function of their inheritance rank. Ranks were revealed by repeatedly removing rank 1 individuals and allowing the next individual in the queue to inherit (see Cant et al. 2006a for details). Points show means ± s.e.
The data reviewed above imply that variation in costs and benefits, rather than variation in genetic relatedness, primarily determines variation in behaviour within groups of primitively eusocial wasps. We emphasize, however, that we cannot conclude from this that kin selection is unimportant in general. Most groups of primitively eusocial wasps comprise relatives, so that helpers gain indirect fitness through boosting the reproductive success of the dominant. The reason that behavioural fine-tuning reflects variation in costs and benefits is probably that there are informational constraints. Cues such as rank or group size, which are correlated with an individual's future fitness, may be more easily detected than cues correlated with relatedness (Keller 1997; Field et al. 2006). It is possible, however, that in other situations where cues correlated with within-group variation in relatedness are readily available, relatedness could be a determinant of within-group variation in behaviour. For example, if it was systematically correlated with rank or group size, relatedness could reinforce or oppose the effects of rank or group size on behaviour. Relatedness is not strongly correlated with rank or group size in the wasp populations reviewed above (Bridge & Field 2007; Zanette & Field 2008, 2009), but is likely to be correlated with rank in other social queues (e.g. Dierkes et al. 2005).
3. Queue stability
(a). The queue should be stable
The foregoing results imply that at least in primitively eusocial wasps, the queuing rule must be adhered to reasonably closely. In the hairy-faced hover wasp, for example, if relative age was only a weak predictor of inheritance payoff, variation in behaviours such as helping effort would not be expected to map so well onto queue position. Experimental removal of dominants from groups of individuals of known relative age suggests that age is indeed a good predictor of inheritance order (Bridge & Field 2007). After 90 per cent of removals (n = 69), the oldest subordinate was the one to inherit. Wasps that inherited naturally were the oldest in a similar fraction of cases. Bridge & Field (2007) identified seven out of 69 individuals that jumped the queue, in the sense that they inherited ahead of one or more older individuals. Before they inherited, these queue jumpers had worked significantly less hard than expected for their rank, suggesting that their accession might not simply be the result of winning a fight with an older wasp at the moment when the previous dominant was removed. The sample size was small, but there was no indication that queue jumpers were larger than the individuals supplanted, or that they had an especially large incentive to jump the queue because they were unrelated to their nest-mates (Bridge & Field 2007). It is possible that the queuing system can support a small proportion of ‘cheats’ that break the rules, perhaps by mimicking cues associated with age. However, an obvious question then is why more individuals do not cheat, unless mimicry is costly. Alternatively, perhaps there are no cheats: the queueing rules may just be more complex than we realize. Instead of being based purely on age, the rule might be that the oldest wasp inherits unless another unknown variable takes particular values.
(b). What behavioural mechanisms stabilize the queue?
In an inheritance queue, individuals wait their turn, and so risk dying before they inherit. This begs the question of how the queuing rules are enforced. All else being equal, each individual should prefer itself to produce the offspring reared by the group. Yet reproductive skew is often high in primitively eusocial wasps, perhaps especially in hover wasps (Field & Cant 2009). Why do low-ranked individuals not challenge those ranked above them?
One kind of explanation for queue stability is that even after successfully challenging for reproductive status, a former subordinate might end up with a larger slice of a smaller ‘cake’. In other words, there could be group-level costs of violating the queueing rules, paralleled in several well-known scenarios such as the tug-of-war model of reproductive skew (Reeve et al. 1998). These costs would translate into reduced group productivity, perhaps because group-mates are injured or leave the group following their demotion, and more generally because time and energy were wasted in competition. In social insects, precious time can be wasted when a new dominant takes over, simply because she must often mate and develop physiologically before attaining full reproductive capacity (e.g. Strassmann et al. 2004).
By definition, group-level costs are paid by all members of the group. A potentially more potent disincentive against breaking the queuing rules would be the existence of personal costs involved in doing so. In meerkat (Suricata suricatta) social groups, such costs are suggested by observations of dominants temporarily expelling pregnant subordinates from the group. Such subordinates, which might otherwise kill the dominant's own pups or produce competing litters, lose weight and fertility while they are away from the group, and usually abort their litters (Clutton-Brock et al. 1998; Young et al. 2006). The ideal way to measure costs, however, is to manipulate individuals into breaking the rules and then measuring the consequences. Some of the best work in this area has involved queues of fishes in which, as in the hairy-faced hover wasp, only the dominants breed (Buston 2003a; Heg et al. 2004; Wong et al. 2007, 2008). In these queues, there is a breeding pair rather than just a breeding female, and the queue appears to be based primarily on size (although size is perfectly correlated with age in natural queues). The breeding pair are typically the largest fish in the group, and a young fish joins the end of the queue because it is the smallest. More interestingly, there tends to be a constant size ratio between pairs of individuals that occupy adjacent positions in the queue, and if one individual is removed experimentally, the individuals ranked below start to grow (Buston 2003a; Heg et al. 2004; Buston & Cant 2006). As in the hairy-faced hover wasp, knowing the queuing rule means that we can predict the order of inheritance. But size-based queueing provides the added advantage that individuals can be manipulated into breaking the rules. Wong et al. (2008) achieved this by giving a low-ranked goby (Paragobiodon) extra food in each of nine social groups in laboratory aquaria. The result was that five of the nine manipulated gobies, having grown by eating the supplementary food, were evicted from the group. Wong et al.'s (2008) interpretation of these results was that by growing, food-supplemented fish approached the size of those ranked above them, and so became a threat to their status. The hidden threat that normally maintains queue stability was then revealed: the threat of eviction for rule breakers. Eviction is likely to be costly, at least in nature, because an evicted goby probably has only a small chance of finding a new breeding site (Wong et al. 2007). Normally, subordinates avoid eviction through exhibiting self-restraint: by starving themselves, they avoid becoming a threat to higher ranked individuals. Direct interference in feeding by higher ranked individuals could also help to maintain size ratios, at least in other fish queues (Heg et al. 2004).
In the hairy-faced hover wasp, because queue position is based on age rather than size, it is less obvious how to manipulate subordinates into breaking the rules of the queue. Cant et al. (2006b) instead induced subordinates to challenge the dominant breeder physically, this time in natural spring cofoundress associations of the paper wasp Polistes dominulus. Polistes dominulus foundresses again appear to queue for egg-laying positions: the subordinates that will inherit earliest are also the laziest subordinates, implying that there are detectable cues correlated with inheritance rank (Cant & Field 2001). In P. dominulus, however, the rules that determine inheritance rank are not known (Zanette & Field 2009). Although there is frequent low-level aggression between foundresses, escalated conflicts are rarely observed (J. Field & M. A. Cant 2006, unpublished observations of video recordings). Yet, molecular parentage data suggest that there are at least occasional role reversals in Polistes, in which a previously dominant wasp has become a subordinate (e.g. Peters et al. 1995; Field et al. 1998a). In order to induce escalated conflicts, Cant et al. (2006b) placed the dominant (rank 1) female temporarily in a refrigerator and allowed rank 2 to begin establishing herself as the new dominant. After a few days, rank 1 was released and, on her return to the nest, her interaction with rank 2 recorded. Two kinds of interaction were observed. In 11 cases, rank 2 simply submitted to the returning rank 1 without a fight. In another 17 cases, however, there was a serious escalated contest, sometimes lasting several minutes, involving biting, grappling and sometimes attempted stinging. At the end of all but one of these contests, however, rank 2 submitted to rank 1 without any obvious signs of injury and without being expelled from the group.
To the extent that these contests in Polistes mimic challenges that subordinates could mount naturally, they suggest that subordinates do not queue peacefully because they risk serious injury or expulsion during a challenge. Nevertheless, escalated challenges presumably are costly, and may not be worth mounting simply because the dominant usually wins them. The contrast with Wong et al.'s (2008) results, in which subordinates were expelled from the group if they became a threat, may reflect idiosyncratic differences between the two study systems—perhaps it is harder to expel or injure a wasp than a fish, for example. The difference could also reflect the lower value of subordinate gobies to dominants: mean relatedness is lower in gobies than in wasps, perhaps close to zero. And subordinate gobies, unlike subordinate wasps, do not enhance the fitness of the dominant (Shreeves et al. 2003; Wong et al. 2007). However, expulsion has also been observed when subordinate Neolamprologus fishes were manipulated so that they appeared to be lazy. Relatedness is comparable in Neolamprologus and Polistes, and Neolamprologus helpers may increase breeder fitness (Balshine-Earn et al. 1998; Dierkes et al. 2005; but see Bergmüller & Taborsky 2005). How closely the contests induced by Cant et al. (2006b) resemble natural challenges to the dominant is less clear than in the case of Wong et al.'s (2008) gobies. In nature, a rank 1 P. dominulus rarely leaves the nest for more than a few minutes at a time (e.g. Cant & Field 2001). After a long, artificially enforced absence, rank 2 might need to seriously test rank 1 to check that she can still hold her position.
That P. dominulus subordinates rarely win escalated contests with dominants raises the question of whether they have any leverage at all in the group. Must they simply wait in the hope of one day inheriting the dominant position? Or can they somehow induce the dominant to grant them at least a small share of the current reproduction? This question is particularly pertinent in P. dominulus, in which a large proportion of subordinates are unrelated to the dominant and so cannot obtain indirect fitness benefits through rearing her offspring (Queller et al. 2000; Zanette & Field 2008). One possibility is that subordinates might threaten to leave the group if no reproduction is ceded to them, as in the concessions model of reproductive skew (Vehrencamp 1983; Reeve & Ratnieks 1993). Especially if subordinates normally boost the dominant's fitness by helping, this might be an effective sanction. In the allodapine bee Exoneura, and in the cichlid fish Neolamprologus, both of which have subordinates that provide help, the payoff through leaving has been experimentally increased by providing vacant breeding sites for subordinates to use. In both cases, however, although some individuals did leave their groups to take up the vacancies provided, there was no effect on the reproductive share obtained by subordinates that stayed (Langer et al. 2004; Heg et al. 2006). Thus, even though leaving was clearly an option—some individuals did leave—a greater payoff through leaving did not enable subordinates to extract a larger share of reproduction from the dominant. One possible explanation for these results is that unlike subordinates, dominants could not reliably assess the threat of leaving for themselves (Field & Cant 2009). Indeed, in Heg et al.'s (2006) experiment, only subordinates had access to the vacancies provided. In most wasps and bees, the dominant leaves the nest infrequently and only briefly, and so may be unable to track changes in the social environment very effectively. Thus, subordinates that can benefit by leaving may do so, while those that stay are those that do better to accept the prevailing reproductive skew. In analogous experiments on the hairy-faced hover wasp, and another experiment on the same species of Exoneura, provision of vacancies induced few or no extra subordinates to leave their groups (Bull & Schwarz 1996; Field et al. 1998b). In these cases, it would seem that leaving is not even a credible threat, probably because a subordinate on her own is unlikely to survive long enough to rear offspring through to adulthood (e.g. Field et al. 2000; Shreeves et al. 2003). In such situations, there would be no need for the dominant to cede reproduction to the subordinate in order to retain her in the group (Reeve & Ratnieks 1993).
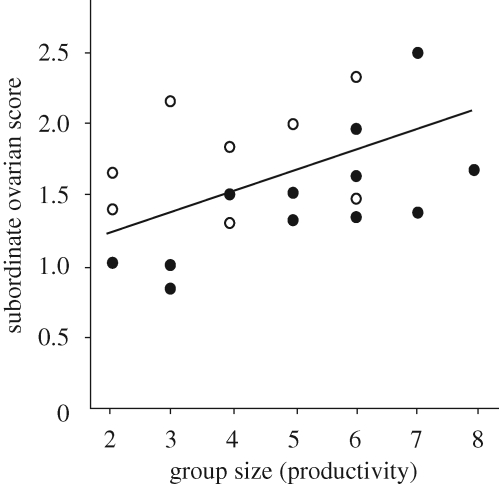
An alternative source of leverage for subordinates might be the threat of aggression. This could take the form of low-level harassment (e.g. ‘lunges’ in Polistes) or escalated fights. In either case, the potential cost might be enough to induce appeasement in the form of reproductive concessions from the dominant. This is similar to the idea of ‘peace incentives’, where it was postulated that a dominant might cede some reproduction to a subordinate in order to reduce her motivation to risk a challenge in the form of a fatal fight (Reeve & Ratnieks 1993). Cant et al. (2006b) tested the counterpart of this argument, that high skew will lead to more aggression by subordinates, using data from the contests that they induced in P. dominulus. Rank 2s appeared to control whether an escalated contest occurred, because a contest ended when rank 2 exhibited stereotyped submissive behaviour (Cant et al. 2006b). Cant et al. (2006b) predicted that if rank 2s were granted little or no direct reproduction (high skew), so that they had more to gain from reversing roles with the dominant, they should be more likely to engage in escalated contests with her. This prediction was supported by the data (figure 2). What rank 2 stood to inherit if she could maintain her newly dominant position was estimated by the group size: larger groups with more helpers are more productive. As expected, rank 2s were more likely to engage in escalated conflict with the returning rank 1 when the winner stood to inherit a more valuable group (figure 2). But more interestingly, rank 2s that had less ovarian development—suggesting a smaller share of the direct reproduction—were also more likely to escalate (figure 2). This suggests that the threat of escalation could give subordinates a way of extracting reproduction: by ceding reproduction, the dominant might avoid escalation. The underlying cause of variation in the rank 2s’ ovarian development is only partially clear. Rank 2s in larger groups had better developed ovaries (figure 2). This could again be consistent with rank 2s being able to extract more reproduction when they had more incentive to overthrow the dominant, although other explanations are possible (Cant et al. 2006b). But the variation in ovarian development that was correlated with the decision to escalate was present after controlling for group size. It seems unlikely that subordinates with better-developed ovaries were simply subordinates of better quality: better quality subordinates should also be more likely to win an escalated contest, yet were less likely to initiate one (figure 2).
Figure 2.
Results of a dominant removal–reintroduction experiment designed to induce contests over the rank 1 position in P. dominulus (Cant et al. 2006b). Rank 1 foundresses were removed for several days to allow rank 2 foundresses to inherit and establish themselves as replacement dominants. Mean ovarian development of rank 2s is plotted as a function of group size, which is an index of productivity. The line shows the significant least-squares regression of ovarian development on group size. Filled circles represent trials in which these newly promoted rank 2 individuals entered into an escalated fight with reintroduced rank 1 individuals; open circles represent trials in which rank 2s immediately submitted to reintroduced rank 1s. Both ovarian development and group size had significant effects on the probability of escalated conflict.
It is interesting to note that the correlation between low-level aggression and reproductive skew in two other Polistes species was in the reverse direction to the one found by Cant et al. (2006b) for escalated aggression: subordinate Polistes bellicosus and Polistes carolina exhibited less low-level aggression when skew was high (Field et al. 1998a; Seppä et al. 2002). Manipulative studies are needed, but these contrasting results may indicate that different kinds of aggression have different functions.
4. Discussion
Accumulating evidence suggests that the individuals in small animal societies typically queue to inherit reproductive positions. This is implied by the repeated finding that individual behaviour is correlated with queue position (e.g. Monnin & Peeters 1999; Cant & Field 2001; Deshpande et al. 2006; Field & Cant 2006; Field et al. 2006). One apparent exception to this pattern was the primitively eusocial wasp Ropalidia marginata, in which it has so far proved impossible to identify rank 2 female before her accession to rank 1. However, recent experiments suggest that even though humans cannot identify rank 2, the wasps themselves can (Bhadra & Gadagkar 2008).
Social queues create asymmetries in expected future fitness, which appear to strongly influence individual behaviour, perhaps facilitating cooperation (Innocent & West 2006). Nevertheless, each individual would still prefer to be nearer the front of the queue. The stability of these social hierarchies may result more from hidden threats that rarely have to be carried out than from direct, all-out competition of the kind seen in a tug-of-war. This has an obvious efficiency advantage. If threats are rarely carried out, both group-level and personal costs are rarely paid. We expect strong selection to avoid triggering hidden threats because an individual that does so suffers a sudden drop in fitness (or fitness ‘cliff-edge’; Kokko 2003) as a result. For example, a dominant that monopolizes reproduction to the extent that it triggers a subordinate's departure suddenly loses all future help from that subordinate. In order to avoid triggering threats unnecessarily, individuals must gain information about the nature of the threat and the location of the threshold beyond which a threat will be triggered, either by trial-and-error learning or by communicating. In the goby Paragobiodon xanthosomus, for example, subordinates must know the minimum size difference that will be tolerated if they are to avoid triggering eviction unnecessarily. Dominants could signal to growing subordinates that they are approaching the threshold, but here there is considerable scope for deception because a dominant will benefit from exaggerating its willingness to exercise a threat, while a subordinate will be selected to ignore warning signals unless there is some way to evaluate their credibility. One way to guarantee credibility is to use warning signals that are costly to the signaller, such as direct aggression or costly displays. This raises the intriguing possibility that dominance displays and acts of social aggression may function to support the credibility of threats to destabilize the group (e.g. by eviction or departure) so that these threats do not, in the end, have to be carried out (Cant & Johnstone 2009).
Which individuals enforce the queuing rules, given that enforcement itself may have personal costs? At one extreme, a single individual such as the dominant might be able to police the entire queue in a small society. Unless relatedness varies systematically with rank, rank reversals among subordinates might have little effect on the dominant's fitness, but the dominant might police the queue because she stands to lose most if within-group conflict leads to a decline in group productivity. Alternatively, each individual might be policed primarily by the individual just ahead of it in the queue—the individual that will lose rank if queue jumping occurs. Consistent with the latter scenario, Cant et al. (2006a) found that the vast majority of low-level aggressive interactions among P. dominulus foundresses were between wasps at adjacent positions in the queue (but see Cronin & Field 2007a,b in the hairy-faced hover wasp). Similarly, dominants that were returned to their nests after temporary removal interacted only with rank 2s that stood to be displaced by them. An exception was after the single escalated contest where a rank 1 submitted to a rank 2: rank 3 then immediately submitted to the defeated rank 1. Parallel observations exist for queues of fishes. In anemonefish, it is the smallest, lowest-ranking subordinates that are most aggressive towards potential joiners to the end of the queue, and subordinates occasionally attempt to evict the individual ranked immediately below them (Elliott et al. 1995; Mitchell 2005; Buston 2003b). Wong et al. (2008) state that rank 4 gobies that had been manipulated into breaking the queuing rules were evicted by their immediate dominants, the individuals at rank 3, rather than by the breeding pair. Similarly, manipulated Neolamprologus cichlid helpers were attacked by other subordinates, not by the dominant, when they were returned to their groups (Balshine-Earn et al. 1998). Overall, these observations suggest that each individual interacts primarily with the individual adjacent to it in the queue. An interesting exception is the ant Dinoponera quadriceps, in which a challenger may be chemically marked by the adjacent rank 1 female. However, marking causes lower ranking females to restrain the challenger physically (‘immobilization’), which in turn can lead to a loss of rank for the challenger (Monnin & Peeters 1999; Monnin et al. 2002).
The approach that we have taken in this paper and previously (Cant & Field 2001, 2005) implies that variation between individuals in future fitness, as embodied by factors such as group size and queue position, has important consequences for variation in behaviours such as helping effort and aggression. Particularly in the case of aggression, this somewhat reverses the traditional argument that it is resource-holding potential that determines access to resources and hence position in the hierarchy. Could it be that queue position is initially determined by individual variation in resource-holding potential, expressed through aggression, so that resource-holding potential is what ultimately determines variation in behaviours such as helping effort in primitively eusocial wasps? For example, do individuals nearer to the end of the queue carry out most of the risky foraging because higher ranked individuals are physically stronger and can force them into doing so, rather than because low-ranked individuals have little expected future fitness? In the hairy-faced hover wasp, this seems unlikely. The term ‘gerontocracy’, in which older individuals are ranked above younger ones, means that the highest-ranked wasps may sometimes be the smallest in the group. Indeed, to the extent that size reflects resource-holding potential, there is no evidence that rank is correlated with quality (Field et al. 1999; Sumner et al. 2002). There could plausibly be selection on other attributes of quality during the queuing process, so that only higher quality individuals tend to survive to reach the highest ranks. But it seems unlikely that mortality, which probably acts largely stochastically during foraging, could lead to the oldest individual, which inherits 90 per cent of the time, consistently being the individual of highest quality. Furthermore, variation in quality would not explain the results of Field et al. (2006), where future fitness was experimentally manipulated. For example, individual helpers worked harder after their group sizes were reduced, even though the same individuals still occupied the ranks above them. Helping effort was measured only 2 days after the manipulation, so that it is also unlikely that individual condition had changed significantly in the interim.
In P. dominulus, what determines an individual's position in the queue is less clear. The dominant individual tends to be larger than rank 2 in an Italian population (Cervo et al. 2008), but in the population studied by Cant et al. (2006a,b), queue position was not correlated with body size (Cant & Field 2001; Zanette & Field 2009). Correlations between queue position and order of arrival at the nest in spring, genetic relatedness or the presence of black facial marks (another potential indicator of quality; Tibbetts & Dale 2004) are also either weak or non-existent (Zanette & Field 2009). Cant et al. (2006a,b) found no evidence that body size determines rates of aggression between wasps of adjacent rank, or the occurrence and duration of escalated contests. If rank is not correlated with body size, why were 16/17 escalated contests won by rank 1 female? Cant et al. (2006b) attribute the asymmetry in outcomes to an ownership effect. The nest may be more valuable to rank 1 female because it contains mainly her offspring, so that she may be prepared to fight harder to retain control of it. Nevertheless, it remains possible that rank is somehow influenced by individual quality: aggressive interactions characterize the initial stages of group formation in Polistes (Reeve 1991). Alternatively, higher ranked individuals may eventually attain better condition through priority of access to resources and reduced energy expenditure, even if rank was initially established independent of individual quality. A positive feedback loop might then result. Queue position, determined by whatever mechanism, could lead low-ranked individuals to work harder, so that they lose condition. This in turn might further reduce their life expectancy and chance of inheritance, further increasing their incentive to work and further reducing their condition. Variation in resource-holding potential, whether intrinsic or occurring via variation in condition, could then reinforce the effect of variation in future fitness in influencing behaviours such as helping effort. Further work to investigate what ultimately determines queue position in primitively eusocial wasps, especially Polistes, is needed to resolve this issue.
Acknowledgements
We thank A. Bourke, H. Helanterä and F. Ratnieks for constructive comments on the manuscript. J.F. thanks T. H. Clutton-Brock, R. A. Foley, F. L. W. Ratnieks and S. West for inviting him to take part in the Royal Society's ‘Evolution of Society’ Discussion Meeting.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘The evolution of society’.
References
- Balshine-Earn S., Neat F. C., Reid H., Taborsky M.1998Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438 (doi:10.1093/beheco/9.5.432) [Google Scholar]
- Bergmüller R., Taborsky M.2005Experimental manipulation of helping in a cooperative breeder: helpers ‘pay to stay’ by pre-emptive appeasement. Anim. Behav. 69, 19–28 (doi:10.1016/j.anbehav.2004.05.009) [Google Scholar]
- Bhadra A., Gadagkar R.2008We know that the wasps ‘know’: cryptic successors to the queen in Ropalidia marginata. Biol. Lett. 4, 634–637 (doi:10.1098/rsbl.2008.0455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge C., Field J.2007Queuing for dominance: gerontocracy and queue-jumping in the hover wasp Liostenogaster flavolineata. Behav. Ecol. Sociobiol. 61, 1253–1259 (doi:10.1007/s00265-007-0355-9) [Google Scholar]
- Bull N. J., Schwarz M. P.1996The habitat saturation hypothesis and sociality in an allodapine bee: cooperative nesting is not 'making the best of a bad situation'. Behav. Ecol. Sociobiol. 39, 267–274 (doi:10.1007/s002650050289) [Google Scholar]
- Buston P.2003aForcible eviction and prevention of recruitment in the clown anemonefish. Behav. Ecol. 14, 576–582 (doi:10.1093/beheco/arg036) [Google Scholar]
- Buston P.2003bSocial hierarchies: Size and growth modification in clownfish. Nature 424, 145–146 (doi:10.1038/424145a) [DOI] [PubMed] [Google Scholar]
- Buston P. M., Cant M. A.2006A new perspective on size hierarchies in nature: patterns, causes, and consequences. Oecologia 149, 362–372 (doi:10.1007/s00442-006-0442-z) [DOI] [PubMed] [Google Scholar]
- Cant M. A., Field J.2001Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. Lond. B 268, 1959–1964 (doi:10.1098/rspb.2001.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant M. A., Field J.2005Helping effort in a dominance hierarchy. Behav. Ecol. 16, 708–715 (doi:10.1093/beheco/ari051) [Google Scholar]
- Cant M. A., Johnstone R. A.2009How threats influence the evolutionary resolution of within-group conflict. Am. Nat. 173, 759–771 (doi:10.1086/598489) [DOI] [PubMed] [Google Scholar]
- Cant M. A., Llop J. B., Field J.2006aIndividual variation in social aggression and the probability of inheritance: Theory and a field test. Am. Nat. 167, 837–852 (doi:10.1086/503445) [DOI] [PubMed] [Google Scholar]
- Cant M. A., English S., Reeve H. K., Field J.2006bEscalated conflict in a social hierarchy. Proc. R. Soc. B 273, 2977–2984 (doi:10.1098/rspb.2006.3669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo R., Dapporto L., Beani L., Strassmann J. E., Turillazzi S.2008On status badges and quality signals in the paper wasp Polistes dominulus: body size, facial colour patterns and hierarchical rank. Proc. R. Soc. B 275, 1189–1196 (doi:10.1098/rspb.2007.1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Brotherton P. N. M., Smith R., McIlrath G. M., Kansky R., Gaynor D., O'Riain M. J., Skinner J. D.1998Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Brotherton P. N. M., O'Riain M. J., Griffin A. S., Gaynor D., Sharpe L., Kansky R., Manser M. B., McIlrath G. M.2000Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc. R. Soc. Lond. B 267, 301–305 (doi:10.1098/rspb.2000.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin A. L., Field J.2007aRank and colony defense against conspecifics in a facultatively eusocial hover wasp. Behav. Ecol. 18, 331–336 (doi:10.1093/beheco/arl091) [Google Scholar]
- Cronin A. L., Field J.2007bSocial aggression in an age-dependent dominance hierarchy. Behaviour 144, 753–765 (doi:10.1163/156853907781476436) [Google Scholar]
- Deshpande S. A., Sumana A., Surbeck M., Gadagkar R.2006Wasp who would be queen: a comparative study of two primitively eusocial species. Curr. Sci. 91, 332–336 [Google Scholar]
- Dierkes P., Heg D., Taborsky M., Skubic E., Achmann R.2005Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975 (doi:10.1111/j.1461-0248.2005.00801.x) [DOI] [PubMed] [Google Scholar]
- Elliott J. K., Elliott J. M., Mariscal R. N.1995Host selection, location, and association behaviors of anemonefishes in field settlement experiments. Mar. Biol. 122, 377–389 (doi:10.1007/BF00350870) [Google Scholar]
- Field J.2008The ecology and evolution of helping in hover wasps (Hymenoptera: Stenogastrinae). In Ecology of social evolution (eds Korb J., Heinze J.), pp. 85–107 Berlin, Germany: Springer [Google Scholar]
- Field J., Cant M. A.2006Helping effort in primitively eusocial wasps. Ann. Zool. Fenn. 43, 481–487 [Google Scholar]
- Field J., Cant M. A.2009Reproductive skew in primitively eusocial wasps: how useful are current models? In Reproductive skew in vertebrates, vol. 20 (eds Hager R., Jones C.), pp. 773–780 Cambridge, UK: Cambridge University Press [Google Scholar]
- Field J., Solis C. R., Queller D. C., Strassmann J. E.1998aSocial and genetic structure of paper wasp cofoundress associations: tests of reproductive skew models. Am. Nat. 151, 545–563 (doi:10.1086/286140) [DOI] [PubMed] [Google Scholar]
- Field J., Foster W., Shreeves G., Sumner S.1998bEcological constraints on independent nesting in facultatively eusocial hover wasps. Proc. R. Soc. Lond. B 265, 973–977 (doi:10.1098/rspb.1998.0386) [Google Scholar]
- Field J., Shreeves G., Sumner S.1999Group size, queuing and helping decisions in facultatively eusocial hover wasps. Behav. Ecol. Sociobiol. 45, 378–385 (doi:10.1007/s002650050574) [Google Scholar]
- Field J., Shreeves G., Sumner S., Casiraghi M.2000Insurance-based advantage to helpers in a tropical hover wasp. Nature 404, 869–871 (doi:10.1038/35009097) [DOI] [PubMed] [Google Scholar]
- Field J., Cronin A., Bridge C.2006Future fitness and helping in social queues. Nature 441, 214–217 (doi:10.1038/nature04560) [DOI] [PubMed] [Google Scholar]
- Griffin A. S., West S. A.2003Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- Heg D., Bender N., Hamilton I.2004Strategic growth decisions in helper cichlids. Proc. R. Soc. Lond. B 271, S505–S508 (doi:10.1098/rsbl.2004.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heg D., Bergmüller R., Bonfils D., Otti O., Bachar Z., Burri R., Heckel G., Taborsky M.2006Cichlids do not adjust reproductive skew to the availability of independent breeding options. Behav. Ecol. 17, 419–429 (doi:10.1093/beheco/arj056) [Google Scholar]
- Hines H. M., Hunt J. H., O'Connor T. K., Gillespie J. J., Cameron S. A.2007Multigene phylogeny reveals eusociality evolved twice in vespid wasps. Proc. Natl Acad. Sci. USA 104, 3295–3299 (doi:10.1073/pnas.0610140104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocent T. M., West S. A.2006Social evolution: cooperation by conflict. Curr. Biol. 16, R365–R367 (doi:10.1016/j.cub.2006.04.009) [DOI] [PubMed] [Google Scholar]
- Keller L.1997Indiscriminate altruism: unduly nice parents and siblings. Trends Ecol. Evol. 12, 99–103 (doi:10.1016/S0169-5347(96)10065-3) [DOI] [PubMed] [Google Scholar]
- Kokko H.2003Are reproductive skew models evolutionarily stable. Proc. R. Soc. Lond. B 270, 265–270 (doi:10.1098/rspb.2002.2238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P., Hogendoorn K., Keller L.2004Tug-of-war over reproduction in a social bee. Nature 428, 844–847 (doi:10.1038/nature02431) [DOI] [PubMed] [Google Scholar]
- Mitchell J.2005Queue selection and switching by false clown anemonefish, Amphiprion ocellaris. Anim. Behav. 69, 643–652 (doi:10.1016/j.anbehav.2004.05.017) [Google Scholar]
- Monnin T., Peeters C.1999Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10, 323–332 (doi:10.1093/beheco/10.3.323) [Google Scholar]
- Monnin T., Ratnieks F. L. W., Jones G. R., Beard R.2002Pretender punishment induced by chemical signalling in a queenless ant. Nature 419, 61–65 (doi:10.1038/nature00932) [DOI] [PubMed] [Google Scholar]
- Peters J. M., Queller D. C., Strassmann J. E., Solis C. R.1995Maternity assignment and queen replacement in a social wasp. Proc. R. Soc. Lond. B 260, 7–12 (doi:10.1098/rspb.1995.0052) [DOI] [PubMed] [Google Scholar]
- Queller D. C., Zacchi F., Cervo R., Turillazzi S., Henshaw M. T., Santorelli L. A., Strassmann J. E.2000Unrelated helpers in a social insect. Nature 405, 784–787 (doi:10.1038/35015552) [DOI] [PubMed] [Google Scholar]
- Reeve H. K.1991Polistes In The Social biology of wasps (eds Ross K. G., Mathews R. W.), pp. 99–148 Ithaca, NY: Cornell University Press [Google Scholar]
- Reeve H. K., Ratnieks F. L. W.1993Queen–queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In Queen number and sociality in insects (ed. Keller L.), pp. 45–85 Oxford, UK: Oxford University Press [Google Scholar]
- Reeve H. K., Emlen S. T., Keller L.1998Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 9, 267–278 (doi:10.1093/beheco/9.3.267) [Google Scholar]
- Seppä P., Queller D. C., Strassmann J. E.2002Reproduction in foundress associations of the social wasp, Polistes carolina: conventions, competition, and skew. Behav. Ecol. 13, 531–542 (doi:10.1093/beheco/13.4.531) [Google Scholar]
- Shreeves G., Field J.2002Group size and direct fitness in social queues. Am. Nat. 159, 81–95 [DOI] [PubMed] [Google Scholar]
- Shreeves G., Cant M. A., Bolton A., Field J.2003Insurance-based advantages for subordinate co-foundresses in a temperate paper wasp. Proc. R. Soc. Lond. B 270, 1617–1622 (doi:10.1098/rspb.2003.2409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann J. E., Fortunato A., Cervo R., Turillazzi S., Damon J. M., Queller D. C.2004The cost of queen loss in the social wasp Polistes dominulus (Hymenoptera: Vespidae). J. Kans. Entomol. Soc. 77, 343–355 (doi:10.2317/E-15.1) [Google Scholar]
- Sumner S., Casiraghi M., Foster W., Field J.2002High reproductive skew in tropical hover wasps. Proc. R. Soc. Lond. B 269, 179–186 (doi:10.1098/rspb.2001.1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Dale J.2004A socially enforced signal of quality in a paper wasp. Nature 432, 218–222 (doi:10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- Turillazzi S.1991The Stenogastrinae. In The social biology of wasps (eds Ross K. G., Matthews R. W.), pp. 74–98 Ithaca, NY: Cornell University Press [Google Scholar]
- Vehrencamp S. L.1983A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667–682 (doi:10.1016/S0003-3472(83)80222-X) [Google Scholar]
- Wong M. Y. L., Buston P. M., Munday P. L., Jones G. P.2007The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc. R. Soc. B 274, 1093–1099 (doi:10.1098/rspb.2006.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Y. L., Munday P. L., Buston P. M., Jones G. R.2008Fasting or feasting in a fish social hierarchy. Curr. Biol. 18, R372–R373 (doi:10.1016/j.cub.2008.02.063) [DOI] [PubMed] [Google Scholar]
- Young A. J., Carlson A. A., Monfort S. L., Russell A. F., Bennett N. C., Clutton-Brock T.2006Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 001 (doi:10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette L. R. S., Field J.2008Genetic relatedness in early associations of Polistes dominulus: from related to unrelated helpers. Mol. Ecol. 17, 2590–2597 (doi:10.1111/j.1365-294X.2008.03785.x) [DOI] [PubMed] [Google Scholar]
- Zanette L. R. S., Field J.2009Cues, concessions and inheritance: dominance hierarchies in the paper wasp Polistes dominulus. Behav. Ecol. 20, 773–780 [Google Scholar]