Abstract
The effects of temperature on pollen germination and pollen tube growth rate were measured in vitro in thermogenic skunk cabbage, Symplocarpus renifolius Schott ex Tzvelev, and related to floral temperatures in the field. This species has physiologically thermoregulatory spadices that maintain temperatures near 23°C, even in sub-freezing air. Tests at 8, 13, 18, 23, 28 and 33°C showed sharp optima at 23°C for both variables, and practically no development at 8°C. Thermogenesis is therefore a requirement for fertilization in early spring. The narrow temperature tolerance is probably related to a long period of evolution in flowers that thermoregulate within a narrow range.
Keywords: thermogenic, flower, pollen, germination, skunk cabbage, Symplocarpus renifolius
1. Introduction
Thermogenesis by flowers occurs in several families of ancient seed plants, including Araceae, Arecaceae, Aristolochiaceae, Annonaceae, Cycadaceae, Cyclanthaceae, Magnoliaceae, Nelumbonaceae and Nymphaeaceae (Seymour & Schultze-Motel 1997). The usual explanation for thermogenesis is to enhance fragrance production and volatility (Fægri & van der Pijl 1979; Meeuse & Raskin 1988), but it may have other functions, particularly because heat production often persists far longer than the period of insect attraction and some species physiologically regulate heat production. Physiologically, thermoregulatory plants adjust respiration inversely with ambient temperatures, to maintain constant floral temperature in the face of a changing environment (Nagy et al. 1972; Knutson 1974; Seymour & Schultze-Motel 1998). Thermogenesis is known to be a direct energy reward for insect visitors by enhancing their activities (Seymour et al. 2003). In the case of eastern skunk cabbage, Symplocarpus foetidus, heating over a period of a week or two was suggested to protect it from freezing and enable it to bloom early in spring (Knutson 1974, 1979). Temperature affects the timing of morphological and physiological developments in flowers in general (Kinet et al. 1985), but there are almost no experimental studies on thermogenic species that show how heating affects floral development. One important aspect is pollen function, which has been investigated by quantifying germination success and pollen tube growth in many species, especially agricultural plants.
Inflorescences of skunk cabbage are the most precise thermoregulators studied so far. S. foetidus can maintain spadix temperature within a 3.5°C range (22.7–26.2°C) over an ambient air temperature range of 37.4°C (−10.3 to 27.1°C) (Seymour 2004). Precise temperature regulation also occurs in Symplocarpus renifolius from Japan (Onda et al. 2008). The inflorescence of both species consists of a fleshy, reddish-brown-coloured spathe around a spadix having approximately 50–100 bisexual florets (Uemura et al. 1993; Seymour & Blaylock 1999). Blooming in early spring, often under snow, the plants can maintain spadix temperatures above 20°C, despite ambient temperatures down to −14°C (Knutson 1974, 1979). Thermogenesis begins before the spathe opens, continues throughout the period of stigma receptivity and diminishes during subsequent pollen release (Seymour & Blaylock 1999). The bisexual florets go through a stigma phase (mean 6.8 days), a transitional phase (2 days) and a staminate phase (16.7 days) (Uemura et al. 1993). Although there is some overlap in phases, outcrossing is apparently required for good seed set.
This study examined the effect of temperature on in vitro pollen germination and pollen tube growth in S. renifolius. The aims were to determine whether pollen function was optimal at the regulated temperature of the spadix and whether thermogenesis was essential for effective pollination. We made measurements of both germination and tube growth, because they are two distinct physiological mechanisms that do not necessarily have the same optima (Weinbaum et al. 1984). Although in vitro measurements of the maximum tube growth length can be less than in vivo measurements (Taylor & Hepler 1997), they provide useful comparisons with the literature.
2. Material and methods
Symplocarpus renifolius was studied near Hakuba, Nagano prefecture (36°39′ N, 137°50′ E) and Omori, Akita prefecture (39°19′ N, 141°20′ E), Japan. On 4 days between 13 and 22 April 2002, at Hakuba, spadix temperature was measured with a thermocouple in 40 thermoregulating inflorescences, and the spadix was removed and weighed (Onda et al. 2008). Fresh pollen was collected in March and April 2008, and taken to the laboratory in Morioka, where experiments were begun immediately. In vitro germination assays were carried out according to a slightly modified technique of Dafni (1992). The liquid medium contained 10 per cent [w/v] sucrose, 2 mM H3BO3 and 6 mM Ca(NO3)2. Fresh pollen was added to the medium (100 mg/200 μl) and gently mixed. A well was made with petroleum jelly on the inner side of a Petri dish cover, and 10 μl of the mixture were placed into it, while 5 ml was added to the bottom plate. The cover and base were sealed with petroleum jelly to prevent evaporation. Five dishes with pollen from each of four spadices were placed in incubators at temperatures of 8, 13, 18, 23, 28 and 33°C. All temperatures were tested concurrently, with pollen from the same spadices. Germination and pollen tube length were measured at 3, 6, 9, 12 and 24 hours of incubation. One dish was opened at a time and a drop of methylene blue was added to the mixture in the well. With a pipette, each mixture was transferred to a single concave slide and examined under a light microscope (BX51, Olympus) and a digital camera. Measurements of pollen germination rates and tube length were performed manually with ImageJ software (Rasband 1997–2007). For each replicate, at least 300 pollen grains were counted for germination and 100 pollen tubes were measured, except at 8°C (where few pollen grains germinated). A pollen grain was considered to have germinated if the tube length was at least the diameter of the pollen grain (Boavida & McCormick 2007).
3. Results
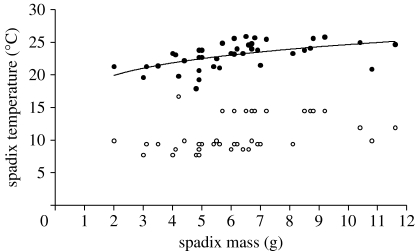
Spadix temperatures of 40 inflorescences that were measured in the stigma stage averaged 23.0±0.6 (95% CI)°C, when the daytime air temperature averaged 10.7°C (figure 1). Smaller spadices were slightly, but significantly, cooler than larger ones.
Figure 1.
Daytime temperatures of the spadix (filled circles) and ambient air (open circles) of thermogenic S. renifolius in the field. The relationship between spadix temperature (T s) and spadix mass (M) is given by the regression, T s=18.2 M0.13 (R 2=0.29).
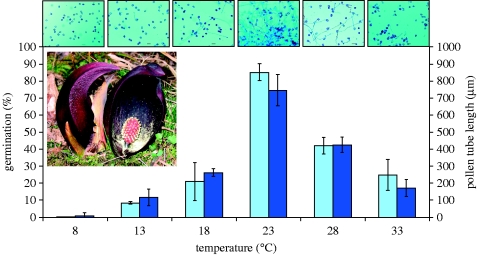
Overall, the experiments showed distinct peaks in pollen germination and pollen tube growth at 23°C (figure 2). The values were approximately twice as high at 23°C than at 18 or 28°C for both measures. There was virtually no pollen germination or pollen tube growth at 8°C.
Figure 2.
Percentage of pollen germination (left bars) and pollen tube length (right bars) of S. renifolius after 24 hours of incubation at selected temperatures. Scale bars, 50 μm. Standard errors represent four replicates. The inset shows the female and male phases of blooming.
Temperature treatment and observation time had significant effects on the fraction of germinated pollen and pollen tube growth. ANOVAs and Tukey post hoc tests revealed significant differences (p<0.05) between all temperature combinations, except between 18 and 33°C, for both measures (table S1, see the electronic supplementary material). Germination fraction increased significantly and apparently linearly during the first 12 hours of observation in all cases, but was not significantly different between 12 and 24 hours, except at 23°C where germination increased from 62±8 to 85±5 per cent (t-tests; figures S1 and S2, see the electronic supplementary material). There was also a significant effect of time on pollen tube length in all cases where observations were 6 hours or more apart and all but two cases at 3 hours.
4. Discussion
This study demonstrates coincidence of the highest pollen germination and pollen tube growth rate with naturally regulated spadix temperature in S. renifolius. Regulated temperatures averaged 23°C (figure 1), similar to those measured in other studies of S. renifolius (Uemura et al. 1993; Ito et al. 2004; Ito & Ito 2005) and also near those of S. foetidus in the field (Knutson 1974; Seymour & Blaylock 1999; Seymour 2004). Initially unknown to us, Shibata (1985) had measured pollen tube growth in S. renifolius and found it maximal between 20 and 25°C, but did not report the germination rate. The results indicate that the regulated temperature of 23°C is optimal, and both aspects of pollen function decrease steeply within a ±5°C range around it (figure 2). Moreover, because pollination is practically impossible below 8°C, thermogenesis is essential for successful reproduction at natural environmental temperatures. S. renifolius commonly proceeds through the stigma stage when average ambient temperatures are below 5°C (Onda et al. 2008). Without thermogenesis or the ability of pollen to adapt to lower temperatures, skunk cabbage could not successfully flower as early in the season as it does. This study provides support for Knutson's (1979) proposal that thermogenesis permits early blooming.
There are two interpretations of the coincidence—either the regulated temperature is a metabolic response to intrinsic optima of the pollen, or the pollen optima have evolved in the regime of regulated floral temperature. The latter explanation seems preferable, given the wide variability of pollen functional optima shown in the literature. Data from 21, non-thermogenic species, which were studied with a broad enough temperature range to indicate clear optima, are summarized in table S2 of the electronic supplementary material. Optima for pollen germination are as low as 7°C and as high as 40°C. Optima for pollen tube growth rate range from 17 to 36°C. In many cases, the optima for the two processes are similar, but not necessarily coincidental. In all cases where natural flower or ambient temperatures are reported, they are lower, sometimes considerably, than the optima. While some species have optima near 25°C as in our study, it is not clear whether the natural floral temperatures correspond to them. It is clear, however, that more studies are necessary to determine whether the responses of pollen development to experimental temperatures correspond to the temperatures of flowers in nature.
Despite calls for ecophysiological studies on flower function (Delph et al. 1997), there are few on thermal niches for floral development in relation to natural variability in floral temperature. A working hypothesis is that a narrow range of pollen thermal tolerance would be associated with floral temperature stability, and vice versa. Our investigation shows that thermoregulating flowers indeed produce pollen with a narrow range of thermal tolerance, matched to the regulated temperature, so they may be termed ‘thermal specialists’. Molecular phylogenies of the Araceae show temperature-regulating, thermogenic genera (Philodendron, Symplocarpus) on both sides of a tree extending back over 100 million years (Nie et al. 2006). However, among the three species of Symplocarpus, S. foetidus and S. renifolius are both highly thermogenic and precise thermoregulators that bloom early in spring, while Symplocarpus nipponicus shows negligible thermogenesis and blooms in summer (R. S. Seymour & K. Ito 2004, unpublished data). Spadix temperatures of six S. nipponicus averaged 17.8°C, but ranged from 10.0 to 26.3°C over 6 days in June, 2004. The S. foetidus–S. renifolius group split from S. nipponicus some 3–11 million years ago (Nie et al. 2006). It is predicted that the pollen of S. nipponicus would have a broader thermal tolerance than the others.
Acknowledgments
The research was supported by the Australian Research Council (DP 0771854) and the Japan Society for the Promotion of Science, with a Grant-in-Aid for Exploratory Research (19658128) and with the twenty-first century COE programme. Robin Seymour provided technical assistance.
Footnotes
Dedicated to Mr Kaoru Ito.
References
- Boavida L.C., McCormick S.2007Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52, 570–582doi:10.1111/j.1365-313X.2007.03248.x [DOI] [PubMed] [Google Scholar]
- Dafni A.Pollen and stigma biology. In Pollination ecology: the practical approach ed. Dafni A.1992. pp. 59–89New York, NY:Oxford University Press [Google Scholar]
- Delph L.F., Johannsson M.H., Stephenson A.G.1997How environmental factors affect pollen performance: ecological and evolutionary perspectives. Ecology 78, 1632–1639doi:10.2307/2266087 [Google Scholar]
- Fægri K., van der Pijl L.The principles of pollination ecology. 1979Oxford, UK:Pergamon Press [Google Scholar]
- Ito K., Ito T., Onda Y., Uemura M.2004Temperature-triggered periodical thermogenic oscillations in skunk cabbage (Symplocarpus foetidus). Plant Cell Physiol 45, 257–264doi:10.1093/pcp/pch038 [DOI] [PubMed] [Google Scholar]
- Ito T., Ito K.2005Nonlinear dynamics of homeothermic temperature control in skunk cabbage, Symplocarpus foetidus. Phys. Rev. E 72, 051909.doi:10.1103/PhysRevE.72.051909 [DOI] [PubMed] [Google Scholar]
- Kinet J.-M., Sachs R.M., Bernier G.The development of flowers. In The physiology of flowering 1985Boca Raton, FL:CRC Press [Google Scholar]
- Knutson R.M.1974Heat production and temperature regulation in eastern skunk cabbage. Science 186, 746–747doi:10.1126/science.186.4165.746 [DOI] [PubMed] [Google Scholar]
- Knutson R.M.1979Plants in heat. Nat. Hist 88, 42–47 [Google Scholar]
- Meeuse B.J.D., Raskin I.1988Sexual reproduction in the arum lily family, with emphasis on thermogenicity. Sex. Plant Reprod 1, 3–15doi:10.1007/BF00227016 [Google Scholar]
- Nagy K.A., Odell D.K., Seymour R.S.1972Temperature regulation by the inflorescence of Philodendron. Science 178, 1195–1197doi: 10.1126/science.178.4066.1195 [DOI] [PubMed] [Google Scholar]
- Nie Z.-L., Sun H., Li H., Wen J.2006Intercontinental biogeography of subfamily Orontioideae (Symplocarpus, Lysichiton, and Orontium) of Araceae in eastern Asia and North America. Mol. Phylogenet. Evol 40, 155–165doi:10.1016/j.ympev.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Onda Y., et al. 2008Functional coexpression of the mitochondrial alternative oxidase and uncoupling protein underlies thermoregulation in the thermogenic florets of skunk cabbage. Plant Physiol 146, 636–645doi:10.1104/pp.107.113563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W.S.ImageJ.Bethesda, MA:US National Institutes of Health; 1997–2007 [Google Scholar]
- Seymour R.S.2004Dynamics and precision of thermoregulatory responses of eastern skunk cabbage Symplocarpus foetidus. Plant Cell Environ 27, 1014–1022doi:10.1111/j.1365-3040.2004.01206.x [Google Scholar]
- Seymour R.S., Blaylock A.J.1999Switching off the heater: influence of ambient temperature on thermoregulation by eastern skunk cabbage Symplocarpus foetidus. J. Exp. Bot 50, 1525–1532doi:10.1093/jexbot/50.338.1525 [Google Scholar]
- Seymour R.S., Schultze-Motel P.1997Heat-producing flowers. Endeavour 21, 125–129doi:10.1016/S0160-9327(97)80222-0 [Google Scholar]
- Seymour R.S., Schultze-Motel P.1998Physiological temperature regulation by flowers of the sacred lotus. Phil. Trans. R. Soc. Lond. B 353, 935–943doi:10.1098/rstb.1998.0258 [Google Scholar]
- Seymour R.S., White C.R., Gibernau M.2003Heat reward for insect pollinators. Nature 426, 243–244doi:10.1038/426243a [DOI] [PubMed] [Google Scholar]
- Shibata O.Altitudinal botany. 1985Tokyo, Japan:Uchida Rokakuho [Google Scholar]
- Taylor L.P., Hepler P.K.1997Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol 48, 461–491doi:10.1146/annurev.arplant.48.1.461 [DOI] [PubMed] [Google Scholar]
- Uemura S., Ohkawara K., Kudo G., Wada N., Higashi S.1993Heat-production and cross-pollination of the Asian skunk cabbage Symplocarpus renifolius (Araceae). Am. J. Bot 806, 635–640doi:10.2307/2445433 [Google Scholar]
- Weinbaum S.A., Parfitt D.E., Polito V.S.1984Differential cold sensitivity of pollen grain germination in two Prunus species. Euphytica 33, 419–426doi:10.1007/BF00021139 [Google Scholar]