Abstract
Recently, plant-derived methane (CH4) emission has been questioned because limited evidence of the chemical mechanism has been identified to account for the process. We conducted an experiment with four treatments (i.e. winter-grazed, natural alpine meadow; naturally restored alpine meadow eight years after cultivation; oat pasture and bare soil without roots) during the growing seasons of 2007 and 2008 to examine the question of CH4 emission by plant communities in the alpine meadow. Each treatment consumed CH4 in closed, opaque chambers in the field, but two types of alpine meadow vegetation reduced CH4 consumption compared with bare soil, whereas oat pasture increased consumption. This result could imply that meadow vegetation produces CH4. However, measurements of soil temperature and water content showed significant differences between vegetated and bare soil and appeared to explain differences in CH4 production between treatments. Our study strongly suggests that the apparent CH4 production by vegetation, when compared with bare soil in some previous studies, might represent differences in soil temperature and water-filled pore space and not the true vegetation sources of CH4.
Keywords: CH4 consumption by alpine ecosystem, CH4 emission by plant community, soil temperature, soil moisture, land-use change
1. Introduction
Recently, serious debates have focused on methane (CH4) emission by living plants and plant communities under aerobic conditions (Keppler et al. 2006, 2008; Butenhoff & Khalil 2007; Dueck et al. 2007; Beerling et al. 2008; Cao et al. 2008; Kirschbaum & Walcroft 2008; Wang et al. 2008). Although some field observations pointed to evidence of aerobic CH4 emission from plants (Cao et al. 2008), these studies did not consider the changes (i.e. soil moisture and soil temperature) caused by environmental treatments, which may control CH4 fluxes by regulating methanogenesis and oxidation processes (Pearce & Clymo 2001; Zhuang et al. 2007). Therefore, plant-derived CH4 emission is still questioned because limited evidence of the chemical mechanism has been identified to account for the process (Keppler et al. 2008; McLeod et al. 2008; Vigano et al. 2008; Messenger et al. 2009). Using a closed, opaque chamber technique, we conducted an experiment with four treatments during the growing seasons of 2007 and 2008 to examine the hypothesis that abiotic (i.e. soil moisture and soil temperature) rather than biotic (i.e. alpine vegetation) factors resulted in the difference in CH4 consumption between plots with vegetation and plots with bare soil.
2. Material and methods
The study site was the same as the alpine Kobresia meadow studied by Cao et al. (2008) during the growing seasons of 2007 and 2008. A completely randomized design was employed, with four replicate plots of each of four treatments as follows: (i) native, natural alpine meadow; (ii) naturally restored alpine meadow eight years after cultivation in the 1960s; (iii) bare soil with roots removed from 0–20 cm soil depth in May 2007; and (iv) annual oat sown with 600 kg seeds per hectare in mid-June 2007 and by the end of May 2008. Each plot (4 × 4.5 m) was separated by a 2 m buffer zone. The total rainfall was 352 and 290 mm from June to September in 2007 and 2008, respectively.
Fluxes of CH4 were measured weekly inside opaque, static, stainless steel chambers using the methods of flux calculation described by Ma et al. (2006). CH4 concentrations of gas samples were analysed by gas chromatography (HP Series 4890D, Hewlett Packard, USA) within 24 h. The fluxes of CH4 between 9.00 a.m. and 11.00 a.m. local time were used to represent 1 day's average flux as described by Cao et al. (2008).
During each gas-sampling occasion in 2007, soil temperature was measured using digital thermometers in situ at 5 cm depth in all plots. The volumetric soil moisture (%) at 5 cm depth was measured during both 2007 and 2008 using time domain reflectometry (CS615) for each plot to calculate the water-filled pore space (WFPS):
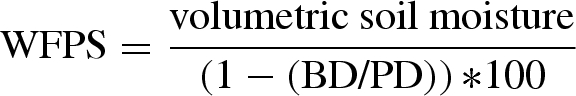 |
where BD is the soil bulk density and PD is the soil particle density.
A general linear model repeated-measures define factors procedure (SPSS 12.0, SPSS Inc., Chicago, IL, USA) was used to assess the significance of the impacts of experimental year, sampling day, treatment and their interaction on soil water content, soil temperature and CH4 fluxes, treating the experimental year and sampling day as within-subject variables within similar sampling dates for two years. For each measured variable, the significant difference between treatments was assessed by one-way ANOVA and least significant difference. Pearson's correlations were calculated between soil temperature and WFPS and CH4 fluxes. All significances mentioned in the text were at 0.05 level.
3. Results
(a). Environmental changes
Soil WFPS was affected significantly by treatment, sampling date, year and their interaction (table 1). The average soil water content for both years in the bare soil plots (31.8 ± 1.0%) was significantly lower (by approx. 24%) than in the native, natural alpine meadow and naturally restored alpine meadow plots, whereas higher (by 11%) than in the oat plots. The average soil temperature at 5 cm soil depth was not significantly different between all treatments during the study period in 2007 (data not shown).
Table 1.
Soil WFPS and methane (CH4) consumption rates from repeated-measures ANOVA using year and sampling date as repeated measures (between-subjects).
| WFPS |
CH4 consumption rate |
|||||
|---|---|---|---|---|---|---|
| model | MS | F | p | MS | F | p |
| treatment (T) | 1381.57 | 34.42 | <0.001 | 2808.65 | 2.431 | 0.14 |
| year (Y) | 10 456.5 | 467.49 | <0.001 | 340.58 | 0.426 | 0.532 |
| Y × T | 297.64 | 13.307 | 0.002 | 231.23 | 0.289 | 0.832 |
| date (D) | 1076.46 | 106.33 | <0.001 | 4876.28 | 4.491 | <0.001 |
| D × T | 24 | 2.371 | 0.009 | 1066.9 | 0.983 | 0.502 |
| Y × D | 557.82 | 47.421 | <0.001 | 1730.15 | 2.011 | 0.05 |
| Y × D × T | 13.89 | 1.18 | 0.314 | 1353.48 | 1.573 | 0.066 |
(b). Methane consumption rate
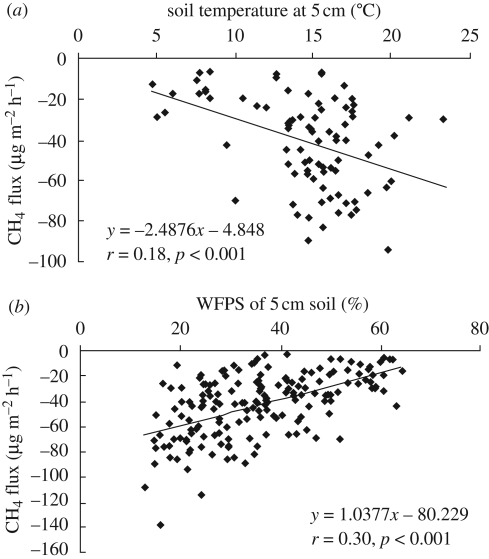
The effect of different land-use (treatment effect) on CH4 consumption rate for every sample date was not significant for either year (table 1). However, multiple comparison analysis between mean values for each sampling period showed significant differences in CH4 consumption rate between native, natural alpine meadow and oat plots in 2007, and between native, natural alpine meadow and oat and bare soil plots in 2008 (electronic supplementary material). CH4 consumption rate in 2007 increased with an increase in soil temperature at 5 cm depth (figure 1a). However, increasing WFPS significantly decreased CH4 consumption (figure 1b).
Figure 1.
Relationships between methane (CH4) consumption rate and (a) soil temperature in 2007 and (b) soil WFPS for both years
(c). Methane emission by alpine communities
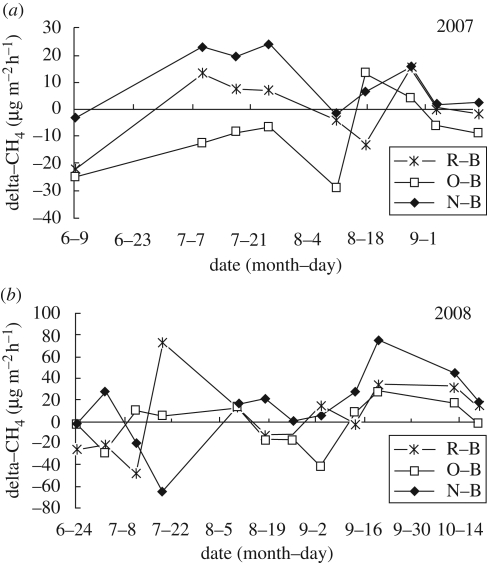
Our study showed that bare soil plots were a net sink for atmospheric CH4, with an average of approximately 40.7 µg CH4 m−2 h−1 (range: −14.8 ∼ −63.7) in 2007 and 52.5 µg CH4 m−2 h−1 (−17 ∼ −79) in 2008 during the study periods. Our calculation assumed that the CH4 emission rate by plant communities was the difference between the plots treated with vegetation and bare soils (Cao et al. 2008) (which we have called apparent emission rate by plants). The average apparent CH4 emission rates were 15.0 µg CH4 m−2 h−1 (9.9∼20.2) and 5.1 µg CH4 m−2 h−1 (0.3∼9.9), with great seasonal variations of −65.0∼75.0 µg CH4 m−2 h−1 in 2007 and −48.0∼72.0 µg CH4 m−2 h−1 in 2008 for the native, natural alpine meadow community and the naturally restored alpine meadow community, respectively. In contrast, annual oat vegetation apparently consumed atmospheric methane at an average rate of 4.8 µg CH4 m−2 h−1 (−0.8 to −8.7 µg CH4 m−2 h−1), with great seasonal variations of −5.8∼13.4 µg CH4 m−2 h−1 in 2007 and −3.0∼27.0 µg CH4 m−2 h−1 in 2008 (figure 2).
Figure 2.
Dynamics of difference (Delta) of methane (CH4) emission between the treatments with vegetation and bare soil during the study periods in (a) 2007 and (b) 2008. R-B, ‘naturally restored alpine meadow for eight years after cultivation in 1960s’ minus ‘bare soil’; O-B, ‘annual oat’ minus bare soil; N-B, ‘native, natural alpine meadow’ minus bare soil.
4. Discussion
(a). Methane emissions by plants
Our results appear to indicate possible CH4 production by alpine meadow vegetation because the two types of meadow vegetation reduced CH4 consumption compared with bare soil. In contrast, the oat pasture increased CH4 consumption compared with bare soil. These results seem to support the apparent conclusion that the intact Kobresia meadow emitted CH4 as reported by Cao et al. (2008). However, the response of CH4 consumption is very sensitive to changes in soil moisture and temperature in the field. In our study, we calculated a regression equation between CH4 consumption rate and soil WFPS and soil temperature in 2007, which was:
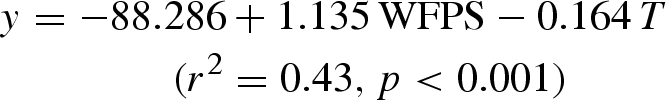 |
where T is the soil temperature at 5 cm depth. Thus, native, natural meadow plots could consume more CH4 (approx. 11.8 µg CH4 m−2 h−1) through calibration based on the same soil WFPS and temperature as bare soil plots. This effect would explain the original difference of the CH4 consumption rate between native, natural alpine meadow (−30.8 µg CH4 m−2 h−1) and bare soil (−40.7 µg CH4 m−2 h−1) in 2007. Similar results were observed in 2008 and for other treatments in our study. Therefore, our results strongly suggest that the apparent CH4 production by vegetation, when calculated in comparison with bare soil in some previous studies, might represent differences in soil temperature and WFPS and not the true vegetation sources of CH4.
(b). Effect of soil temperature and moisture on CH4 consumption
Removal of roots from soil often alters its physico-chemical characteristics, which are of critical importance for CH4 uptake (Smith et al. 2000). Soil temperature measured on nine occasions in 2007 varied between 7 and 24°C and was linearly correlated with soil WFPS (r = −0.45, p < 0.01) in all treatments. Our study showed a clear positive relationship between CH4 consumption rate and soil temperature for all treatments (figure 2a). Probably, when fewer soil pores are water-filled, more atmospheric CH4 could diffuse into the soil and reach methanotrophic micro-organisms, which might respond positively to the temperature increase (Pearce & Clymo 2001; Zhuang et al. 2007).
In our study, CH4 consumption appeared to increase linearly with decreases in soil moisture (figure 2b). We found that the response of CH4 consumption to soil moisture was greater in 2008 than in 2007 (the slopes of the regression equations between CH4 consumption rate and WFPS were 1.04 and 2.27 in 2007 and 2008, respectively). This may have resulted from more drought in 2008 than in 2007, which would limit the diffusive transport of methane through the soil gas phase when soil moisture is high (King 1997; Castaldi & Fierro 2005). In these soils, where gas diffusion represents the main controlling factor of CH4 oxidation, soil water content is of critical importance in determining the potential of the ecosystem to be a CH4 sink (Striegl 1993).
(c). Effect of solar radiation on CH4 emission
Many studies show that solar radiation stimulates some CH4 emission from plant foliage (Keppler et al. 2006, 2008; McLeod et al. 2008; Vigano et al. 2008; Messenger et al. 2009), whereas the study of Cao et al. (2008) reported methane emissions from whole plants in plots when compared with bare soil. Our method showed, however, that the CH4 production was different from that reported by Cao et al. (2008). In this study, we observed a new explanation for the apparent methane emissions reported by Cao et al. (2008). We used closed, opaque chambers (i.e. without solar radiation available to plants), whereas Cao et al. (2008) used transparent chambers shaded with white plastic (i.e. with some solar radiation available to the plants). The difference between our results and those of Cao et al. (2008) may partially derive from the different experimental methods used to assess CH4 emissions. However, we also detected that apparent CH4 emissions may arise from treatments owing to changes in soil temperature and WFPS and do not represent a true vegetation source.
Therefore, the question of aerobic methane production from vegetation in the Qinghai-Tibetan Plateau still remains open. Further studies should evaluate the effects of soil conditions on CH4 emission by plant communities and the role of solar radiation, which was excluded from our study of the alpine meadow.
Acknowledgements
This research was funded by the Knowledge Innovation Programs (KZCX2-XB2-06-01, KSCX2-YW-N-040) and the ‘100-Talent Program’ of Chinese Academy of Sciences and Chinese National Natural Science Foundation Commission (30871824).
References
- Beerling D. J., Gradiner T., Leggett G., McLeod A., Quick W. P.2008Missing methane emissions from leaves of terrestrial plants. Global Change Biol. 14, 1–6 (doi:10.1111/j.1365-2486.2008.01613.x) [Google Scholar]
- Butenhoff G. L., Khalil M. A. K.2007Global methane emissions from terrestrial plants. Environ. Sci. Technol. 41, 4032–4037 (doi:10.1021/es062404i) [DOI] [PubMed] [Google Scholar]
- Cao G. M., Xu X. L., Long R. J., Wang Q. L., Wang C. T., Du Y. G., Zhao X. Q.2008Methane emissions by alpine plant communities in the Qinghai-Tibet Plateau. Biol. Lett. 4, 681–684 (doi:10.1098/rsbl.2008.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldi S., Fierro A.2005Soil–atmosphere methane exchange in undisturbed and burned Mediterranean shrubland of Southern Italy. Ecosystem 8, 182–190 (doi:10.1007/s10021-004-0093-z) [Google Scholar]
- Dueck T. A., et al. 2007No evidence for substantial aerobic methane emission by terrestrial plants: a 13C-labelling approach. New Phytol. 175, 29–35 (doi:10.1111/j.1469-8137.2007.02103.x) [DOI] [PubMed] [Google Scholar]
- Keppler F., Hamilton T. G., Braβ M., Röckmann T.2006Methane emissions from terrestrial plants under aerobic conditions. Nature 439, 187–191 (doi:10.1038/nature04415) [DOI] [PubMed] [Google Scholar]
- Keppler F., Hamilton T. G., McRoberts W. C., Vigano I., Braβ M., Röckmann T.2008Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytol. 178, 808–814 (doi:10.1111/j.1469-8137.2008.02411.x) [DOI] [PubMed] [Google Scholar]
- King G. M.1997Responses of atmospheric methane consumption by soils to global climate change. Global Change Biol. 3, 351–362 (doi:10.1046/j.1365-2486.1997.00090.x) [Google Scholar]
- Kirschbaum M. U. F., Walcroft A.2008No detected aerobic methane efflux from plant material, nor from adsorption/desorption processes. Biogeosci. Discuss. 5, 2773–2794 [Google Scholar]
- Ma X. Z., Wang S. P., Wang Y. F., Jiang G. M., Nyren P.2006Short-term effects of sheep excreta on carbon dioxide, nitrous oxide and methane fluxes in typical grassland of Inner Mongolia. N. Z. J. Agric. Res. 49, 285–297 [Google Scholar]
- McLeod A. R., Fry S. C., Loake G. J., Messenger D. J., Reay D. S., Smith K. A., Yun W.2008Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 180, 124–132 (doi:10.1111/j.1469-8137.2008.02571.x) [DOI] [PubMed] [Google Scholar]
- Messenger D. J., McLeod A. R., Fry S. C.2009The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 32, 1–9 (doi:10.1111/j.1365-3040.2008.01892.x) [DOI] [PubMed] [Google Scholar]
- Pearce D. M. E., Clymo R. S.2001Methane oxidation in a peatland core. Global Biogeochem. Cycles 15, 709–720 [Google Scholar]
- Smith C.K., Coyea M. R., Munson A. D.2000Soil carbon, nitrogen, and phosphorus stocks and dynamics under disturbed black spruce forests. Ecol. Appl. 10, 775–788 (doi:10.1890/1051-0761(2000)010[0775:SCNAPS]2.0.CO;2) [Google Scholar]
- Striegl R. G.1993Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere 26, 715–720 [Google Scholar]
- Vigano I., Weelden H. V., Holzinger R., Keppler F., McLeod A., Röckmann T.2008Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeoscience 5, 937–947 [Google Scholar]
- Wang Z. P., Han X. G., Wang G. G., Song Y., Gulledge J.2008Aerobic methane emission from plants in the Inner Mongolia steppe. Environ. Sci. Technol. 42, 62–68 (doi:10.1021/es071224l) [DOI] [PubMed] [Google Scholar]
- Zhuang Q., Melillo J. M., McGuire A. D., Kicklighter D. W., Prinn R. G., Steudler P. A., Felzer B. S., Hu S.2007Net emission of CH4 and CO2 in Alaska: implications for the region's greenhouse gas budget. Ecol. Appl. 17, 203–212 (doi:10.1890/1051-0761(2007)017[0203:NEOCAC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]