Abstract
Histological sections of the mammalian striatum reveal a “matrix” that is histochemically distinguishable from patches, or “striosomes”. The latter are cross sections of a compartment that consists primarily of tube-shaped structures radiating through the matrix. As a test of the hypothesis that the function of the striosome/patch compartment includes the mediation of behaviors related to reward, the present study examined electrical self-stimulation of the caudoputamen in rats with electrodes in either of the two compartments. Rats acquired and maintained bar-pressing responses that were contingent on stimulation through electrodes making contact with striosomes/patches more reliably than animals with electrodes terminating exclusively in the matrix. The results provide in vivo evidence that the striosome/patch compartment is functionally differentiated from the matrix compartment: Stimulation centered in or around the striosome/patch compartment but not in the matrix led to rapid acquisition of a new behavior.
Keywords: caudate nucleus, putamen, matrix, reward, reinforcement, neural plasticity
The striatum, a major component of the basal ganglia, is of interest because of its apparent involvement in movement disorders (1–3), motivated behaviors (4–6), and drug addiction (7, 8). The dorsal part of the striatum, or caudoputamen in the rat, is composed of at least two distinct compartments: the “matrix” and the “striosomes” or “patches” (9–12), which are surrounded by the matrix. These compartments are distinguishable by their differing distributions of neurotransmitters and neuropeptides and by differences in other brain areas with which they are connected (1, 13, 14). The matrix is enriched in met-enkephalin cells (1, 15, 17) and acetylcholinesterase (10, 11) and receives afferents from the superficial layers of the medial prefrontal cortex (16) and motor, somatosensory, and visual cortices (18). In contrast, the striosome/patch compartment is enriched in fibers immunoreactive for leu-enkephalin (19) and calretinin (20) and in μ opiate receptor binding sites (11) and receives projections from the deep layers of the medial prefrontal (16) and limbic cortices (16, 21, 22) and their related cortical regions (23). The dopaminergic innervation of the two compartments also differs (14, 24, 25).
In vitro studies have provided evidence that the anteromedial and posterolateral caudoputamen in the rat differ in their responsiveness to glutamate, gamma-aminobutyric acid, and opiate ligands, which might reflect a reported higher density of striosome/patches in the anteromedial caudoputamen than in the posterolateral caudoputamen (26–28). Gauchy et al. (29) demonstrated in vitro that the matrix- and striosome/patch-enriched zones in cat striatal slices also differ in responsiveness to cholinergic and opiate ligands.
Notwithstanding this anatomical and neurochemical information, little is known about the behavioral functions of these striatal compartments. Mainly on the basis of its anatomical relationships, it has been suggested that the matrix mediates some form of sensorimotor processing (19, 30), and there is behavioral evidence (31–35) that the dorsolateral part of the caudoputamen, which is relatively enriched in matrix (19, 36), may mediate responsiveness to external stimuli (for example, ref. 37).
Conjecture about the function of the striosome/patch compartment has been even more limited. Two aspects of the relationships of this striatal compartment to other structures have led to the speculation that this compartment may have reward-related functions (see refs. 1 and 38 and references therein). First, certain limbic structures implicated in the mediation of affective behaviors project to the striosome/patch compartment. Several reports (13, 16, 18, 23, 39) describe a preferential projection from the medial prefrontal cortex, which has been implicated in electrical self-stimulation of the brain (40–42) and in cocaine self-administration (43), to the striosome/patch compartment. The basolateral nucleus of the amygdala also projects preferentially to the striosome/patch compartment (44, 45) and has been implicated in behaviors involving primary reward (46), conditioned reward (47–50) and aversion (for example, ref. 51). Second, although there are a number of differences, there are also certain similarities between the innervation of nucleus accumbens and the areas of the striatum that contain heavy concentrations of striosomes/patches (1, 38). The nucleus accumbens is implicated in the mediation of behaviors such as drug self-administration (52–54) and electrical self-stimulation of the brain (55–57) that are thought to depend on reward. These observations led to the suggestion that the striosome/patch compartment may also have reward-related functions and to the present test of this hypothesis (58).
Since its discovery (59), electrical self-stimulation of the brain has been used to localize areas involved in reward- and reinforcement-related functions, usually in experimental situations that allow animals to control the stimulation they receive by pressing a bar. The fact that animals learn to press the bar to receive stimulation is suggestive of the function of brain areas that support this behavior.
Although self-stimulation of the caudoputamen in the rat has been reported by several workers (60–63), the bar-pressing behavior sustained by electrodes in this part of the brain is weak and inconsistent compared with the robust responding observed with electrodes in areas such as the septum, the ventral tegmental area, the lateral hypothalamus, or the nucleus accumbens (55, 65). The hypothesis that a reward- or reinforcement-related process is initiated by electrical stimulation of the striosome/patch but not of the matrix compartment could explain the unreliability of the caudoputamen self-stimulation observed in these studies because the electrodes were placed without reference to the striatal compartments. The hypothesis suggests that self-stimulation would be observed only if the stimulation activated some neural element that is part of the striosome/patch compartment. The finding that, in individual rats, stimulation sustained responding at one locus but not another in the caudoputamen (63, 64) is also consistent with this notion.
In the present study, we examined this hypothesis by testing a group of rats with electrodes in caudoputamen for self-stimulation. Using appropriate histochemical techniques, we examined the locations of the electrodes with respect to striosomes/patches and compared these locations with the animals’ self-stimulation behavior.
MATERIALS AND METHODS
Subjects.
We used 75 male Long–Evans rats (200–400 g) as subjects. They were housed individually in hanging, wire mesh cages in a 12-hr light/dark cycle with food and water ad libitum. Under 60 mg/kg sodium pentobarbital anesthesia, a single electrode aimed at one of the following sets of coordinates, measured from bregma (67): 0.2 (anterior–posterior), 2.0 (lateral), 4.0 (vertical); 0.2, 2.0, 5.0; 0.2, 3.75, 5.0; 1.0, 2.0, 5.0; 1.7, 2.3, 5.0; 0.7, 3.5, 5.0; 0.8, 4.2, 6.0; 1.6, 1.5, 5.3; 0.8, 2.0, 6.0, using standard stereotoxic techniques.
Apparatus.
Untwisted two-wire electrodes (Plastics One, Roanoke, VA) were used. One of the insulated wires was implanted into the brain where the uninsulated, smoothed, cross-sectional area of the tip served as the electrode surface. Wires of two different diameters were used: 0.27 and 0.30 mm. The reference electrode was made by removing the insulation from the other wire and wrapping it around three skull screws. Thus, the rats received monopolar stimulation from the tip of the implanted wire with reference to the skull screws.
Animals were tested in two identical wooden cages (19.7 × 19.7 × 40.6 cm) with wire mesh floors and the lower half of the front wall made of Plexiglas. The open top of each cage was fitted with a commutator that allowed free movement when a rat’s electrode was connected. A Plexiglas lever (3.8 × 7.6 cm) protruded from one of the side walls of the cage. The lever closed a microswitch that produced a 0.5-sec train of 60-Hz constant current sine-wave stimulation. Sine-wave stimulation was used because of its relative lack of selectivity (compared with the discrete square-wave pulses used in parametric studies of self-stimulation), thus maximizing the possibility of observing self-stimulation. The stimulation current was adjusted using a potentiometer to change the resistance in series with the electrode. The same trains of stimulation could also be delivered by the experimenter. A counter recorded the number of trains received by the animal.
Procedure.
Behavioral testing began 7–10 days after surgery. The rats were first given a “shaping” procedure for 5–30 min per day. The experimenter delivered a train of stimulation when the rat approached and/or touched the bar. The stimulation current was raised gradually from 10 to 30 μA until the rat showed an apparent approach response, began to press the bar spontaneously (self-stimulation) or, in a few rats, until the stimulation elicited a motor response that was incompatible with bar pressing. The latter usually consisted of a forced contralateral turning movement that was precisely coincident with the stimulation. The stimulation current was decreased when these responses were observed. After the shaping part of each session, the rat was left in the experimental cage for 30 min. The number of experimenter-administered trains of stimulation during the shaping part of the session and the number of spontaneous bar presses (self-stimulation) during the final 30 min part of the session were recorded separately. These sessions continued daily until a rat responded spontaneously with >100 bar presses in the final 30-min part of the session on each of 3 consecutive days or for a maximum of 11–18 days.
Some of the rats that met the criterion of 100 responses on 3 consecutive days were given extinction sessions on the following 2–3 days. During these sessions, the animals were placed into the test cages with the stimulation current turned off. All other conditions were identical to those during the test sessions. No experimenter-administered stimulation was given, and the number of spontaneous bar presses was counted.
Immunohistochemistry.
After behavioral testing was complete, the rats were anesthetized with an overdose of sodium pentobarbital (600 mg/kg, i.p.) and perfused with 0.9% saline followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 2 hr and were cryoprotected in 20% glycerol overnight. The brains were cut at 20 or 30 μm using a sliding microtome, and every section containing evidence of the track left by the electrode was saved. The sections were stored in phosphate buffer containing 0.1% sodium azide. Floating sections were then stained with monoclonal anti-calbindinD antibody (1:1,000; Sigma) or with polyclonal anti-calretinin antiserum (1:2,500, Chemicon) following the standard Adivin/Biotinylated Enzyme Complex method for calbindin or the nickel intensification method for calretinin (20). A calbindin antibody was used to stain the matrix (68), and a calretinin antiserum was used to mark striosomes/patches in the caudoputamen (20). Omission of the primary antibodies eliminated specific staining.
Anatomical Classification.
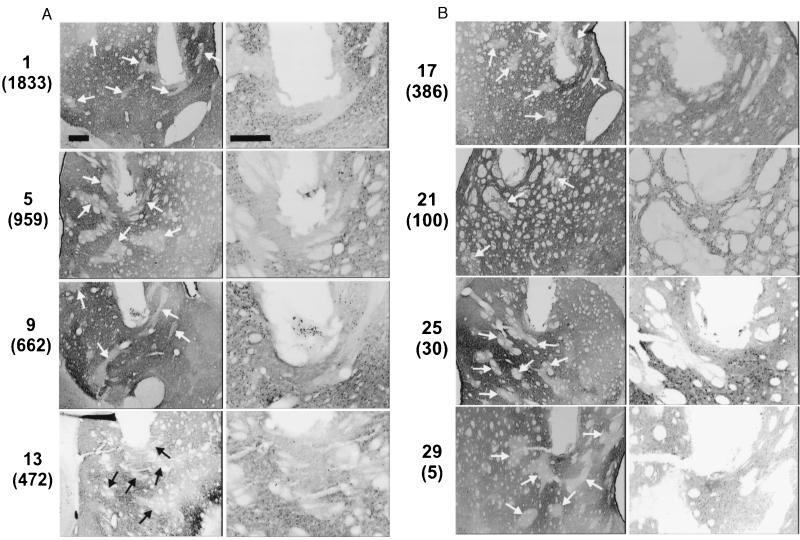
All analysis of histological material was done without reference to the behavioral data. For each rat, every brain section containing evidence of the track left by the electrode was available, and three to seven of these sections were scrutinized to divide the cases into two groups. Rats were assigned to the “identified” group using two criteria: (i) availability of a brain section with damage produced by the tip of the electrode (these were distinguishable from sections with damage produced by the shaft of the electrode because the holes made by the tips were more rectangular and more ventral than the holes in adjacent sections) and (ii) at least one feature clearly identifiable as a striosome/patch [that is, an irregularly shaped calbindin-poor area with clearly defined borders (see Fig. 3)] in the area of the tip and in sections anterior and posterior to it.
Figure 3.
Illustrations of electrode tip locations in relation to striosomes/patches for cases ranked 1, 5, 9, 13, 17, 21, 25, and 29 in Fig. 2 (self-stimulation rates in parentheses). (A, Left and B, Left) A relatively dark background stained for calbindin-D 28k (matrix) with irregularly shaped and placed lighter areas marked by arrows not expressing this stain (striosomes/patches). (A, Right and B, Right) Higher magnification illustrations in which individual cells expressing calbindin are visible. The absence or presence of these cells between the electrode track and a striosome/patch was an important criterion for determining whether a tip contacted or did not contact a striosome/patch. (Bars = 250 μm.)
Cases not meeting these criteria were assigned to the “unidentified” group. In some of these cases, a section containing the tip of the electrode track could not be identified positively. In other cases, mainly with tips in the lateral caudoputamen, the borders of the striosomes/patches were not defined clearly. In a few cases, a spherical calbindin-poor area surrounded the electrode tip, more-or-less conforming to its shape. Because striosomes/patches are highly irregular in shape and because of the possibility that the calbindin-poor area was caused by the stimulation, these cases were eliminated.
The brains in the identified group were divided further into two groups. In one group (“striosome/patch”), the electrode tip could be seen inside of or in contact with the surface of a striosome/patch in at least one section. As illustrated in Fig. 3, a major criterion for this judgement was the absence of individual cells expressing calbindin between the electrode track and the striosome/patch. In the other group (“matrix”), no such contact was visible in any section.
RESULTS
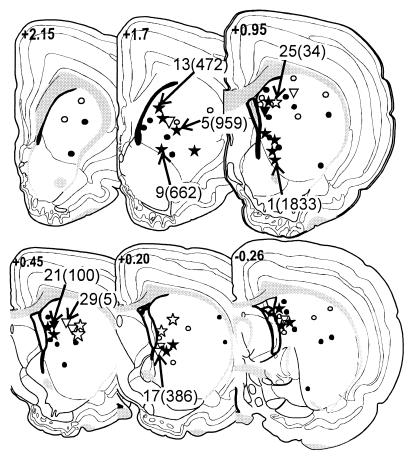
The locations of the electrode tips for the 75 rats in the experiment are shown in Fig. 1. Most of the tips were located between the anterior part of the caudoputamen and the posteromedial part of the structure, in its medial and ventral parts. The relationship between the stimulating electrode and striosomes/patches was identified in the histological material for 31 rats (Fig. 3); the remaining 44 cases failed to meet the criteria described in Materials and Methods.
Figure 1.
Placement of electrode tips for 75 subjects. In 22 cases (stars), the tip was localized in a striosome/patch (see text and Fig. 3), and in 9 cases (inverted triangles), the tip was in the matrix; the remaining 44 cases (circles) were unidentified. Filled symbols represent subjects that made >99 responses in three sessions (self-stimulators); open symbols indicate lower response rates. The cases identified by their rank-order and bar-press rates in parentheses (see Fig. 2) are illustrated in Fig. 3.
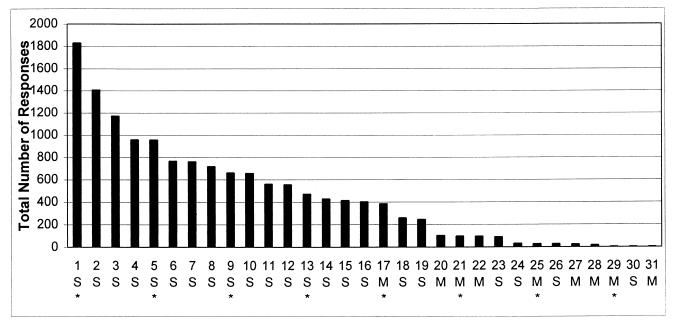
The total number of responses made by each rat during the three consecutive test sessions that yielded the highest response rates was used to rank-order the identified (Fig. 2) and unidentified (data not shown) rats. The rank-ordered rates for each group were divided into quartiles. The mean response rates of the quartiles for the identified rats were 1073.0, 520.1, 165.8, and 19.0; for the unidentified rats the corresponding values were 1011.8, 407.6, 107.7, and 26.3. A two-way ANOVA on these means revealed no significant difference between the groups [F(1, 67) = 1.37, P > 0.2] and no significant interaction between groups and quartiles [F(3, 67) = 0.27, P > 0.8], showing that identification or lack of identification of the relationship of electrode tips to striosome/patches was unrelated to the distribution of the rats’ response rates.
Figure 2.
Rank-ordering of self-stimulation rates for 31 rats in which the location of the electrode tip with respect to a striosome/patch was determined (see text and Fig. 3). The numbers of responses shown for each rat are the totals made during the three consecutive 30-min sessions with the highest rates of responding. Cases in which the electrode tip contacted a striosome/patch (S) and those with the tip in the matrix (M) are indicated. Asterisks identify cases illustrated in Fig. 3 (every fourth case is illustrated). A median split (ranks 1–15 vs. ranks 17–31) of the rank-ordered data was used to determine the statistical relationship of self-stimulation rate to electrode location (see text).
As is typical in previous reports (60–64) of self-stimulation in the caudoputamen, there are large, individual differences in rates of responding that have no obvious relationship to the locations of the electrode tips (Fig. 1). The intermingling of self-stimulation and non-self-stimulation sites, especially in the medial parts of the structure, suggests functional heterogeneity.
Fig. 3 illustrates cases of electrode tips judged to be in contact with a striosome/patch or in the matrix (“S” or “M,” respectively, in Fig. 2). Starting with the animal ranked 1, every fourth case is shown. The illustrated cases are indicated by asterisks in Fig. 2.
The rank-ordered identified cases (Fig. 2) were divided into two groups using a median split. In the upper group, 15 cases were identified as striosome/patch and none as matrix. In the lower group, six cases were identified as striosome/patch and nine were identified as matrix. This distribution is significantly different from chance (χ2 = 12.86, p < .0003). Thus, self-stimulation is far more likely to occur at a much higher response rate with electrodes in or in contact with striosomes/patches than with electrodes that do not make contact with these structures.
Two cases in the lower group (ranks 18 and 19) had electrodes that were in contact with striosomes/patches and made a total of >200 responses, a low but consistent rate of self-stimulation. Three cases (ranks 23, 24, and 30) with electrode tips in contact with striosomes/patches failed to exhibit self-stimulation. Possible explanations for these exceptions include electrode failure, differences in the functions of the striosomes/patches contacted, and collateral elicitation of behaviors incompatible with self-stimulation (see below).
Eight self-stimulators were given extinction sessions. During their final 3 self-stimulation sessions, these rats made an average of 282.7 responses per session. During the extinction sessions they made an average of 44.8 responses per session [t(7) = 4.48, P < .01]. This observation confirms that the high rates of bar pressing observed during the self-stimulation sessions were contingent on the stimulation.
The stimulation currents used during the shaping and test sessions and the numbers of trains of experimenter-administered stimulation administered to the rats during the shaping sessions were compared for the rats in the upper and lower groups defined by the median split used to compare electrode locations. The mean stimulation currents used for the upper group were 26.6 μA; for the lower group the mean was 24.2 μA. These values were not significantly different [t(28) = 1.14, P > .05]. When the stimulation elicited movements incompatible with bar pressing (Fig. 2, cases 14, 20, and 24), the current was kept below the level at which these effects occurred. These cases occurred at about the same rate in striosome/patch and matrix cases, and the currents used were usually sufficient to maintain bar pressing if the rat was a self-stimulator.
The animals in the upper group required an average of 9.2 shaping sessions before starting to bar press spontaneously, and they received an average of 42.3 experimenter-administered trains of shaping stimulation per session. The rats in the lower group received an average of 14.5 shaping sessions before starting to bar press or until the sessions were discontinued, with an average of 43.8 experimenter-administered trains of stimulation. There was no significant difference between the mean numbers of experimenter-administered trains per session [t(28) = 0.11, P > .05] suggesting that neither differences in the amount of training nor number of experimenter-administered trains of stimulation can account for the difference in the effect of the stimulation on the rats in the two groups.
DISCUSSION
The present results suggest that electrodes contacting the striosomes/patches of the rat caudoputamen sustain electrical self-stimulation more reliably than electrodes in the matrix that surrounds them. This observation provides in vivo evidence for a functional difference on the behavioral level between these two neurochemically and anatomically differentiated systems.
Some rats in this study never self-stimulated, even after 11–18 days of training. Although this amount of training makes it highly unlikely that they would ever have started, this possibility cannot be eliminated categorically. Accordingly, the data permit the conclusion that self-stimulation is acquired more readily with electrodes in striosomes/patches than in matrix.
We used calbindin immunohistochemistry to identify the two compartments in the caudoputamen based on evidence (68) that areas poor in this marker correspond to striosomes/patches originally identified as areas that, among other things, are poor in acetylcholinesterase (10, 11) and express opiate receptor binding (11). However, the matrix marker calbindin does not reliably stain the dorsal and lateral sectors of the middle and posterior parts of the caudoputamen in the rat (69), even though these sectors are differentiated into the two compartments by other markers (11, 69). Because our ability to locate electrode tips with respect to the compartments was limited to the medial, central, and ventral areas of the caudoputamen, the present analysis is limited to these areas of the caudoputamen.
It is unlikely that the features we identified as striosomes/patches were produced by the stimulation. First, as shown in Fig. 3, similarly shaped calbindin-poor areas appeared both in contact with and at a distance from the tips of the stimulating electrodes in all cases. Second, damage produced by the stimulation should have been symmetrical with respect to the tips, and a few cases with such symmetrical calbindin-poor areas were eliminated from the identified group. Third, stimulation-produced calbindin-poor areas would have been the result of tissue damage or lesions, which should have increased resistance around the electrode tips, eliminating self-stimulation or at least resulting in an increase in the stimulation current required to maintain self-stimulation. Neither of these effects was observed. Finally, in several cases (data not shown), we used an immunohistochemical stain for calretinin to identify intact striosomes/patches at the tips of electrode tracks in the brains of animals that had self-stimulated. This marker for striosomes/patches (20) would have disappeared if stimulation-produced lesions had damaged tissue around the tip of the electrode.
The effective spread of stimulation current from the tips of the stimulating electrodes is an issue affecting the conclusion that electrodes in striosomes/patches produced self-stimulation because of their action on neural elements that are parts of these structures. Ranck’s (70) analysis suggests that the maximum radius of effective stimulation current in the conditions of the present experiment was probably <500 μm because of our use of relatively large, low resistance electrodes. Although we do not know exactly how far the effective stimulation spread, it seems likely that the neural elements, activation of which was critical for producing self-stimulation, were associated with striosome/patch tissue. If such elements were located in the matrix, self-stimulation should have been observed reliably with electrodes that completely failed to contact this compartment, and this was not the case.
The present data do not indicate which elements of the striosomes/patches are critical for self-stimulation. Previous studies (63, 64, 71) suggest that self-stimulation sites in the striatum are uncorrelated with the density of dopaminergic innervation (63) and that the stimulated elements have refractory periods between 0.65 and 6.00 msec (64), somewhat longer than those observed for self-stimulation in the medial forebrain bundle (71). These findings suggest that some portion of the neural elements activated by the effective stimulation may consist of cell bodies, implying that activation of neurons intrinsic to the striosomes/patches could be involved in the observed self-stimulation.
Cell bodies in striosomes/patches are preferentially innervated by projections from limbic cortex (45), and these cells could be the critical neurons activated by the stimulation electrodes. The fact that a few of these afferent neurons also innervate cells in the matrix could account for the four relatively weak cases of matrix self-stimulation we observed. The idea that self-stimulation results from activation of cell bodies in the striosome/patch compartment suggests the possible involvement of strionigral neurons (19) terminating in pars compacta, which also supports self-stimulation (72, 73). In turn, a portion of the nigrostriatal dopaminergic neurons innervate the striosomes/patches (24, 25). Thus, stimulation of striosomes/patches could produce dopamine release in the same structures. Dopaminergic activity in nucleus accumbens has been associated with several kinds of reward, including self-stimulation, drug addiction, and eating (7, 58, 74, 79). If dopamine release in striosomes/patches also activates some reward-like process, the rats could have learned to press the bar to obtain these rewarding effects. The observation that stimulants with rewarding effects (for example, ref. 54) induce gene expression in striosomes/patches (80, 81), is consistent with this hypothesis.
Finally, the present findings provide a possible explanation for the inconsistent results of previous studies of caudoputamen self-stimulation. Stimulation of this brain area has been reported to sustain responding (60, 62), not to sustain it (61), or to sustain it at one locus but not another (63, 64). It seems likely that in these studies, self-stimulation was observed in cases where the electrode tips contacted a striosome/patch but not when they were in the matrix.
Acknowledgments
We thank Corrine McDonald, Stephanie Cassie, and Janet Raymond for technical assistance. This research was supported by grants from the Natural Sciences and the Engineering Research Council of Canada and from Fonds pour la Formation de Chercheurs et l’Aide à la Recerche, Province of Quebec, to N.M.W.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Graybiel A M. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 2.Graybiel A M. Curr Opin Neurobiol. 1991;1:644–651. doi: 10.1016/s0959-4388(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 3.Graybiel A M, Hirsch E C, Agid Y. Adv Neurol. 1990;53:17–29. [PubMed] [Google Scholar]
- 4.Mogenson G J, Yang C R. Adv Exper Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 5.Mogenson G, Yim C C. In: Neuromodulatory Functions of the Mesolimbic Dopamine System: Electrophysiological and Behavioral Studies. Willner P, Scheel-Kruger J, editors. Philadelphia: Wiley; 1991. pp. 105–130. [Google Scholar]
- 6.Le Moal M, Simon H. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 7.White N M, Hiroi N. Semin Neurosci. 1993;5:329–336. [Google Scholar]
- 8.Koob G F, Bloom F E. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic P S. J Comp Neurol. 1982;205:398–413. doi: 10.1002/cne.902050408. [DOI] [PubMed] [Google Scholar]
- 10.Graybiel A M, Ragsdale C W. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herkenham M, Pert C B. Nature (London) 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- 12.Gerfen C R. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 13.Gerfen C R. Nature (London) 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 14.Gerfen C R. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 15.Graybiel A M, Chesselet M F. Proc Natl Acad Sci USA. 1984;81:7980–7984. doi: 10.1073/pnas.81.24.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerfen C R. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Moratalla R, Gold L H, Hiroi N, Koob G F, Graybiel A M, Tonegawa S. Cell. 1994;79:728–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 18.Donoghue J P, Herkenham M. Brain Res. 1986;365:397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- 19.Gerfen C R. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- 20.Hiroi N. Neurosci Lett. 1995;197:223–226. doi: 10.1016/0304-3940(95)11942-p. [DOI] [PubMed] [Google Scholar]
- 21.Berendse H W, Galis-de Graaf Y, Groenewegen H J. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 22.Ragsdale C W, Graybiel A M. J Comp Neurol. 1991;311:134–167. doi: 10.1002/cne.903110110. [DOI] [PubMed] [Google Scholar]
- 23.Ragsdale C W, Graybiel A M. Proc Natl Acad Sci USA. 1990;87:6196–6199. doi: 10.1073/pnas.87.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerfen C R, Herkenham M, Thibault J. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer L F, Graybiel A M. Brain Res. 1989;498:344–350. doi: 10.1016/0006-8993(89)91114-1. [DOI] [PubMed] [Google Scholar]
- 26.Krebs M O, Trovero F, Desban M, Gauchy C, Glowinski J, Kemel M L. J Neurosci. 1991;11:1256–1262. doi: 10.1523/JNEUROSCI.11-05-01256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs M O, Gauchy C, Desban M, Glowinski J, Kemel M L. J Neurosci. 1994;14:2435–2443. doi: 10.1523/JNEUROSCI.14-04-02435.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs M O, Kemel M L, Gauchy C, Desban M, Glowinski J. Neuroscience. 1993;57:249–260. doi: 10.1016/0306-4522(93)90060-s. [DOI] [PubMed] [Google Scholar]
- 29.Gauchy C, Desban M, Krebs M O, Glowinski J, Kemel M L. Neuroscience. 1991;41:449–458. doi: 10.1016/0306-4522(91)90340-t. [DOI] [PubMed] [Google Scholar]
- 30.Graybiel A M, Baughman R W, Eckenstein F. Nature (London) 1986;323:625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- 31.Dunnett S B, Iversen S D. Brain Res. 1982;248:121–127. doi: 10.1016/0006-8993(82)91153-2. [DOI] [PubMed] [Google Scholar]
- 32.Whishaw I Q, O’Connor W T, Dunnett S B. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 33.Whishaw I Q, Tomie J. Behav Neurosci. 1987;101:603–616. doi: 10.1037//0735-7044.101.5.603. [DOI] [PubMed] [Google Scholar]
- 34.Fairley P C, Marshall J F. Behav Neurosci. 1986;100:652–663. doi: 10.1037//0735-7044.100.5.652. [DOI] [PubMed] [Google Scholar]
- 35.White N M. Neurosci Biobehav Rev. 1986;10:15–36. doi: 10.1016/0149-7634(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 36.Pert C B, Kuhar M J, Snyder S H. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson L W. Acta Morphol Neerl Scand. 1988;26:165–176. [PubMed] [Google Scholar]
- 38.White N M. Life Sci. 1989;45:1943–1957. doi: 10.1016/0024-3205(89)90569-9. [DOI] [PubMed] [Google Scholar]
- 39.Morino P, Mascagni F, McDonald A, Hökfeldt T. Neuroscience. 1994;59:939–952. doi: 10.1016/0306-4522(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 40.Robertson A, Mogenson G J. Psychopharmacology. 1979;65:149–154. doi: 10.1007/BF00433041. [DOI] [PubMed] [Google Scholar]
- 41.Corbett D, Laferriere A, Milner P M. Physiol Behav. 1982;28:531–534. doi: 10.1016/0031-9384(82)90151-2. [DOI] [PubMed] [Google Scholar]
- 42.Schenk S, Shizgal P. Physiol Behav. 1982;28:133–138. doi: 10.1016/0031-9384(82)90114-7. [DOI] [PubMed] [Google Scholar]
- 43.Goeders N E, Smith J E. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- 44.Ragsdale C W, Graybiel A M. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- 45.Wright C I, Beljer A V J, Groenewegen H J. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wurtz R H, Olds J. J Comp Physiol Psychol. 1963;56:941–949. doi: 10.1037/h0042033. [DOI] [PubMed] [Google Scholar]
- 47.Spiegler B J, Mishkin M. Behav Brain Res. 1981;3:303–317. doi: 10.1016/0166-4328(81)90002-4. [DOI] [PubMed] [Google Scholar]
- 48.Everitt B J. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 49.Hiroi N, White N M. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Everitt B J, Morris K A, O’Brien A, Robbins T W. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 51.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 52.Olds M E. Brain Res. 1979;168:351–360. doi: 10.1016/0006-8993(79)90175-6. [DOI] [PubMed] [Google Scholar]
- 53.Olds M E. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- 54.Hoebel B G, Monaco A P, Hernandez L, Aulisi E F, Stanley B G, Lenard L G. Psychopharmacology. 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- 55.Phillips A G, Brooke S M, Fibiger H C. Brain Res. 1975;85:13–22. doi: 10.1016/0006-8993(75)90998-1. [DOI] [PubMed] [Google Scholar]
- 56.Robertson A, Mogenson G J. Can J Psychol. 1978;32:67–76. doi: 10.1037/h0081677. [DOI] [PubMed] [Google Scholar]
- 57.Fiorino D F, Coury A, Fibiger H C, Phillips A G. Behav Brain Res. 1993;55:131–141. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- 58.White N M. Neurosci Biobehav Rev. 1989;13:181–186. doi: 10.1016/s0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- 59.Olds J, Milner P M. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 60.Routtenberg A. J Comp Physiol Psychol. 1971;75:269–276. doi: 10.1037/h0030927. [DOI] [PubMed] [Google Scholar]
- 61.Prado-Alcala R A, Kent E W, Reid L D. Brain Res. 1975;84:531–540. doi: 10.1016/0006-8993(75)90770-2. [DOI] [PubMed] [Google Scholar]
- 62.Phillips A G, Carter D A, Fibiger H C. Brain Res. 1976;104:221–232. doi: 10.1016/0006-8993(76)90615-6. [DOI] [PubMed] [Google Scholar]
- 63.Prado-Alcala R, Wise R A. Brain Res. 1984;297:265–273. doi: 10.1016/0006-8993(84)90567-5. [DOI] [PubMed] [Google Scholar]
- 64.Trzcinska M, Bielajew C. Behav Brain Res. 1992;48:1–8. doi: 10.1016/s0166-4328(05)80132-9. [DOI] [PubMed] [Google Scholar]
- 65.Olds M E, Olds J. J Comp Neurol. 1963;120:259–295. doi: 10.1002/cne.901200206. [DOI] [PubMed] [Google Scholar]
- 66.Roberts D C S, Bennett S A L. Psychopharmacology. 1993;111:215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- 67.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- 68.Gerfen C R, Baimbridge K G, Miller J J. Proc Natl Acad Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoen S W, Graybiel A M. J Comp Neurol. 1992;322:566–576. doi: 10.1002/cne.903220410. [DOI] [PubMed] [Google Scholar]
- 70.Ranck J B. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 71.Gallistel C R, Shizgal P, Yeomans J S. Psychol Rev. 1981;88:228–273. [PubMed] [Google Scholar]
- 72.Clavier R M, Fibiger H C. Brain Res. 1977;131:271–286. doi: 10.1016/0006-8993(77)90520-0. [DOI] [PubMed] [Google Scholar]
- 73.Vaccarino F, Franklin K B J. Behav Brain Res. 1982;5:281–295. doi: 10.1016/0166-4328(82)90034-1. [DOI] [PubMed] [Google Scholar]
- 74.Wise R A, Bauco P, Carlezon W A, Jr, Trojniar W. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- 75.Koob G F. Semin Neurosci. 1992;4:139–148. [Google Scholar]
- 76.Blackburn J R, Pfaus J G, Phillips A G. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- 77.Hiroi N, White N M. Brain Res. 1991;552:141–152. doi: 10.1016/0006-8993(91)90672-i. [DOI] [PubMed] [Google Scholar]
- 78.Hiroi N, White N M. Brain Res. 1990;510:33–42. doi: 10.1016/0006-8993(90)90724-p. [DOI] [PubMed] [Google Scholar]
- 79.Salamone J D. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 80.Graybiel A M, Moratalla R, Robertson H A. Proc Nat Acad Sci. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moratalla R, Elibol B, Vallejo M, Graybiel A M. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]