Abstract
Nest-mate recognition is fundamental for protecting social insect colonies from intrusion threats such as predators or social parasites. The aggression of resident females towards intruders is mediated by their cuticular semiochemicals. A positive relation between the amount of cues and responses has been widely assumed and often taken for granted, even though direct tests have not been carried out. This hypothesis has important consequences, since it is the basis for the chemical insignificance strategy, the most common explanation for the reduction in the amount of semiochemicals occurring in many social parasites. Here we used the social wasp Polistes dominulus, a model species in animal communication studies and host of three social parasites, to test this hypothesis. We discovered that different amounts of cuticular hydrocarbons (CHC) of a foreign female evoke quantitatively different behavioural reactions in the resident foundress. The relation between CHC quantity and the elicited response supports the idea that a threshold exists in the chemical recognition system of this species. The chemical insignificance hypothesis thus holds in a host–parasite system of Polistes wasps, even though other explanations should not be discarded.
Keywords: nest-mate recognition, social parasitism, chemical communication, social wasps
1. Introduction
Social insect colonies are highly defended fortresses. The great investment in time and resources they represent have posed the challenge of defending this treasure properly. Besides heterospecific predators and parasites, a major threat is usually represented by homospecific intruders, who get into the nest looking for an available ‘source of protein and sugar’ or for a well-developed colony to exploit. Colonies are thus usually extremely xenophobic, heavily rejecting non-nest-mates. Behavioural and chemical studies have provided overwhelming evidence that nest-mate–non-nest-mate discrimination mainly depends on a chemical recognition system (Vander Meer et al. 1998). The chemical cues involved are basically the cuticular lipids, mainly composed of linear or branched long-chained hydrocarbons (Lockey 1988). The colonial-specific cuticular profile is acquired by newly emerged individuals in the first hours or days of life, and it constitutes an internal template, which will be compared with the odour of any encountered animal (Lenoir et al. 1999). If the two profiles match, the individual will be accepted; otherwise it will be aggressively rejected (Vander Meer et al. 1998).
The amount of cues clearly plays a fundamental role in the discrimination process. Individuals having a low amount of cues (newly emerged individuals or individuals experimentally deprived of the lipid layer with a solvent) evoke no aggressive response, so that it is possible for ‘callows’ to get accepted in foreign colonies (Lenoir et al. 2001). This mechanism may represent a pitfall in the recognition system: intruders could have poor chemical profiles to decrease the chance of being recognized and attacked, a strategy which seems to be used by many social parasites. These species penetrate the nests of social insects and exploit them (Wilson 1971). Bearing a small amount of semiochemicals when approaching the host colony could help in fooling the host recognition system, in not being recognized or, at least, in reducing the evoked aggression (the ‘chemical insignificance’ hypothesis; Lenoir et al. 1999). Indeed, many social parasites show poor chemical profiles at the moment of usurpation, and chemical insignificance represents the most used explanation for this semiochemical's reduction (Lenoir et al. 2001; Lorenzi & Bagnères 2002; Lorenzi et al. 2004). However, the decrease in aggression in response to a reduction in the chemical cues' quantity on the intruder's body has not been directly tested, as far as we know, in any social insects. The reduction in cues could also have other explanations as, for example, to facilitate the acquisition of host-specific compounds by social parasites (Lorenzi et al. 2004) in the widely occurring process of chemical resemblance (Howard & Blomquist 2005).
For a chemical insignificance strategy to be useful, the host response should decrease with the reduction in the parasite cuticular hydrocarbon (CHC) amount. Here we use the social wasp Polistes dominulus to test this hypothesis. We conducted behavioural essays presenting increasing fractions of a foreign wasp's chemical profile to foundresses in the workers' pre-emergence period. We expected an increased aggressive response as the amount of cues increased.
2. Material and methods
(a). The biological model
Polistes dominulus colonies are founded in spring by one or several overwintering mated females and colonies are highly aggressive towards intruders (Dani et al. 1996). Polistes dominulus is a widely used biological model in sociobiological and chemical communication studies (Starks & Turillazzi 2006), and it is the host of three species of congeneric social parasites (Cervo 2006), some of which seem to use the chemical insignificance strategy to increase their success in usurpation (Lorenzi 2006).
(b). Collection and rearing of animals
Twenty-two single-foundress pre-emergence colonies were collected in spring in the surroundings of Florence, from four different populations. Foundresses and nests were transferred to the laboratory and reared from cubic glass cages of 15 cm in each dimension. Water, sugar and fly maggots were provided ad libitum. Twenty-two foraging foundresses were collected at the same time and in the same places as the colonies. They were killed by freezing and their cuticular compounds were used to prepare lures (discussed subsequently).
(c). Lure preparation
We obtained 22 extracts of P. dominulus foundresses by washing each wasp in 300 µl of pentane for 15 min. Extracts were thendried at room temperature and resuspended in 200 µl of pentane to obtain higher concentrations. Lures were obtained by placing different amounts of the extract of the same wasp on a square piece of clean filter paper (rinsed with the solvent) ofapproximately 0.8 cm side. The first lure (hereafter called ‘one-third lure’) was prepared by putting one-third (approx. 67 µl) of the total extract on the filter paper. After the behavioural session, the lure was covered with another third of the extract toobtain the ‘two-third lure’. Finally, after the following behavioural session, the paper was covered with the last third of theextract to obtain the ‘total extract lure’. Preliminary GC–MS analyses have shown that this protocol effectively provides fractions with the expected amount of CHC without altering the chemical profile. The control lure was obtained by using the solvent only.
(d). Behavioural essays
The lure was set on a long stick and was slowly brought closer to the nest. It was held at 1 cm distance from the nest for 1 min after the first interaction of the foundress with the lure. Four presentations, with a 60 min interval between them, were made in the following order: blank lure, one-third lure, two-third lure and total extract lure. Tests were carried out when colonies had at least one larva and one pupa. Essays were made between 11:00 and 17:00, and wasp behaviour was video-recorded. Since we did not expect a ‘piece of paper’ lure without the physical presence of the intruder to be sufficient to evoke very aggressive responses, we calculated an aggressive response index by summing the total time spent in biting, climbing on and antennating the lure during the first 60 s of presentation. Data were analysed with SPSS 15.0.
Since it was impossible to subtract CHC from the piece of paper to perform essays in a random order, we performed the further control experiment to check for possible sensibilization or habituation effects on the wasp's response. We made behavioural essays using the same lure for the four presentations without changing the cues' quantity (a one-third fraction of the total extract of a foreign wasp, N = 15 colonies). This experiment also allowed us to check for a possible effect of interactions between foundresses and lures, which are often bitten.
In order to avoid high relatedness between ‘resident females’ and ‘intruders’, and be confident that they had not previously interacted, we coupled each resident female with an extract obtained by a parasympatric wasp.
3. Results
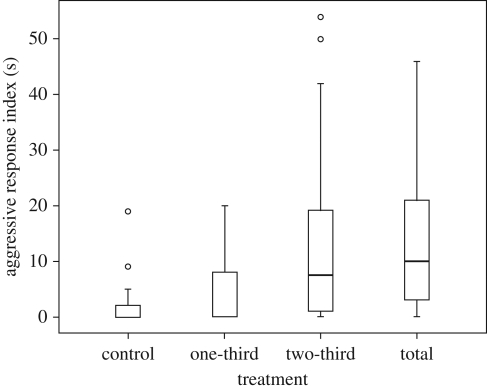
Foundresses increased their response according to theincrease in the CHC amount. The time spent in the aggressive response differed between the treatments (Friedman test, χ2 = 14.18, d.f. = 3, p = 0.003, N = 22 colonies; see figure 1). The full concentration elicited a significantly stronger response than the one-third concentration. The one-third extract did not elicit more aggressive responses than the control, while the two-third and the full amount extracts did. There was no difference in the responses evoked by the one-third and two-third extracts (see table 1 for post hoc results). Repeated presentation per se did not influence the aggressive response, i.e. the response did not change if wasps were tested four consecutive times with the same amount of CHC each time (mean ± s.d.: 5.4 ± 9.60, 9.13 ± 19.63, 8.87 ± 10.33, 9.73 ± 15.35 s; Friedman test, χ2 = 1.856, d.f. = 3, p = 0.603, N = 15 colonies).
Figure 1.
Foundresses' behavioural responses towards the presentation of stimuli with different amounts of a total foreign foundress' CHC quantity. Foundresses (N = 22) increase their responses according to the increase in CHC amount. Time spent in the behavioural responses differs between the treatments. The full concentration elicited significantly stronger responses than the one-third concentration. The one-third extract did not elicit more aggressive responses than the control, while the two-third and the full amount extracts did. Box plots represent the medians (thick horizontal lines), the interquartile range (boxes), the top and lowest quartiles (horizontal lines) and the outliers (circles).
Table 1.
Results of Wilcoxon signed-rank test, Monte Carlo method (Z- and p-values), testing for differences in the aggressive responses of foundresses towards presentation of stimuli with different amounts of foreign CHC (d.f. = 3, N = 22). (Bold numbers refer to significant p-values.)
| pair | Z-value | p-value |
|---|---|---|
| control versus one-third | −1.469 | 0.151 |
| control versus two-third | −2.881 | 0.003 |
| control versus total | −3.322 | <0.001 |
| one-third versus two-third | −1.815 | 0.058 |
| one-third versus total | −2.653 | 0.006 |
| two-third versus total | −0.430 | 0.680 |
4. Discussion
Our results show that different amounts of cuticular lipids of a foreign female evoke different aggressive responses in the resident foundresses in the social wasp P. dominulus. The full amount elicited significantly stronger responses than the one-third fraction and the blank control, while a fraction smaller than the full amount (two-thirds of the total) is treated as the full amount and small quantities (one-third of the total) evoke the same responses as the blank control. Our findings thus support the idea of a threshold mechanism in the chemical recognition system, with discrimination occurring only above a certain amount of cues.
Our results show a great variation in the individual response levels to the same cues' amounts. Several factors could account for these differences, from heterogeneity in chemical stimuli to individual differences in perceptive abilities or aggressiveness. We believe that the threshold could be plastic and dependent on colonial and population features in addition to individual-level peculiarities. Nest-mate recognition is indeed strongly dependent on the context in P. dominulus (Starks et al. 1998). Future work would be useful to evaluate the consequences of this great variability such as differences in susceptibility to social parasitism among colonies.
We believe that these results could be important in the study of social parasitism in Hymenoptera. Our study provides the first direct evidence that the chemical recognition system of a host species depends on the semiochemicals' amounts. Under a certain threshold (around one-third of the total cuticular amount in this study), non-nest-mate rejection seems to be impaired. Bearing-reduced cue quantities of CHC when approaching the colony could hence be really advantageous for the parasites. Indeed, this is what the social parasites of our model species do. Polistes atrimandibularis and P. semenowi show a reduction in the amount of their chemical profile during the usurpation period, when they show reduced amounts of CHC compared with their hosts P. biglumis and P. dominulus (Lorenzi & Bagnères 2002; Lorenzi et al. 2004). The presence of a threshold implies that the chemical insignificance strategy could be advantageous only if the reduction decreases the chemical profile under the threshold. This is indeed what P. semenowi seems to do: usurping parasites possess only 40 per cent of the amount of CHC of their P. dominulus hosts (Lorenzi et al. 2004).
Our study expands knowledge on the chemical recognition system of a model species in animal communication studies, deepening the knowledge of the quantitative aspects of chemical recognition—an often neglected approach, if compared with the qualitative ones. The results are moreover interesting for the study of social parasitism, a very widespread phenomenon which evolved multiple times in social insects as well as in birds, fishes and mammals (Cervo 2006).
Acknowledgements
We thank Claudia Bruschini for useful suggestions during theexperimental work, David Baracchi, Leonardo Dapporto, Maria Cristina Lorenzi, Stefano Turillazzi and two anonymous referees for their helpful comments on the manuscript and Carlotta Cini for linguistic revision. Financial support was provided by the University of Florence.
References
- Cervo R.2006Polistes wasps and their social parasites: an overview. Ann. Zool. Fennici 43, 531–549 [Google Scholar]
- Dani F. R., Fratini S., Turillazzi S.1996Behavioural evidence for the involvement of Dufour's gland secretion in nestmate recognition in the social wasp Polistes dominulus (Hymenoptera: Vespidea). Behav. Ecol. Sociobiol. 38, 311–319 (doi:10.1007/s002650050247) [Google Scholar]
- Howard R. W., Blomquist G. J.2005Ecological, behavioral and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393 (doi:10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- Lenoir A., Fresneau D., Errard C., Hefetz A.1999Theindividuality and the colonial identity in ants: theemergence of the social representation concept. InInformation processing in social insects (eds Detrain C., Deneubourg J. L., Pasteels J.), pp. 219–237 Basel: Birkhäuser Verlag [Google Scholar]
- Lenoir A., D'Ettorre P., Errard C., Hefetz A.2001Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599 (doi:10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- Lockey K. H.1988Lipids of the insect cuticle: origin, composition and function. Comp. Biochem. Physiol. 89B, 595–645 (doi:10.1016/0305-0491(88)90305-7) [Google Scholar]
- Lorenzi M. C.2006The result of an arms race: the chemical strategies of Polistes social parasites. Ann. Zool. Fennici 43, 550–563 [Google Scholar]
- Lorenzi M. C., Bagnères A. G.2002Concealing identity and mimicking hosts: a dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 125, 507–512 (doi:10.1017/S003118200200238X) [DOI] [PubMed] [Google Scholar]
- Lorenzi M. C., Cervo R., Zacchi F., Turillazzi S., Bagnères A. G.2004Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera: Vespidae). Parasitology 129, 643–651 (doi:10.1017/S0031182004005992) [DOI] [PubMed] [Google Scholar]
- Starks P. T., Turillazzi S.2006Polistes paper wasps: emergence of a model genus. Ann. Zool. Fennici 43, 385–386 [Google Scholar]
- Starks P. T., Fisher D. J., Watson R. E., Melikian G. L., Nath S. D.1998Context-dependent nestmate discrimination in the paper wasp, Polistes dominulus: a critical test of the optimal acceptance threshold model. Anim. Behav. 56, 449–458 (doi:10.1006/anbe.1998.0778) [DOI] [PubMed] [Google Scholar]
- Vander Meer R. K., Breed M. D., Winston M. L., Espelie K. E. (eds) 1998Pheromone communication in social insects: ants, wasps, bees and termites Boulder, Colorado: Westview [Google Scholar]
- Wilson O. E.1971The insect societies Cambridge: Harvard University Press [Google Scholar]