Abstract
Seahorses (Syngnathidae: Hippocampus) are iconic marine teleosts that are readily identifiable by their upright posture. The fossil record is inadequate to shed light on the evolution of this trait because it lacks transitional forms. There are, however, extant syngnathid species (the pygmy pipehorses) that look like horizontally swimming seahorses and that might represent a surviving evolutionary link between the benthic seahorses and other, free-swimming members of the family Syngnathidae. Using sequence data from five nuclear loci, we confirm the sister taxon relationship between seahorses and pygmy pipehorses. Molecular dating indicates that the two taxa diverged during the Late Oligocene. During this time, tectonic events in the Indo-West Pacific resulted in the formation of vast amounts of new shallow-water areas and associated expansion of seagrass habitats that would have favoured the seahorses’ upright posture by improving their camouflage while not affecting their manoeuvrability negatively. The molecular techniques employed here provide new insights into the evolution of a taxon whose fossil record is incomplete, but whose evolutionary history is so recent that the major stages of morphological evolution are still represented in extant species.
Keywords: pygmy pipehorse, Idiotropiscis, evolutionary link, nuclear DNA phylogeny, relaxed molecular clock, biogeography
1. Introduction
Seahorses (Syngnathidae: Hippocampus) today are common throughout the world's tropical, subtropical and temperate marine regions (Kuiter 2000), but fossil seahorses are exceptionally rare. The genus is represented at only two well-documented sites, namely Tunjice in Slovenia (Middle Miocene, approx. 13 Myr; Žalohar et al. 2009) and Marecchia in Italy (Late Pliocene, approx. 3 Myr; Sorbini 1988). In both cases, the fossil seahorses are considered to be morphologically similar to certain extant species rather than being primitive transitional forms, suggesting that seahorses must have evolved earlier.
The genus Hippocampus is one of four genera in the subfamily Hippocampinae, which also includes three genera of pygmy pipehorses (Acentronura, Amphelikturus and Idiotropiscis) that some authors treat as a single genus (Kuiter 2004). Pygmy pipehorses are morphologically very similar to seahorses, but all lack the upright posture. This suggests that they could be a surviving evolutionary link between seahorses and the remaining members of the family Syngnathidae, all of which have a horizontal posture. The species of the temperate Australian pygmy pipehorse genus Idiotropiscis are by far the most seahorse-like in appearance (Kuiter 2004), and the time when these shared a common ancestor with the seahorses is therefore likely to be close to the time when the seahorses’ upright posture evolved. An Australasian origin of seahorses is supported by the fact that the most basal and second most basal Hippocampus lineages occur in the Indo-West Pacific and in Australia, respectively (Teske et al. 2004).
Central to understanding why the seahorses’ upright posture was favoured by natural selection is to determine what environmental conditions prevailed in Australasia during the time when seahorses and pygmy pipehorses diverged from their common ancestor. To this end, we reconstructed phylogenetic relationships among species of Hippocampus and Idiotropiscis, determined their placement among six other syngnathid genera representing the major evolutionary lineages of the family Syngnathidae (Wilson et al. 2003) and dated the split between seahorses and pygmy pipehorses using a relaxed molecular clock.
2. Material and methods
(a). Phylogeny reconstruction
Phylogenetic relationships among seahorses and other syngnathids were reconstructed using a dataset 3386 nucleotides in length that comprised four nuclear genes (RAG1, myh6, Rhodopsin and Tmo4c4) and a fifth nuclear marker (a region spanning intron 1 of the S7 ribosomal protein) that comprised elements of both exon and intron regions. Details about primers, PCR conditions and sequence alignments, as well as museum collection numbers and GenBank accession numbers, are listed in the electronic supplementary material.
Phylogenetic trees were constructed using maximum likelihood (Treefinder; Jobb et al. 2004), parsimony (Mega 4; Tamura et al. 2007) and Bayesian inference (MrBayes 3.1; Ronquist & Huelsenbeck 2003). For the first two methods, support for nodes was assessed by generating 10 000 bootstrap replications. The Bayesian inference was carried out by running four chains simultaneously for 3 million generations and discarding the first 10 per cent of trees as burn-in. Posterior probabilities of nodes were assessed by constructing a 50 per cent majority rule consensus tree. To check for consistency of results, the analyses were repeated three times. For both maximum likelihood analysis and Bayesian inference, the dataset was divided into four partitions: codon positions 1–3 of the nuclear genes, as well as S7. Rates were allowed to vary among partitions, and the GTR + I+ G model was specified for each.
(b). Molecular dating
Molecular dating was performed using a Bayesian method (Beast 1.4.8; Drummond & Rambaut 2007; see electronic supplementary material for methodological details). Two to three calibration points were specified. In each case, a normal prior was used, and its mean and standard deviations were set in such a way that 95 per cent confidence intervals corresponded to the upper and lower bounds of each calibration point. In this way, uncertainty concerning the exact dates of the calibration points could be accounted for. The age of the oldest syngnathid fossils (Monte Bolca formation, Early Eocene) (Patterson 1993; Bellwood 1996) was used as the first calibration point. These fossils date from the boundary between the Ypresian and Lutetian ages (approx. 48–50 Myr). To account for the possibility that they are younger than the origin of the family, we specified the beginning of the Eocene as an upper bound (mean = 52.2, s.d. = 2.3, 95% confidence interval: 48–56). The other two calibration points were based on genetic evidence for divergence events in a seahorse phylogeny that resulted from the formation of land bridges that separated formerly continuous marine habitats (Teske et al. 2007). The best documented of these is the closure of the Central American Seaway during the Late Pliocene, which resulted in the divergence of Hippocampus reidi (West Atlantic) and Hippocampus ingens (East Pacific) from a common ancestor (Teske et al. 2007). The final closure of this seaway occurred approximately 3.1–3.7 Myr (Duque-Caro 1990), but to account for the possibility that these two seahorse species diverged prior to this date as a result of ocean current reorganization in the region (approx. 4.6 Myr; Haug & Tiedemann 1998), we specified the older date as an upper bound (mean = 3.85, s.d. = 0.45, 95% confidence interval: 3.1–4.6). The date of the third calibration point, the closure of the Tethyan Seaway that once connected the Atlantic Ocean with the Indian Ocean, is comparatively vague because there were several phases of closing and reopening prior to complete closure. Previous analyses indicate that both Late Early Miocene (Adams et al. 1983) and Middle Miocene (Rögl & Steininger 1983) closures may have resulted in divergence of seahorse lineages (Teske et al. 2007). We therefore allowed for a wide calibration range that included both these dates (mean = 15.85, s.d.= 2.85, 95% confidence interval: 11.2–20.5), but also dated the syngnathid phylogeny without this calibration point.
3. Results
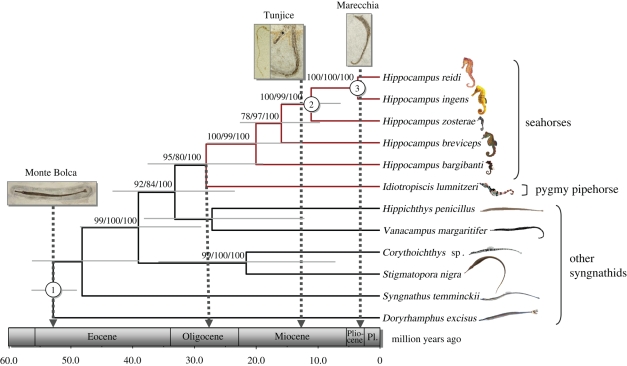
Phylogenetic reconstruction confirmed the sister taxon relationship between the seahorses and the pygmy pipehorse genus Idiotropiscis (figure 1). A divergence time estimate of 28 Myr (three calibration points; s.d.= 0.4, 95% confidence interval: 18–37 Myr; figure 1) or 25 Myr (two calibration points; s.d. = 0.25 Myr; 95% confidence interval: 16–34 Myr) indicates that the seahorses’ upright posture most likely evolved during the Late Oligocene (confidence intervals range from the Early Oligocene to the Early Miocene). This estimate considerably predates the age of the oldest known seahorse fossils.
Figure 1.
A chronogram scaled to geological time reconstructed from DNA sequence data of seahorses, and other species representing the major genetic lineages of the family Syngnathidae. Nodal support is indicated as bootstrap values from maximum likelihood analysis, bootstrap values from parsimony analysis and posterior probabilities from Bayesian inference. Numbers in white circles indicate nodes used as calibration points and include the age of the oldest syngnathid fossils (1) and the closures of the Tethyan and Central American seaways (2 and 3). Light grey bars are 95 per cent highest posterior density intervals of estimated node ages. Grey arrows indicate the age of the oldest syngnathid fossils, the ages of the only two well-documented fossil sites that contain seahorses and the time when seahorses and pygmy pipehorses diverged from their common ancestor (Pl. = Pleistocene).
4. Discussion
A Late Oligocene estimate of the divergence of seahorses and the endemic Australian pygmy pipehorse genus Idiotropiscis indicates that the evolution of the seahorses’ upright posture was likely to have benefitted from geological changes that occurred in the Indo-West Pacific during this time. Following climatic cooling and lowering of sea levels during the Early Oligocene approximately 34 Myr (Miller et al. 2008), tectonic events in the Indo-West Pacific (the most important one being the collision of Australia/New Guinea with the Eurasian plate; Hall 1998) during the Late Oligocene and Early Miocene (approx. 25–20 Myr) resulted in the formation of vast areas of shallow-water habitat between Australia and Indonesia (Wilson & Rosen 1998). This facilitated expansion of seagrass habitats (Brasier 1975).
All three species of Idiotropiscis described to date occur in temperate Australian waters (Kuiter 2004), suggesting that the common ancestor of Hippocampus and Idiotropiscis may have originated in this region. Off northeastern Australia, conditions were temperate during the Late Oligocene (Davies et al. 1991), indicating that this species must have occurred in close proximity to the Indo-West Pacific during the Oligocene–Miocene period of tectonic changes. The earliest seahorses would have greatly benefited from the expansion of seagrass habitats. Not only can seahorses manoeuver exceptionally well in such a habitat (Flynn & Ritz 1999), but the vertical seagrass blades would also have provided good camouflage for their upright bodies, and in that way afforded them both protection from predators and an improved ability to ambush prey. Pygmy pipehorses would not have benefited from the seagrass radiation, and for that reason probably remained restricted to the macroalgal reefs in which they still occur today. Divergence of the two taxa may initially have been driven by different selection pressure in seagrass and algal reef habitats. When water temperature increased during the Early Miocene (Davies et al. 1991), adaptation to higher temperatures in the case of the seahorses that by now would have been widespread in the Indo-Pacific may then have resulted in divergence from their sister taxon.
The molecular techniques employed here provide new insights into the evolution of a taxon whose fossil record is uninformative because it lacks transitional forms, but whose evolutionary history is so recent that the major stages of morphological evolution are still represented in extant species.
Acknowledgements
For samples, we thank Kelley Whitaker, Mark McGrouther (Australian Museum, Sydney), Monica Mwale and Paul Cowley (South African Institute for Aquatic Biodiversity, SAIAB), Sara Lourie (Redpath Museum, Montreal) and Mark Erdmann (UC Berkeley). Jure Žalohar (University of Ljubljana, Slovenia), Kerryn Parkinson and Simon Dakin (Australian Museum), Phil Heemstra (SAIAB) and Karen Havenstein (Lowcountry Geologic) kindly allowed us to use their syngnathid images. P.R.T. was supported by a postdoctoral research fellowship for overseas study by the NRF and by a research grant from the Ernest Oppenheimer Memorial Trust. This is a manuscript of MEGMAR, a research group initially supported by a Macquarie University Research Innovation Fund grant (MQA006162 grant to L.B. Beheregaray).
References
- Adams C. G., Gentry A. W., Whybrow P. J.1983Dating the terminal Tethyan event. In Reconstruction of marine environments (ed. Meulenkamp F.), pp. 273–298 Utrecht, The Netherlands: Micropalaeontological Bulletins [Google Scholar]
- Bellwood D. R.1996The Eocene fishes of Monte Bolca: the earliest coral reef fish assemblage. Coral Reefs 15, 11–19 [Google Scholar]
- Brasier M. D.1975An outline history of seagrass communities. Paleontology 18, 681–702 [Google Scholar]
- Davies P. J., Symonds P. A., Feary D. A., Pigram C. J.1991The evolution of the carbonate platforms of northeast Australia. In The Cainozoic in Australia: a re-appraisal of the evidence (eds Williams M. A. J., De Dekker P., Kershaw A. P.), pp. 44–78 Sydney, Australia: Geological Society of Australia, Special Publication 18. [Google Scholar]
- Drummond A. J., Rambaut A.2007Beast: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Caro H.1990Neogene stratigraphy, palaeoceanography, and palaeobiology in northwestern South America and the evolution of the Panama seaway. Palaeogr. Palaeoclimatol. Palaeoecol. 777, 203–234 [Google Scholar]
- Flynn A. J., Ritz D. A.1999Effect of habitat complexity and predatory style on the capture success of fish feeding on aggregated prey. J. Mar. Biol. Assoc. UK 79, 487–494 (doi:10.1017/S0025315498000617) [Google Scholar]
- Hall R.1998The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In Biogeography and geological evolution of SE Asia (eds Hall R., Holloway J. D.), pp. 99–131 Leiden, The Netherlands: Backhuys Publishers [Google Scholar]
- Haug H. H., Tiedemann R.1998Effect of the formation of the Isthmus of Panama on Atlantic Ocean thermohaline circulation. Nature 393, 673–676 (doi:10.1038/31447) [Google Scholar]
- Jobb G., von Haeseler A., Strimmer K.2004Treefinder: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4, 18 (doi:10.1186/1471-2148-4-18) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuiter R. H.2000Seahorses, pipefishes & their relatives: a comprehensive guide to Syngnathiformes Chorleywood, UK: TMC Publications [Google Scholar]
- Kuiter R. H.2004A new pygmy pipehorse (Pisces: Syngnathidae: Idiotropiscis) from Eastern Australia. Rec. Aust. Mus. 56, 162–165 [Google Scholar]
- Miller K. G., Browning J. V., Aubrey M.-P., Wade B. S., Katz M. E., Kulpecz A. A., Wright J. D.2008Eocene–Oligocene global climate and sea level changes in St Stephens Quarry, Alabama. GSA Bull. 120, 34–53 (doi:10.1130/B26105.1) [Google Scholar]
- Patterson C.1993Osteichthyes: Teleostei. In The Fossil Record 2 (ed. Benton M. J.), pp. 621–665 London, UK: Chapman and Hall [Google Scholar]
- Rögl F., Steininger F. F.1983Vom Zerfall der Tethys zu Mediterran und Paratethys. Ann. Naturhist. Mus. Wien 85, 135–163 [Google Scholar]
- Ronquist F., Huelsenbeck J. P.2003MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- Sorbini L.1988Biogeography and climatology of Pliocene and Messinian fossil fish of Eastern Central Italy. Boll. Mus. Civ. Storia Nat. Verona 14, 1–85 [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S.2007Mega4: Molecular Evolutionary Genetics Analysis (Mega) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (doi:10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]
- Teske P. R., Cherry M. I., Matthee C. A.2004The evolutionary history of seahorses (Syngnathidae: Hippocampus): molecular data suggest an Indo-Pacific origin and two invasions of the Atlantic Ocean. Mol. Phylogenet. Evol. 30, 273–286 (doi:10.1016/S1055-7903(03)00214-8) [DOI] [PubMed] [Google Scholar]
- Teske P. R., Hamilton H., Matthee C. A., Barker N. P.2007Signatures of seaway closures and founder dispersal in the phylogeny of a circumglobally distributed seahorse lineage. BMC Evol. Biol. 7, 138 (doi:10.1186/1471-2148-7-138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E. J., Rosen B. R.1998Implications of paucity of corals in the Paleogene of SE Asia: plate tectonics or centre of origin? In Biogeography and geological evolution of SE Asia (eds Hall R., Holloway J. D.), pp. 165–195 Leiden, The Netherlands: Backhuys Publishers [Google Scholar]
- Wilson A. B., Ahnesjö I., Vincent A., Meyer A.2003The dynamics of male brooding, mating patterns and sex-roles in pipefishes and seahorses (Family Syngnathidae). Evolution 57, 1374–1386 [DOI] [PubMed] [Google Scholar]
- Žalohar J., Hitij T., Križnar M.In pressTwo new species of seahorses (Syngnathidae, Hippocampus) from the Middle Miocene (Sarmatian) Coprolitic Horizon in Tunjice Hills, Slovenia: the oldest fossil record of seahorses. Ann. Paléontol. [Google Scholar]