Abstract
Climate change is advancing the onset of the growing season and this is happening at a particularly fast rate in the High Arctic. However, in most species the relative fitness implications for males and females remain elusive. Here, we present data on 10 successive cohorts of the wolf spider Pardosa glacialis from Zackenberg in High-Arctic, northeast Greenland. We found marked inter-annual variation in adult body size (carapace width) and this variation was greater in females than in males. Earlier snowmelt during both years of its biennial maturation resulted in larger adult body sizes and a skew towards positive sexual size dimorphism (females bigger than males). These results illustrate the pervasive influence of climate on key life-history traits and indicate that male and female responses to climate should be investigated separately whenever possible.
Keywords: body size, carapace width, life cycle, Pardosa glacialis, sexual size dimorphism
1. Introduction
Global warming is advancing physical and biological indicators of spring across the Earth (Parmesan 2006) and this is happening at a particularly fast rate in the far north (Høye et al. 2007). Yet, the consequences of an expanded growing season for most Arctic animal species are not well studied and, in particular, long-term studies of Arctic ectotherms are virtually absent (Callaghan et al. 2005). Recent evidence suggests that Arctic populations of ungulates respond differently (Post & Forchhammer 2008) to earlier spring phenology than populations at lower latitudes (Pettorelli et al. 2005). Hence, the effects of climate on phenotypic variation in the rapidly changing Arctic region are largely unknown, particularly in ectotherms.
Adult body size is a key life-history trait and considerable effort has been devoted to demonstrate the existence of general rules of body size clines, e.g. Rensch's rule of larger variability of males than females among species and Bergmann's rule of increasing body size with latitude within species (Chown & Klok 2003; Karl & Fischer 2008). However, more empirical evidence is needed to support a potentially unifying theory to explain large-scale patterns of body size variation and sexual size dimorphism (SSD) (Blankenhorn et al. 2006). It has been shown that male and female body size respond differently to environmental variation in strongly dimorphic polygynous mammals (Post et al. 1999; Garel et al. 2006), but it remains to be tested whether climate has the capacity to affect SSD in weakly dimorphic species. Here, we demonstrate that adult body size and SSD in 10 successive cohorts (1996–2005) of the weakly dimorphic wolf spider Pardosa glacialis in High-Arctic Greenland was significantly related to the timing of snowmelt during both years of its biennial maturation.
2. Material and methods
(a). Study area and data
The specimens used in this study were collected as part of the Zackenberg basic monitoring programme (Klitgaard & Rasch 2008). The sampling was carried out at Zackenberg, northeast Greenland (74°28′ N; 20°34′ W), which is in the High-Arctic climatic zone. Arthropods were monitored during 10 consecutive years (1996–2005) with samples from six pitfall trap plots (1–6) collected weekly during June, July and August. Plots 5 and 6 were only in operation during the periods 1996–1998 and 1999–2005, respectively. Each plot (10 × 20 m) consisted of eight pitfall traps. Trapping started in June once the snow at each trap had melted (see Høye & Forchhammer (2008) for additional information). At the onset of each season, the timing of snowmelt in each plot was recorded. The area hosts eight species of spiders. Here, we focus on the wolf spider P. glacialis (Thorell, 1872), which is the only lycosid of the region. A total of 27 516 specimens of P. glacialis have been caught in the traps over the 10-year period. We measured the width of the carapace on a subsample of 5000 specimens, 500 from each sampling year. The width of the carapace has previously been identified as the most generally useful measure to identify body size variation in lycosids (Hagstrum 1971; Pickavance 2001). Digital images were taken through a dissecting microscope using a Nikon Coolpix 990 digital camera. Subsequently, the measurements were obtained using the software ImageJ (Rasband 2008).
(b). Statistical analyses
First, we constructed a linear mixed model to test for an effect of capture date on variation in carapace width (mm) among all adult individuals, with sex and year as fixed factors, plot as a random factor and capture date as a covariate. Subsequently, we averaged carapace width of males, females and large juveniles (>1.7 mm carapace width) for each plot in each year to avoid pseudo-replication when analysing effects of environmental variation between years (sensu Post et al. 1999). Juveniles with carapace widths larger than 1.7 mm were assumed to be 1 year old, whereas adults are assumed to be 2 years old (electronic supplementary material). Hence, juveniles should not be sensitive to the timing of snowmelt in the year prior to capture, whereas adults potentially could be. We used linear mixed models with sample size for each average value as weight. The full models included plot as a random factor and the timing of snowmelt (day of the year) in current (snowt) and previous (snowt −1) year as covariates and all possible interactions. We used the same model structure to analyse variation in SSD estimated as ((female size/male size) − 1) within each plot in each year with at least five specimens of each sex. We characterized the timing of snowmelt in each year by averaging the timing of snowmelt for the pitfall trap plots, which were in operation throughout the study period. Model reductions were based on F-tests (α = 0.05) on type III sums of squares for fixed effects and log-likelihood ratio tests for random effects (Quinn & Keough 2002).
3. Results
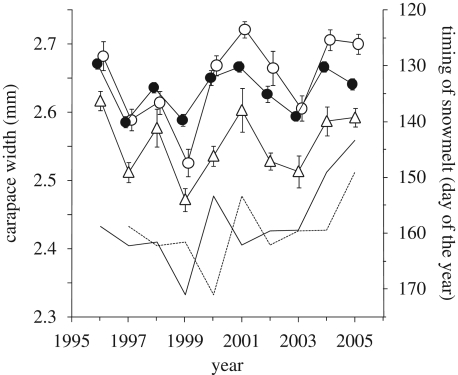
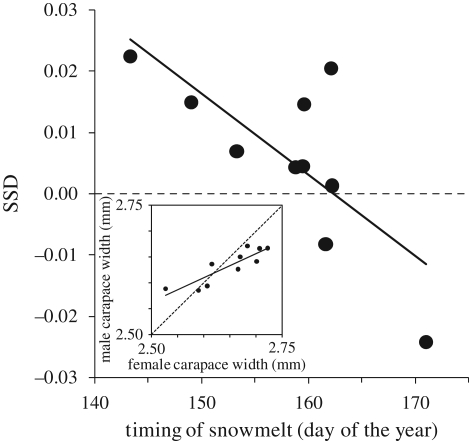
Body size of adults indexed by carapace width did not vary through the summer season when corrected for sex, year and plot effects (F1,3248 = 0.86, p = 0.355). The annual average carapace width of juveniles and adults varied in concert between years, with large body sizes occurring in years of early snowmelt (figure 1). The reduced weighted linear mixed models included all main effects and the interaction between snowt and snowt −1 for males, females and juveniles except that there was no significant effect of plot and snowt −1 in females and juveniles, respectively (table 1). SSD did not differ significantly between plots, but annual estimates of adult body size varied more in females than in males, and SSD was significantly related to snowt (figure 2).
Figure 1.
Inter-annual variation in body size of Pardosa glacialis males (black circles), females (white circles) and juveniles (triangles) given as the annual average width of the carapace in mm (±s.e.) and average timing of snowmelt in the trap plots in yeart (solid line) and yeart −1 (dotted line). For juveniles, the average is based on individuals larger than 1.7 mm. The averages of juvenile carapace widths were added to 0.4 mm to facilitate visual comparison among groups.
Table 1.
The effects of timing of snowmelt in current (snowt) and previous (snowt −1) year on carapace width of males, females and juveniles (>1.7 mm) of Pardosa glacialis. Parameter estimates and p-values are given for final reduced models. In males and juveniles these included plot as a random factor. The full linear mixed models included snowt, snowt −1, plot and all interaction terms and observations were weighted according to sample size within plot and year
| intercept |
snowt |
snowt −1 |
snowt × snowt −1 |
|||||
|---|---|---|---|---|---|---|---|---|
| group | parameter | p-value | parameter | p-value | parameter | p-value | parameter | p-value |
| males | −8.05 | 0.057 | 0.070 | 0.0025 | 0.070 | 0.0019 | −0.00046 | 0.0018 |
| females | −19.39 | 0.0001 | 0.147 | <0.0001 | 0.146 | <0.0001 | −0.00097 | <0.0001 |
| juveniles | 2.68 | <0.0001 | −0.0034 | 0.0098 | — | — | — | — |
Figure 2.
The annual average body size of males plotted against annual average body size of females (inset: R2 = 0.76, p < 0.001) and SSD ((female size/male size) − 1) plotted against timing of snowmelt (R2 = 0.54, p < 0.015).
4. Discussion
The annual average body size of males, females and large juveniles (>1.7 mm carapace width) of P. glacialis fluctuated synchronously across all 10 years. For adults, snowmelt in the year of maturation as well as in the previous year were significant predictors of body size, whereas for 1 year old juveniles, only snowmelt in the year individuals were trapped was significant. The inter-annual size difference is probably caused by higher growth ratios (size after moult/size before moult) in years of early snowmelt. An increased growth ratio has also previously been documented for lycosids in response to increased food availability (Miyashita 1968). Alternatively, the variation in body size could arise by completion of an additional moult in early years. However, if all individuals completed an additional moult, the inter-annual size difference would have been larger than observed and if only some individuals completed an additional moult it would have been indicated by larger intra-annual variation in those years.
The timing of snowmelt could also explain the variation in SSD among cohorts of males and females. Sexual selection on male size and natural selection on female body condition is believed to shape the male-biased size dimorphism observed in many species (Fairbairn 1997). However, even within wolf spiders such sex-specific life-history strategies differ (e.g. male-biased allocation of resources to growth in Lycosa tarantula (Fernández-Montraveta & Moya-Laraño 2007) and female-biased allocation of resources to growth in Hygrolycosa rubrofasciata (Kotiaho et al. 1996; Vertainen et al. 2000)). The stronger response in body size of P. glacialis females to variation in timing of spring snowmelt than in males suggests that size is also a more important predictor of reproductive success in females than in males in this species. Egg mass production is likely to be limited by female size and hence larger females may produce more and/or larger eggs (Simpson 1993, 1995). In the highly seasonal Arctic environment, a major determinant of reproductive success in males may be the timing of their final moult. Early moulting males may be able to mate with more females than late moulting males. This would favour early maturation over attaining large size in males.
While snow depth at the end of winter has varied unpredictably during the study period, spring temperatures have increased dramatically (Klitgaard & Rasch 2008). Further warming is therefore likely to exacerbate SSD. Our study illustrates the pervasive influence of climate on fitness-related phenotypic traits. We suggest that male and female responses to climate should be analysed separately in studies of the ecological effects of climate change.
Acknowledgements
We acknowledge the Zackenberg basic monitoring programme, University of Aarhus for providing access to ecosystem monitoring data and the Zoological Museum, University of Copenhagen for access to specimens. T.F. received funding through SYNTHESYS grant dk-taf 1952. Michael Nickel, Eric Post and two anonymous referees are thanked for insightful comments.
References
- Blankenhorn W. U., Stillwell R. C., Young K. A., Fox C. W., Ashton K. G.2006When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution 60, 2004–2011 (doi:10.1111/j.0014-3820.2006.tb01838.x) [PubMed] [Google Scholar]
- Callaghan T. V., et al. 2005Arctic tundra and polar desert ecosystems. In Arctic climate impact assessment, pp. 243–352 New York, NY: Cambridge University Press [Google Scholar]
- Chown S. L., Klok C. J.2003Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography 26, 445–455 (doi:10.1034/j.1600-0587.2003.03479.x) [Google Scholar]
- Fairbairn D. J.1997Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 28, 659–687 (doi:10.1146/annurev.ecolsys.28.1.659) [Google Scholar]
- Fernández-Montraveta C., Moya-Laraño J.2007Sex-specific plasticity of growth and maturation size in a spider: implications for sexual size dimorphism. J. Evol. Biol. 20, 1689–1699 (doi:10.1111/j.1420-9101.2007.01399.x) [DOI] [PubMed] [Google Scholar]
- Garel M., Solberg E. J., Sæther B. E., Herfindal I., Høgda K. A.2006The length of growing season and adult sex ratio affect sexual size dimorphism in moose. Ecology 87, 745–758 (doi:10.1890/05-0584) [DOI] [PubMed] [Google Scholar]
- Hagstrum D. W.1971Carapace width as a tool for evaluating the rate of development of spiders in the laboratory and the field. Ann. Entomol. Soc. Am. 64, 757–760 [Google Scholar]
- Høye T. T., Forchhammer M. C.2008Phenology of High-Arctic arthropods: effects of climate on spatial, seasonal and inter-annual variation. Adv. Ecol. Res. 40, 299–324 (doi:10.1016/S0065-2504(07)00013-X) [Google Scholar]
- Høye T. T., Post E., Meltofte H., Schmidt N. M., Forchhammer M. C.2007Rapid advancement of spring in the High Arctic. Curr. Biol. 17, R449–R451 (doi:10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- Karl I., Fischer K.2008Why get big in the cold? Towards a solution to a life-history puzzle. Oecologia 155, 215–225 (doi:10.1007/s00442-007-0902-0) [DOI] [PubMed] [Google Scholar]
- Klitgaard A. B., Rasch M.2008Zackenberg ecological research operations 13th annual report. Ministry of Science, Technology and Innovation, Copenhagen, Denmark [Google Scholar]
- Kotiaho J., Alatalo J. M., Mappes J., Parri S.1996Sexual selection in a wolf spider: male drumming activity, body size, and viability. Evolution 50, 1977–1981 (doi:10.2307/2410755) [DOI] [PubMed] [Google Scholar]
- Miyashita K.1968Growth and development of Lycosa T-insignita Boes. et Str. (Aranea: Lycosidae) under different feeding conditions. Appl. Entomol. Zool. 3, 81–88 [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Systemat. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Pettorelli N., Weladji R. B., Holand Ø., Mysterud A., Breie H., Stenseth N. C.2005The relative role of winter and spring conditions: linking climate and landscape-scale plant phenology to alpine reindeer body mass. Biol. Lett. 1, 24–26 (doi:10.1098/rsbl.2004.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickavance J. R.2001Life-cycles of four species of Pardosa (Araneae, Lycosidae) from the island of Newfoundland, Canada. J. Arachnol. 29, 367–377 (doi:10.1636/0161-8202(2001)029[0367:LCOFSO]2.0.CO;2) [Google Scholar]
- Post E., Forchhammer M. C.2008Climate change reduces reproductive success of an arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375 (doi:10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E., Langvatn R., Forchhammer M. C., Stenseth N. C.1999Environmental variation shapes sexual dimorphism in red deer. Proc. Natl Acad. Sci. USA 96, 4467–4471 (doi:10.1073/pnas.96.8.4467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn G. P., Keough M. J.2002Experimental design and data analysis for biologists Cambridge UK: Cambridge University Press [Google Scholar]
- Rasband W. S.2008ImageJ. http://rsb.info.nih.gov/ij/.Bethesda, MD: US National Institutes of Health [Google Scholar]
- Simpson M. R.1993Reproduction in two species of arctic arachnids, Pardosa glacialis and Alopecosa hirtipes. Can. J. Zool. 71, 451–457 (doi:10.1139/z93-065) [Google Scholar]
- Simpson M. R.1995Covariation of spider egg and clutch size: the influence of foraging and parental care. Ecology 76, 795–800 (doi:10.2307/1939345) [Google Scholar]
- Vertainen L., Alatalo J. M., Mappes J., Parri S.2000Sexual differences in growth strategies of the wolf spider. Hygrolycosa rubrofasciata. Evol. Ecol. 14, 595–610 (doi:10.1023/A:1011080706931) [Google Scholar]