Abstract
Social organization among human foragers is characterized by a three-generational system of resource provisioning within families, long-term pair-bonding between men and women, high levels of cooperation between kin and non-kin, and relatively egalitarian social relationships. In this paper, we suggest that these core features of human sociality result from the learning- and skill-intensive human foraging niche, which is distinguished by a late age-peak in caloric production, high complementarity between male and female inputs to offspring viability, high gains to cooperation in production and risk-reduction, and a lack of economically defensible resources. We present an explanatory framework for understanding variation in social organization across human societies, highlighting the interactive effects of four key ecological and economic variables: (i) the role of skill in resource production; (ii) the degree of complementarity in male and female inputs into production; (iii) economies of scale in cooperative production and competition; and (iv) the economic defensibility of physical inputs into production. Finally, we apply this framework to understanding variation in social and political organization across foraging, horticulturalist, pastoralist and agriculturalist societies.
Keywords: intergenerational transfers, sexual division of labour, cooperation, economic defensibility, egalitarianism, leadership
1. Introduction
This paper considers the evolutionary and ecological bases of human social organization and is designed to provide a broad overview of the topic. It offers a general theory based on two central theses. The first is that there is an evolved, modal pattern of traditional human social organization that has co-evolved with the characteristics of our species' specialized foraging niche. This pattern is characterized by a three-generational system of resource provisioning within families, long-term pair-bonding between men and women, high levels of cooperation between kin and non-kin and relatively egalitarian social relationships. We suggest that these features of human sociality are a function of the learning- and skill-intensive human foraging niche, which is distinguished by a late age-peak in caloric production, high complementarity between male and female inputs to offspring viability, high gains to cooperation in production and risk-reduction, and a lack of economically defensible resources.
The second thesis is that major shifts away from this modal pattern of social organization are driven by changes in four key ecological and economic variables: (i) the role of skill in resource production; (ii) the degree of complementarity in male and female inputs into production; (iii) economies of scale in cooperative production and competition; and (iv) the economic defensibility of physical inputs into production. We propose that the interaction of these four factors explains both why human social organization is distinctive in a comparative species context, and also much of the variation in social organization across human societies.
2. The social organization of foragers in relation to other primates
This section addresses the social organization of human foragers with respect to other primates in terms of a larger set of coevolved traits, which we refer to as the human adaptive complex (Kaplan et al. 2009). Compared with other primates, the human life history exhibits a number of remarkable characteristics: (i) an exceptionally long lifespan; (ii) a large brain relative to body size; (iii) an extended period of juvenile dependence, resulting in families with multiple dependent children of different ages; (iv) multi-generational resource flows and support of reproduction by post-reproductive individuals; (v) male support of reproduction through the provisioning of women and their offspring; and (vi) substantial cooperation in production and food-sharing between kin and non-kin. Our theory is that these extreme life-history characteristics and extensive cooperative relationships within and between nuclear families are co-evolved responses to an equally extreme commitment to learning-intensive foraging strategies and a dietary shift towards high-quality, nutrient-dense and difficult-to-acquire food resources (Kaplan 1997; Kaplan et al. 2000; Kaplan & Robson 2002). The following logic underlies our proposal.
First, high levels of knowledge, skill, coordination and strength are required to exploit the suite of high-quality, difficult-to-acquire resources humans consume. The time investment in skill acquisition and knowledge leads to selection for lowered mortality rates and greater longevity, because the returns on the investments in development occur at older ages. The extended learning phase during which productivity is low is compensated for by higher productivity during the adult period, with an intergenerational flow of food from old to young. Second, the feeding niche specializing in the capture of large, valuable food packages—particularly through hunting—promotes cooperation between men and women and high levels of male parental investment, because it favours sex-specific specialization in skill investments and generates a complementarity between male and female inputs. Third, the returns to cooperative production and food-sharing promote cooperative relationships within and between families. Food-sharing reduces the risk of food shortfalls due both to the vagaries of foraging luck and to stochastic variance in family size due to mortality and fertility.
In addition, we propose that the relative egalitarianism and lack of clearly delineated dominance hierarchies in most forager groups (Boehm 1999) is primarily the result of the same socio-ecological factors; we suggest that this egalitarianism results mainly from the importance of pair-bonding and voluntarily cooperative relationships (especially between non-kin), and a lack of easily defensible resources in the human foraging niche. The following subsections present the logic and evidence for each component of this socio-ecological framework in sequence.
(a). Age-specific production and intergenerational transfers
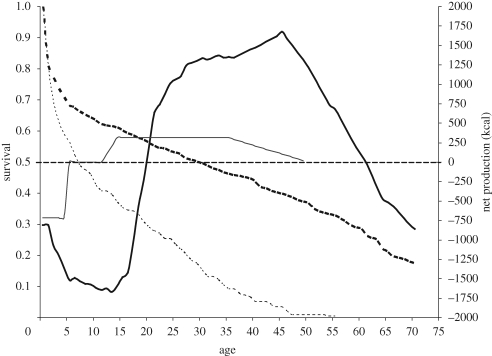
The age-profiles of net food production (food produced minus food consumed) differ dramatically between chimpanzees (Pan troglodytes) and human foragers (figure 1). Among chimpanzees, net production before age 5 is negative, representing complete, then partial, dependence upon mother's milk. The second phase is independent juvenile growth, lasting until adulthood, during which net production is zero. The third phase is reproductive, during which females, but not males, produce a surplus of calories that they allocate to nursing. The slow-growth, learning-intensive human life history, in contrast, generates large calorie deficits until age 20, followed by great calorie surpluses later in life. Humans produce less than they consume for some 15–22 years, depending on the population. Adult net production in humans is about five times as high as in chimpanzees and peaks at about 35–45 years of age.
Figure 1.
Net food production and survival in human foragers and chimpanzees (adapted from Kaplan et al. 2000). Broken lines, human survival; dotted lines, chimpanzee survival; thick solid line, net production humans; thin solid lines, net production chimpanzees.
The human age-pattern of production, with its long period of dependency and late peak in productivity, could only be evolutionarily viable with substantial transfers from producers to consumers. Unlike other mammals, for which transfers from mothers to offspring are limited largely to lactation during infancy, human parents provision their children until adulthood. Moreover, the sharing of food between human parents and their children continues bi-directionally until death in traditional non-market societies. Men, who produce a majority of calories in foraging economies (Kaplan et al. 2001) play a particularly active role in provisioning offspring. Post-reproductive individuals also contribute significant resources towards offspring and grand-offspring fertility and survivorship. The possibility of high-volume transfers from older to younger generations in fact obviates the tight linkage between the age-schedules of fertility and survival that constrains other mammalian life histories, allowing the evolution of significant post-reproductive (i.e. post-menopausal) lifespan (Kaplan & Robson 2002; Lee 2003; Kaplan & Robson 2009). These contributions to offspring fitness may also come in the form of non-material resources, such as help in childcare or the transfer of knowledge and skills.
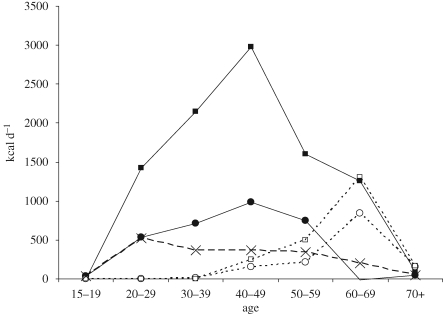
To realize this transfer of resources, human societies exhibit a three-generational residential pattern including offspring, parents and grandparents. Figure 2 shows the implications of this pattern of age-specific production and co-residence for food transfers among Tsimane' forager–horticulturalists. This figure displays net food transfers between parents and children and between grandparents and grandchildern. It shows that for both males and females, parents give more to children than children give to parents, even into old age, and that flows from grandparents to grandchildren become increasingly significant during the post-reproductive period.
Figure 2.
Net caloric resource flows from parents, grandparents and husbands among Tsimane forager–horticulturalists (adapted from Gurven & Kaplan 2008). Filled circles, mother → children; filled squares, father → children; open circles, grandmother → grandchildren; open squares, grandfather → grandchildren; crosses, husband → wife.
(b). Pair-bonding and the sexual division of labour
The human foraging niche, and particularly hunting, promotes cooperation between men and women and high levels of male parental investment because it favours sex-specific economic specialization and generates a complementarity between male and female inputs. Unlike most other mammals, men in foraging societies provide the majority of the energy necessary to support reproduction. Among the 10 foraging societies for which quantitative data are available; on an average men acquire 68 per cent of the calories and almost 88 per cent of the protein; women acquire the remaining 32 per cent of calories and 12 per cent of protein (Kaplan et al. 2001).
Hunting game, as opposed to gathering animal protein in small packets (e.g. insects), is largely incompatible with the evolved commitment among primate females to intensive mothering, carrying of infants and lactation-on-demand. First, it is often risky, involving rapid travel and encounters with dangerous prey. Second, it is often most efficiently practised over relatively long periods of time rather than in short stretches, due to search and travel costs. Third, it is extremely skill-intensive, with improvements in return rate occurring over two decades of daily hunting (Kaplan et al. 2000; Walker et al. 2002; Gurven et al. 2006). The first two qualities make hunting a high-cost activity for pregnant and lactating females. The third quality, in interaction with the first and second, generates life-course effects such that gathering is a better option for females, even when they are not lactating, and hunting is a better option for males. Since women spend a majority of their reproductive lives either nursing or more than three months pregnant, it would not pay to hunt, and they therefore never get enough practice to make it worthwhile, even when they are not nursing or pregnant, or are post-reproductive (Kaplan et al. 2001).
This sex-specific specialization in hunting (for men) and gathering and childcare (for women) yields a complementarity between male and female roles, increasing the returns to economic and reproductive cooperation between men and women and encouraging the formation of long-term pair bonds. The fact that humans are unique in raising multiple dependent offspring of different ages additionally reduces the payoffs to desertion and increases the benefits for men and women to link their economic and reproductive lives over the long run (Kaplan et al. 2003; Winking et al. 2007). Men and women who divorce and remarry during the time they are raising offspring will face conflicts of interest with new spouses over the division of resources. Those conflicts increase the benefits of spouses staying together and having all or most of their children together. As a result, monogamy is the predominant form of marriage across human foraging societies (Kaplan & Lancaster 2003).
(c). Cooperation in production and risk-reduction
While many other animals actively share food—including eusocial insects, social carnivores and some species of birds and bats—the volume and complexity of food-sharing among humans is unique. In addition to within-family food transfers, food-sharing in human foraging societies often extends beyond the nuclear family. Widespread pooling of large game animals, for example, is common among the Hadza (Hawkes et al. 2001), Dobe !Kung (Lee 1979), G/wi (Silberbauer 1981), Ifaluk (Sosis 2000), Ache (Kaplan & Hill 1985) and Gunwinggu (Altman 1987).
Because foragers specialize on the largest, highest quality, most nutrient-dense foods available in their environments, they experience high variance in foraging luck due to the difficulty in acquiring these items. For example, individual Ache hunters return empty-handed on 40 per cent of days they hunt, but some days return with several hundred thousand calories of meat (Hill et al. 1987). Hunting success is even more sporadic among large-game hunters, such as the Hadza, who only acquire meat on less than 3 per cent of hunting days (Hawkes et al. 1991). Since there are diminishing returns to consumption of large quantities of food, especially in environments where spoilage is a problem, and because food portions are very valuable to hungry individuals, reciprocal sharing can significantly reduce variation in day-to-day consumption and maximize the intertemporal utility of food. Reciprocal altruism therefore allows people to devote time and energy to the pursuit of large, asynchronously acquired, high-quality packages (Smith 1988). Widespread sharing of game and other foods may also signal one's quality as a social partner or mate and indebt others to you during critical periods of disability, injury or sickness (Gurven 2004).
Human foragers also experience high gains to cooperation in other aspects of economic production. Many species can be prevented from escaping predation by groups of cooperating hunters. By coordinating their approach, for example, a group of two to five Ache hunters can corral and kill a full troop of capuchin monkeys. Across Amazonia groups of men, women and children can extract large volumes of fish by collectively damming a section of river and intoxicating the fish with plant-derived poisons. Marine hunters, such as the Inuit of North Alaska and Lamalera of Indonesia, work together in 8- to 12-man boats to secure whales that can yield several tons of protein and fat (Spencer 1959; Alvard & Nolin 2002).
(d). Forager egalitarianism
The human feeding niche—with its high returns to cooperation between men and women and between producers—also drives the relative egalitarianism characteristic of foraging societies. This egalitarianism contrasts with both the clearly recognized hierarchies typical of the other apes, as well as the political and economic inequalities characteristic of many post-agricultural human societies. We suggest that forager egalitarianism results primarily from the interaction of (i) the lack of economically defensible resources; (ii) the returns to male provisioning; and (iii) the importance of long-term cooperative partners in human foraging economies.
The key productive resources of foraging economies—game, honey, fruits, nuts and tubers—are rarely concentrated in stable, predictable patches across the landscape, and are difficult to monopolize as a result. Although there are often territorial delineations and non-trivial clashes between neighbouring groups, there is generally open access to foraging sites within a group's territory (Dyson-Hudson & Smith 1978). Because men's productive ability is determined by work effort and ability rather than material wealth, there is comparatively low variance in men's production.
The central importance of cooperative relationships in the human foraging economy further limits overt dominance behaviour and exploitation (Cashdan 1980; Wiessner 1996). The mobility of foragers across largely homogeneous landscapes allows dissatisfied social partners to easily escape the reach of selfish aggrandizers. Would-be dominants must take into account the loss of future cooperation that would result from an intolerably selfish division of cooperatively produced resources. This social accounting becomes more critical as the value of cooperation increases relative to the solitary, non-cooperative alternative.
An additional hypothesis for the evolution of forager egalitarianism that has received significant attention is that humans, more than chimpanzees or other primates, can more effectively form levelling coalitions that counter exploitation by physically dominant individuals (Boehm 1999; Pandit & van Schaik 2003). While this is most often phrased in terms of collective aggression against dominants, we suggest that the ability to coordinate collective emigration away from dominants may be a more important and commonly exercised strategy for imposing costs on aggrandizing and anti-social personalities.
(e). Evolved modal human social organization
Synthesizing these themes, there are four distinctive features of the evolved human production system that have direct implications for social organization:
(i) First, it is skill-based, resulting in a long period of net negative production during infancy, childhood and adolescence, and then a long period of net positive production during adulthood and post-reproductive old age. This results in a three-generational system of wealth flows.
(ii) Second, there is sex-based complementarity in skill acquisition in both production and reproduction. This results in cooperation between males and females in food production and childrearing and relatively stable nuclear families where the reproductive careers of husbands and wives are effectively linked.
(iii) Third, gains from risk-reduction and cooperative resource pursuits generate linkages between nuclear families, based on voluntary labour inputs and resource redistributions.
(iv) Fourth, for the most part, inputs into resource production are not economically defensible, since foraging territories are large and the most important inputs are labour. This—in combination with the gains to monogamous pair-bonding and the importance of voluntarily cooperating partners—leads to relatively egalitarian, dominance-free social relationships.
Together, these social ties produce the basic unit of human social organization: small-scale bands or residential clusters of nuclear families, related through consanguinal and affinal ties, and engaging in cooperative production, altruistic provisioning, and reciprocal sharing in the relative absence of dominance. We refer to this as the evolved modal human social organization, since we propose that most human social groups over the last several tens of thousands of years lived this way. However, we also expect that variation in each of the ecological factors highlighted here has existed throughout human evolutionary history As a result, some groups may have diverged significantly from this basic pattern, and there is much room for subtle variation in social formations within this overall structure (see Foley & Gamble in press, for a discussion of the long-term evolutionary history of human social organization).
3. Major ecological shifters away from the modal pattern
Important changes in social organization occur as the result of changes in the production system of human societies. The introduction of new inputs into production—such as land for agriculture or cattle for pastoralism—can affect social organization through changes in the relative importance of skill-based labour inputs, the nature of male–female complementarity, returns-to-scale in cooperative production and competition, and the ability to establish relations of coercive social dominance (table 1). In the subsequent sections, we discuss some of the important variations in production and their implications for human social organization across foraging, horticulturalist, pastoralist and agrarian societies.
Table 1.
Subsistence ecology and major dimensions of social organization.
| subsistence system (resource base) | intergenerational relations | male–female relations | scale of cooperation, leadership | inequality |
|---|---|---|---|---|
| foragers (mobile prey and widely distributed gathered resources) | intergenerational provisioning, little inheritance | predominant monogamy, bride service | cooperative production and risk reduction, small-scale leadership | relative egalitarianism |
| stratified foragers (concentrated and predictable foraging sites) | intergenerational provisioning, inheritance of foraging sites | some polygyny, bride capture | cooperation and leadership in production and warfare | stratification, slavery, unequal access to prime foraging sites |
| horticulturalists (labour-limited cultivation) | intergenerational provisioning, little inheritance | some polygyny, bride capture | cooperative field labour, big men manage conflict over land | relative egalitarianism |
| pastoralists (livestock) | intergenerational provisioning, inheritance of herds | significant polygyny, bride wealth and bride capture | cooperative husbandry, chiefs manage conflict over herds and grazing land | significant inequality in herd-based wealth |
| agriculturalists (concentrated, high-quality land) | intergenerational provisioning, inheritance of land, primogeniture | significant polygyny, female claustration and dowry | cooperation and leadership in large-scale warfare and public works | stratification, slavery, high inequality in land-based wealth |
(a). Forager variation
The economies of foraging peoples vary on each of the four dimensions discussed above.
(i). Skill
The importance of resources requiring high levels of skill varies seasonally, and from place to place. When there are valuable resources, such as fruits and fish, that are abundant and easy to harvest, children are more productive and less dependent on resource flows from parents and grandparents (Bliege Bird & Bird 2002; Tucker & Young 2005). Nevertheless, there appear to be no cases in which the basic pattern of three-generational families is not fundamental.
(ii). Complementarity
There are specific examples of deviations from the basic pattern of male–female complementarity in production and reproduction. In Australia, high rates of polygyny are found in the Northern Territories and Queensland, with the most extreme case being the Tiwi, where 51 per cent of marriages are polygynous. In a sense, they are the exception that proves the rule because Tiwi women are actively involved in hunting and fishing (Hart & Pilling 1960; Goodale 1971). In that coastal environment, there are fishing and small-game hunting opportunities that are more like gathering than hunting—for example, catching small animals such as lizards, snakes, opossums, rats and bandicoots—and women can be more economically self-sufficient.
(iii). Scale of cooperation
The scale of cooperation also varies ecologically among foragers, across seasons and from one place to another. For example, Shoshoni foragers of the Great Basin in eastern California spent large parts of the year in isolated nuclear families, due to a diet based on pine nuts and scarce game (Steward 1938). On the other hand, game drives and the hunting of large mammals often involve the cooperation of many individuals.
In the case of large-scale cooperation, leaders sometimes emerge, whose power derives from the benefits they provide to fellow group members. We suggest that leadership effort, by encouraging pro-sociality, facilitating coordination and mitigating conflict, can reduce the likelihood that cooperation fails due to free-riding or coordination errors. When the gains to collective action in production, aggression or defence are high but untenable in an unsupervised group, group members will sometimes prefer to cooperate under the direction of a leader and take on the costs he or she might impose rather than operate solitarily (Smith & Choi 2007; Hooper et al. submitted). Among marine hunters, Lamalera and Inuit boat managers provide cases of leadership arising in response to economies of scale when many individuals are required to successfully hunt whales (Spencer 1959; Alvard & Nolin 2002).
(iv). Dominance in production
The nature of resource patches appears to be critical in producing deviations from the standard forager pattern. In general, the resources pursued by human foragers tend to either be mobile or distributed widely in space. However, when there are superabundant patches of resources, such as oak groves and salmon runs in rivers, those inputs into production can become ‘economically defensible’ (Brown 1964; Dyson-Hudson & Smith 1978; Boone 1992). Resource patches tend to be owned, defended with force, and inherited from parents to offspring. Associated with ownership are differences in status, power and wealth with implications for differential survival and reproduction (Sellen & Hruska 2004). In such cases, societies are socially stratified (sometimes including slave classes) and are more similar to land-based agricultural societies. Examples can be found throughout the Pacific coast of North America, being particularly elaborate in British Columbia (Ames 2003).
Forager social organization was also transformed in western North America during the relatively short period during which men maintained herds of horses to hunt bison (Ewers 1958). Because these groups engaged in animal husbandry, their social organization more closely resembled that of pastoral societies, including bride price in the form of ponies, bride capture and frequent warfare.
(b). Tribal horticulture
Subsistence based on horticulture rests on land-extensive, slash-and-burn cultivation or polyculture without significant use of irrigation, terracing, ploughs or draft animals (Bates 2001). People reside together in small villages, larger than hunter–gatherer bands but similarly scaled in terms of face-to-face, kinship-laden interactions. Contemporary examples include the manioc and plantain farmers of South America (such as the Tsimane' and Yanomomö), the millet farmers of sub-Saharan Africa, and the island horticulturalists of Oceana.
(i). Skill
The relative importance of skill in the horticulturalist subsistence strategy varies. For example, among South American forager–horticulturalists, skill development remains highly important for two reasons. First, the same foraging skills that hunter–gatherers employ must still be learned (Gurven et al. 2006); second, the yearly clearing of forest and field preparation are also demanding. In contrast, economies that depend more exclusively on horticulture and in which fields are cleared less frequently because the soil remains fertile, as in many African horticulturalists, the relative importance of skill may be diminished.
(ii). Complementarity
Local ecology affects complementarity in the male and female division of labour in horticultural societies. Garden production by women using the dibble and hoe can provide the carbohydrate and caloric base of the diet and is easily combined with childcare (Boserup 1970; Goody 1976). Males contribute their labour in clearing fields, in provisioning animal protein through hunting and fishing, and in protection of the village resource base through defence. The relative contribution of male help, however, varies by ecological context. More productive soils can be cultivated for longer periods before abandonment, decreasing the yearly demand for strength- and skill-intensive clearing effort. The availability of vegetable protein can also decrease the demand for male hunting effort. As a result of these two factors, gardening based on millet and sorghum that is high in protein and farmed on riverine alluvial soils—as in much of village Africa—is very different from subsistence based on manioc in the thin, lateritic soils of South America. In the African case, each wife is more capable of supporting herself and her children through her own labour, leading to a high frequency of sororal polygyny as sisters form collaborative horticultural work groups (Colson 1960; Lancaster 1981). In the South American case, frequent clearing is necessary and male hunting is critical to acquiring dietary protein, resulting in lower levels of polygyny (Lancaster & Kaplan 1992).
Where the men's primary complementary input is defence, rather than productive labour, it can be supplied as an ‘umbrella’ benefit to multiple women. As a result, males do not have to consider so much whether they can afford to provision additional wives and children, but rather how they can acquire and keep them (Kaplan & Lancaster 2003). The returns to bride capture and threat of bride capture by other groups can create conditions of endemic raiding (Ayres 1974; Low 2000). Polygyny based on bride capture can be understood as harem-defence polygyny in Orians' (1969) distinction between harem-defence and resource-defence polygyny.
(iii). Scale of cooperation
While there are some returns to cooperative in horticultural labour, particularly in clearing and harvesting fields, warfare for bride capture and over land yield the highest returns to scale in horticulturalist societies. In highland New Guinea, the endemic need for military coherence and for diplomacy with both hostile and collaborative groups encouraged the rise of war leaders and big men (Meggitt 1977). These leaders held considerable social power based on their ability to help realize the group's interests, but were relatively constrained compared with the elites of large-scale agricultural societies.
(iv). Dominance in production
The distribution of high-quality land is generally less patchy in horticultural than agricultural systems. Access to land is generally defended by males against outsiders. Within the group, a usufruct system of land tenure gives group members direct rights to the means of production and reproduction (Boserup 1970; Goody 1976). The returns to competition for land within and between groups increase where productive soil is concentrated in high-quality patches, as in the lower alluvial Amazon, or where land becomes the limiting input to production due to population pressure, as in the highlands of New Guinea (Meggitt 1977).
(c). Tribal pastoralism
Pastoral economies are those based on domesticated herd animals such as cattle, camels, horses and sheep. Contemporary examples include the Maasai of East Africa, the Tuareg of North Africa and the Altaic peoples of Inner Asia. The introduction of livestock as the key input to economic production has multiple effects on social and political organization. The importance of competition for grazing lands and the ability to dominate the resources of neighbouring agricultural settlements add additional dimensions affecting variability among pastoral societies.
(i). Skill
While successful herd management requires skill and knowledge of animal husbandry and local ecology, the daily supervision of herding animals is often accomplished by boys and adolescent males (Spencer 1998). An adult male's productivity becomes determined more by the size of his herd than his inputs of time and labour. Because cattle are generally inherited (if they are not obtained through warfare), this affords parents more control of their offspring, since offspring require parental transfers to marry and make a living (Borgerhoff Mulder 1988; Spencer 1998). Thus, the three-generational system of wealth flows is still intact, but children are beholden to their parents, which is not true in foraging societies, where individual freedom often extends to family relationships as well.
(ii). Complementarity
The products of herds require intensive processing of meat, milk and hides, labour provided by women. Men contribute their wealth of animal stock and protection from raiding by other groups. The facts that men's contribution to production is limited by herd size—the distribution of which may be highly unequal—and that defence may be offered as an umbrella benefit to multiple wives allows high levels of polygyny in most pastoral economies (Spencer 1998; Kaplan & Lancaster 2003). Because males compete for the resources that females require for successful reproduction, this mating system can be understood as resource-defence polygyny. One expression of the importance of male extra-somatic wealth in the mating process is the institution of bride wealth, by which men provide a significant payment of resources as a preferred substitute for bride service (Borgerhoff Mulder 1988). Thus, among pastoralists, the complementarity in sex roles interacts with the inheritance system and wealth differences among men to produce higher inequalities in male reproductive success and greater asymmetries in male–female relationships than in economies limited by men's labour.
(iii). Scale of cooperation
The frequency and intensity of warfare and the need for diplomacy in negotiating grazing rights creates a demand for significant leadership roles among pastoralists. The prominence of pastoralist chiefs probably reflects both these increased demands for organization in intergroup conflict, as well as the inequalities in social power that accompany the large inequalities in resource holdings possible in pastoral economies. Within pastoralists, some groups—such as the mounted pastoralists of Inner Asia and North Africa—evidence stronger tendencies toward larger-scale political integration than others—such as the pastoralists of sub-Saharan Africa—perhaps due to the former's proximity to resource-rich and militarily tempting agrarian communities (M. Borgerhoff Mulder 2009, personal communication; Turchin 2009).
(iv). Dominance in production
The nature of pastoral resources—individually controllable but easily stolen—plus the returns to bride capture drive the chronic warfare common to pastoralists (White & Burton 1988; Keeley 1996). Here the ‘warrior complex’ is full-blown, with chronic internal warfare, blood feuds, social segregation of a male warrior age class, fraternal interest groups, a geographical flow of women from subordinate to dominant groups, and expansionist, segmentary lineages based on the male line (DiVale & Harris 1976; White & Burton 1988). The differential accumulation and loss of livestock promotes asymmetries in male wealth and reproductive success both within- and between-groups. Variation in both the degree of status inequality and the scale of warfare may also be determined by the importance of high quality grazing lands as an input into livestock production. Where grazing lands are poor, cattle must move extensively, land is often held communally and status differentials may be modest; when they are rich and patchily distributed, larger scale wars and differentials in status and power tend to be found.
(d). Agrarian states and despotism
The rise of the first large-scale agrarian states, beginning about 6000 years ago in Mesopotamia and later in East Asia and the Americas, marked a critical shift in how humans organized themselves in relation to each other and their environments. These early civilizations share a number of common characteristics: (i) the presence of large, stratified social groupings settled on particularly concentrated, high-quality resource patches; (ii) the appearance of social despots, men who use political and military power to defend their wealth and reproduction and warfare to acquire more resource patches and slaves; and (iii) the advent of formal systems of social governance and law (Betzig 1986; Betzig 1993; Diamond 1997; Summers 2005). We argue that these characteristics of agrarian social organization flow from the nature of the territorial resources that provided the primary input into large-scale agricultural production.
(i). Skill
As in the inheritance of livestock in pastoralism, the inheritance of land as an important input into production affects the relative importance of skill in production. Children are more beholden to their parents, who control their primary source of wealth. Because land and other rival sources of wealth can yield greater economic and social returns when kept intact rather than divided, there were sometimes efforts to reduce the number of claimants to the reproductive estate; this was accomplished through distinctions between legitimate and illegitimate offspring and differential inheritance to first-born sons (Goody 1976; Hrdy & Judge 1993).
(ii). Complementarity
For access to the mating market, men must have brought wealth, power and land in order to be favourably placed, or else get wives as high-risk booty in warfare against other groups (Low 2000; Clarke & Low 2001). These despotic males provide an extreme example of resource-defence polygyny; i.e. they controlled access to the resource base for reproduction that females required and, with few competitors, polygynous marriage to them became the only reproductive option for many women.
While women in stratified systems continued to bring their health, fertility and labour to the mating market, the extreme variance in male quality sometimes forced women's families to put down more value on the table in order to access a desirable groom or move a daughter up in the social hierarchy. These extra payments included actual wealth in the form of dowry, as well as guarantees of paternity confidence. Guarantees of a daughter's virginity and chastity took the form of female seclusion and incapacitation—special women's quarters, chaperones, foot-binding, corseting, clitoridectomy and infibulation—and could be substantially costly, barring women from the outside world of productive labour (Dickemann 1979, 1981; Gaulin & Boster 1990).
(iii). Scale of cooperation
In addition to the first-order shift in social inequality and stratification due to the patchiness of territorial resources, the returns to scale in territorial competition and agricultural intensification—and the more general need for peaceful coexistence in large, dense settlements—may have driven the emergence of prominent leadership roles and top-down governance structures typical of agrarian states. Returns to cooperation in expansionist or defensive warfare may have especially encouraged the acceptance of politically ‘legitimate’ elite leadership (Hooper et al. submitted). The Epic of Gilgamesh, in which Gilgamesh consolidates political control by establishing the defensive walls of Uruk, provides an early narrative of this theme (Adams 1966). The advent of formal systems of law—such as the Code of Hammurabi in Mesopotamia—probably reflected both the effort of ruling elites to control subordinate behaviour to their personal advantage, as well as popular demand for the regulation of social life in large-scale politics. While centralized social control was often an instrument of exploitation, the formal definition and punishment of crime, management of property rights and enforced contributions to public goods may have yielded benefits to non-elites as well (Smith & Choi 2007; Hooper et al. submitted).
(iv). Dominance in production
The patches upon which the first agricultural civilizations were settled were highly productive, but set in environments where there was a rapid fall off to unproductive lands such as desert or forest. Intensification of production through irrigation or terracing tended to further increase patchiness in land quality (Adams 1966). Competition for access to high-quality, defensible patches drove both social stratification within groups as well as territorial warfare between competing settlements. Families that were unable to produce on their own land became labourers and share-croppers under socially dominant lineages (Boone 1992; Smith & Choi 2007). Political and military control of resources in these societies was maintained almost exclusively by the power of men. Although non-human primate females often form alliances with their female kin to protect and control access to the resources necessary for their reproduction (Isbell 1991; Sterck et al. 1997), women are not in a position to do so; the very nature of the sexual division of labour and the dependency of multiple children means that women cannot band together and contest men for control of resources.
Variance in male resource holdings in agrarian states was probably the greatest it has ever been in human history (Betzig 1986, 1992, 1993). Despotic males yielded tremendous social power, with the ability to eliminate rivals and their families through edicts, to acquire land, wealth and slaves for labour and reproduction, and to determine political succession for favoured sons. An outcome of such major variance in male quality and male mating success was that many men remained unmarried or had only one wife, so that male celibacy or at least non-marital sex was a prominent feature of the mating-pool structure for men. This extreme variance in male resource-holdings inevitably led to political instability from the creation of too many potential heirs and too many males without access to the means of reproduction (Turchin & Nefedov 2009).
4. Conclusions
To summarize, we propose that details of the resource production system explain much of human social organization, both accounting for our distinctiveness among primates, as well as explaining variation in human sociality across space and time.
Investment in skill and knowledge is the hallmark of human foraging. Such investments diminish in importance when other inputs, especially defensible and inheritable resources such as land and cattle, become important in determining total production. Nevertheless, the three-generational system of downward wealth flows appears to be a universal feature of human social organization. A principal difference when inheritable wealth becomes an important input in production is that differential inheritance within and among families also emerges, and members of the older generation often exert greater power over their descendents.
Complementarity among the inputs of men and women into production and reproduction is another hallmark of our species. This complementarity derives from the forager diet of mobile prey—which requires both skill and dangerous pursuit—and stationary extracted plant resources. Complementarity, coupled with the existence of multiple offspring of different ages, tends to link the reproductive careers of husbands and wives. Thus, among foragers, where wealth variance is general minimal, monogamy is the dominant marital form. As additional material inputs into production become important, however, the nature of complementarity between males and females also changes because males tend to control those physical inputs through dominance, warfare and inheritance. Wealth in the form of defensible, storable and inheritable resources tends to increase the variance among males in what they have to offer females, and the rate and extent of polygyny increases as well. This is true of stratified foragers, and becomes more extreme with pastoralism and agrarian states.
Risk-reduction, food-sharing and cooperative production tend to be nearly universal among foragers (although varying in extent both seasonally and cross-culturally). This is due to the large and highly variable packages acquired by human foragers, and the skill-based production strategy that puts a premium on cooperative pursuits. The mix of cooperation, returns to monogamy, and the lack of defensible inputs into production tends to limit the formation of dominance hierarchies among foragers. There is still some debate as to whether this relative lack of dominance is due to social levelling strategies, or whether, since the major input into production is voluntary skilled labour, the opportunities for coercion are limited.
The importance of defensible material inputs into production and intensification are almost always associated with greater inequality, social stratification and more prominent political elites. The roles of despotic motives versus the demand for group leadership in driving greater stratification in such situations are difficult to disentangle, however. In our view, it is principally the incorporation of defensible assets into production that generates exploitative dominance hierarchies and despotism, whereas it is economies of scale that produce patterns of managerial leadership. It is just that as economies tend to be based on defensible resources that the scale of production and conflict increases as well. Therefore, managerial leadership and dominance relations usually co-occur.
Finally, we suggest that the same principles may explain the transitions in social organization accompanying the shift to modern developed economies. The transition from despotic agrarian states to more representative forms of government appears to involve the rise of commerce in Europe with a new emphasis on managerial and technological skills. This created a transition from an economy based largely on defendable agricultural land to one based on fungible capital and skill-based labour. The efficiency of private, as opposed to state-mandated, economic production led ruling classes to release direct control of the means of production (McNeill 1982). This tipped the balance of economic and political bargaining power, traditionally held firmly by land-based elites, toward a growing commercial middle class, which demanded expanded social and political rights. This trend continued with the industrial revolution and the efficiency of labour markets—with their attendant mobility—in contrast to slavery, peonage and patron–client bonds characteristic of feudal Europe and the plantation economies of the Americas. In some respects, modern skill-based economies and the skill-based economies of foragers share some fundamental similarities, both resulting in more egalitarian political and social institutions and more individual freedom.
Acknowledgements
Jane Lancaster contributed significantly to the development of many of the ideas in this paper. Thanks also to Sam Bowles, Jim Boone, Paul Seabright, Ann Caldwell, Robert Foley and Monique Borgerhoff Mulder for helpful discussions and feedback, to Jeff Winking for the preparation of Tsimane data and to Tim Clutton-Brock for organizing the Royal Society discussion meeting. H.K., P.H. and M.G. were supported by the National Science Foundation (BCS-0422690) and the National Institute on Ageing (R01AG024119-01). P.H. received additional support from the Howard Hughes Medical Institute through the Program in Interdisciplinary Biological and Biomedical Sciences at UNM.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘The evolution of society’.
References
- Adams R. M.1966The evolution of urban society: early Mesopotamia and prehispanic Mexico Chicago, IL: Aldine [Google Scholar]
- Altman J. C.1987Hunter–gatherers today: an aboriginal economy of north Australia Canberra, Australia: Australian Institute of Aboriginal Studies [Google Scholar]
- Alvard M. S., Nolin D. A.2002Rousseau's whale hunt? Coordination among big-game hunters. Curr. Anthropol. 43, 533–559 (doi:10.1086/341653) [Google Scholar]
- Ames K.2003The northwest coast. Evol. Anthropol. 12, 19–33 (doi:10.1002/evan.10102) [Google Scholar]
- Ayres B.1974Bride theft and raiding for wives in cross-cultural perspective. Anthropol. Quart. 47, 238–252 (doi:10.2307/3316978) [Google Scholar]
- Bates D. G.2001Human adaptive strategies: ecology culture and politics, 2nd edn Boston, MA: Allyn and Bacon [Google Scholar]
- Betzig L.1986Despotism and differential reproduction: a Darwinian view of history Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- Betzig L.1992Roman polygyny. Ethol. Sociobiol. 13, 309–349 (doi:10.1016/0162-3095(92)90008-R) [DOI] [PubMed] [Google Scholar]
- Betzig L.1993Sex, succession and stratification in the first six civilizations: how powerful men reproduced, passed power on to their sons, and used their power to defend their wealth, women and children. In Social stratification and socioeconomic inequality (ed. Ellis L.), pp. 37–74 New York, NY: Praeger [Google Scholar]
- Bliege Bird R., Bird D.2002Constraints of knowing or constraints of growing? Fishing and collecting by the children of Mer. Human Nat. 13, 239–267 [DOI] [PubMed] [Google Scholar]
- Boehm C.1999Hierarchy in the forest: the evolution of egalitarian behavior Cambridge, UK: Harvard University Press [Google Scholar]
- Boone J. L.1992Competition, conflict, and the development of social hierarchies. In Evolutionary ecology and human behavior (eds Smith E. A., Winterhalder B.), pp. 301–337 Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- Borgerhoff Mulder M.1988Kipsigis bridewealth payments. In Human reproductive behavior: a Darwinian perspective (eds Betzig L., Borgerhoff Mulder M., Turke P.), pp. 65–82 Cambridge, UK: Cambridge University Press [Google Scholar]
- Boserup E.1970Women's role in economic development London, UK: Allen and Unwin [Google Scholar]
- Brown J. L.1964The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169 [Google Scholar]
- Cashdan E. A.1980Egalitarianism among hunters and gatherers. Am. Anthropol. 82, 116–120 (doi:10.1525/aa.1980.82.1.02a00100) [Google Scholar]
- Clarke A. L., Low B. S.2001Testing evolutionary hypotheses with demographic data. Popul. Dev. Rev. 27, 633–660 (doi:10.1111/j.1728-4457.2001.00633.x) [Google Scholar]
- Colson E.1960Social organization of the Gwembe Tonga Manchester, UK: Rhodes-Livingstone Institute [Google Scholar]
- Diamond J.1997Guns, germs, and steel: the fates of human societies New York, NY: Norton [Google Scholar]
- Dickemann M.1979The ecology of mating systems in hypergynous dowry societies. Soc. Sci. Inform. 18, 163–195 (doi:10.1177/053901847901800201) [Google Scholar]
- Dickemann M.1981Paternal confidence and dowry competition: a biocultural analysis of purdah. In Natural selection and social behavior (eds Alexander R. D., Tinkle D. W.), pp. 417–438 New York, NY: Chiron Press [Google Scholar]
- DiVale W., Harris M.1976Population, warfare, and the male supremacist complex. Am. Anthropol. 80, 21–41 [Google Scholar]
- Dyson-Hudson R., Smith E. A.1978Human territoriality: an ecological reassessment. Am. Anthropol. 80, 21–41 (doi:10.1525/aa.1978.80.1.02a00020) [Google Scholar]
- Ewers J. C.1958The Blackfeet: raiders on the northwestern plains Norman, OK: University of Oklahoma Press [Google Scholar]
- Foley R., Gamble C.In press The ecology of social transitions in human evolution. Phil. Trans. R. Soc. B (doi:10.1098/rstb.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin S., Boster J.1990Dowry as female competition. Am. Anthropol. 92, 994–1005 (doi:10.1525/aa.1990.92.4.02a00080) [Google Scholar]
- Goodale J. C.1971Tiwi wives Seattle, WA: University of Washington Press [Google Scholar]
- Goody J.1976Production and reproduction: a comparative study of the domestic domain Cambridge, UK: Cambridge University Press [Google Scholar]
- Gurven M.2004To give or give not: the evolutionary ecology of human food transfers. Behav. Brain Sci. 27, 543–583 [Google Scholar]
- Gurven M., Kaplan H.2008Beyond the grandmother hypothesis: evolutionary models of human longevity. In The cultural context of aging: worldwide perspectives (ed. Sokolovsky J.), pp. 53–60, 3rd edn Santa Barbara, CA: Greenwood Press [Google Scholar]
- Gurven M., Kaplan H., Guitierrez M.2006How long does it take to become a proficient hunter? Implications on the evolution of delayed growth. J. Human Evol. 51, 454–470 (doi:10.1016/j.jhevol.2006.05.003) [DOI] [PubMed] [Google Scholar]
- Hart C. W. M., Pilling A. R.1960The Tiwi of north Australia New York, NY: Holt, Rinehart and Winston [Google Scholar]
- Hawkes K., O'Connell J. F., Blurton Jones N. G., Oftedal O. T., Blumenschine R. J.1991Hunting income patterns among the Hadza: big game, common goods, foraging goals and the evolution of the human diet. Phil. Trans. R. Soc. Lond. B 334, 243–251 (doi:10.1098/rstb.1991.0113) [DOI] [PubMed] [Google Scholar]
- Hawkes K., O'Connell J. F., Blurton Jones N.2001Hadza meat sharing. Evol. Human Behav. 22, 113–142 (doi:10.1016/S1090-5138(00)00066-0) [DOI] [PubMed] [Google Scholar]
- Hill K., Hawkes K., Kaplan H., Hurtado M.1987Foraging decisions among Ache hunter–gatherers: new data and implications for optimal foraging models. Ethol. Sociobiol. 8, 1–36 (doi:10.1016/0162-3095(87)90055-0) [Google Scholar]
- Hooper P. L., Kaplan H. S., Boone J. L.Submitted A theory of leadership in human cooperative groups. [DOI] [PubMed] [Google Scholar]
- Hrdy S. B., Judge D.1993Darwin and the puzzle of primogeniture: an essay on biases in parental investment after death. Human Nat. 4, 1–45 (doi:10.1007/BF02734088) [DOI] [PubMed] [Google Scholar]
- Isbell L. A.1991Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav. Ecol. 2, 143–155 (doi:10.1093/beheco/2.2.143) [Google Scholar]
- Kaplan H. S.1997The evolution of the human life course. Between Zeus and Salmon: the biodemography of aging (eds Wachter K., Finch C.), pp. 175–211 Washington, DC: National Academy of Sciences [Google Scholar]
- Kaplan H. S., Hill K.1985Food-sharing among Ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–245 (doi:10.1086/203251) [Google Scholar]
- Kaplan H., Robson A.2002The co-evolution of intelligence and longevity and the emergence of humans. Proc. Natl Acad. Sci. USA 99, 10221–10226 (doi:10.1073/pnas.152502899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S., Lancaster J. B.2003An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment. In Offspring: human fertility behavior in biodemographic perspective (eds Wachter K. W., Bulatao R. A.), pp. 270–223 Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- Kaplan H. S., Robson A.2009We age because we grow. Proc. R. Soc. B 276, 1837–1844 (doi:10.1098/rspb.2008.1831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H., Hill K., Lancaster J. B., Hurtado A. M.2000A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [Google Scholar]
- Kaplan H. S., Hill K., Hurtado A. M., Lancaster J. B.2001The embodied capital theory of human evolution. In Reproductive ecology and human evolution (ed. Ellison P. T.), pp. 293–317 Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- Kaplan H., Robson A., Lancaster J. B.2003Embodied capital and the evolutionary economics of the human lifespan. In Lifespan: evolutionary, ecology and demographic perspectives (eds Carey J. R., Tuljapaur S.), Population and Development Review 29(Supplement), 152–182 New York, NY: The Population Council [Google Scholar]
- Kaplan H. S., Gurven M., Winking J.2009An evolutionary theory of human lifespan: Embodied capital and the human adaptive complex. In Handbook of theories of aging (eds Bengtson V. L., Gans D., Putney N. M., Silverstein M.), pp. 53–60 2nd edn New York: Springer [Google Scholar]
- Keeley L. H.1996War before civilization: the myth of the peaceful savage Oxford, UK: Oxford University Press [Google Scholar]
- Lancaster C. S.1981The Goba of the Zambezi: sex roles, economics, and change Norman, OK: University of Oklahoma Press [Google Scholar]
- Lancaster J. B., Kaplan H.1992Human mating and family formation strategies: the effects of variability among males in quality and the allocation of mating effort and parental investment. In Topics in primatology: human origins, vol. 1 (eds Nishida T., McGrew W. C., Marler P., Pickford M., de Waal F.), pp. 21–33 Tokyo, Japan: University of Tokyo Press [Google Scholar]
- Lee R. B.1979The !Kung San: men, women, and work in a foraging society Cambridge, UK: Cambridge University Press [Google Scholar]
- Lee R.2003Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc. Natl Acad. Sci. 100, 9637–9642 (doi:10.1073/pnas.1530303100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. S.2000Why sex matters: a Darwinian look at human behavior Princeton, NJ: Princeton University Press [Google Scholar]
- McNeill W. H.1982The pursuit of power: technology, armed force, and society since A.D. 1000 Chicago, IL: University of Chicago Press [Google Scholar]
- Meggitt M.1977Blood is their argument: warfare among the Mae Enga tribesmen of the New Guinea Highlands Mountain View, CA: Mayfield [Google Scholar]
- Orians G. H.1969On the evolution of mating systems in birds and mammals. Am. Nat. 103, 589–603 (doi:10.1086/282628) [Google Scholar]
- Pandit S. A., van Schaik C. P.2003A model for leveling coalitions among primate males: toward a theory of egalitarianism. Behav. Ecol. Sociobiol. 55, 161–168 (doi:10.1007/s00265-003-0692-2) [Google Scholar]
- Sellen D. W., Hruska D. J.2004Extracted-food resource-defense polygyny in native western North America. Curr. Anthropol. 45, 707–714 (doi:10.1086/425637) [Google Scholar]
- Silberbauer G.1981Hunter and habitat in the Central Kalahari Desert Cambridge, UK: Cambridge University Press [Google Scholar]
- Smith E. A.1988Risk and uncertainty in the ‘original affluent society’: evolutionary ecology of resource-sharing and land tenure. In Hunter gatherers. Vol. 1: History, evolution and social change (eds Ingold T., Riches D., Woodburn J.), pp. 222–251 New York, NY: Berg [Google Scholar]
- Smith E. A., Choi J. K.2007The emergence of inequality in small-scale societies: simple scenarios and agent-based simulations. In The model-based archaeology of socio-natural systems (eds van der Leeuw S., Kohler T.). Santa Fe, NM: SAR Press [Google Scholar]
- Sosis R.2000The emergence and stability of cooperative fishing on Ifaluk Atoll. In Human behavior and adaptation: an anthropological perspective (eds Cronk L., Chagnon N., Irons W.), pp. 437–472 Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- Spencer P.1998The pastoral continuum Oxford, UK: Oxford University Press [Google Scholar]
- Spencer R.1959The north Alaskan Eskimo: a study in ecology and society Washington, DC: Smithsonian Institution [Google Scholar]
- Sterck E. H. M., Watts D. P., van Schaik C. P.1997The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309 (doi:10.1007/s002650050390) [Google Scholar]
- Steward J.1938Basin-plateau aboriginal sociopolitical groups Washington, DC: Smithsonian Institution [Google Scholar]
- Summers K.2005The evolutionary ecology of despotism. Evol. Human Behav. 26, 106–135 (doi:10.1016/j.evolhumbehav.2004.09.001) [Google Scholar]
- Tucker B., Young A. G.2005Growing up Mikea: children's time allocation and tuber foraging in southwestern Madagascar. In Hunter–gatherer childhoods (eds Hewlett B., Lamb M.), pp. 147–171 New York, NY: Aldine de Gruyter [Google Scholar]
- Turchin P.2009A theory for formation of large empires. J. Glob. Hist. 4, 191–217 [Google Scholar]
- Turchin P., Nefedov S. A.2009Secular cycles Princeton, NJ: Princeton University Press [Google Scholar]
- Walker R., Hill K., Kaplan H., McMillan G.2002Age-dependency in skill, strength and hunting ability among the Ache of eastern Paraguay. J. Human Evol. 42, 639–657 (doi:10.1006/jhev.2001.0541) [DOI] [PubMed] [Google Scholar]
- White D. R., Burton M. L.1988Causes of polygyny: ecology, economy, kinship, and warfare. Am. Anthropol. 90, 871–887 (doi:10.1525/aa.1988.90.4.02a00060) [Google Scholar]
- Wiessner P.1996Leveling the hunter: constraints on the status quest in foraging societies. In Food and the status quest: an interdisciplinary perspective (eds Wiessner P., Schiefenhövel W.), pp. 171–192 Providence, RI: Berghahn Books [Google Scholar]
- Winking J., Kaplan H., Gurven M., Rucas S.2007Why do men marry and why do they stray? Proc. R. Soc. B 274, 1643–1649 (doi:10.1098/rspb.2006.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]