Abstract
Bahamas mosquitofish (Gambusia hubbsi) colonized blue holes during the past approximately 15 000 years and exhibit relatively larger caudal regions in blue holes that contain piscivorous fish. It is hypothesized that larger caudal regions enhance fast-start escape performance and thus reflect an adaptation for avoiding predation. Here I test this hypothesis using a three-pronged, experimental approach. First, G. hubbsi from blue holes with predators were found to possess both greater fast-start performance and greater survivorship in the presence of predatory fish. Second, using individual-level data to investigate the morphology–performance–fitness pathway, I found that (i) fish with larger caudal regions produced higher fast-start performance and (ii) fish with higher fast-start performance enjoyed greater survivorship in the presence of fish predators—trends consistently observed across both predator regimes. Finally, I found that morphological divergence between predator regimes at least partially reflects genetic differentiation, as differences were retained in fish raised in a common laboratory environment. These results suggest that natural selection favours increased fast-start performance in the presence of piscivorous fish, consequently driving the evolution of larger caudal regions. Combined with previous work, this provides functional insight into body shape divergence and ecological speciation among Bahamian blue holes.
Keywords: adaptation, biomechanics, locomotion, morphology, population differentiation, predation
1. Introduction
Animals use diverse means of avoiding predation (reviewed in Langerhans 2006). For most fish, the fast-start escape response is the primary mechanism used to evade predator strikes (Domenici in press). The fast start is a rapid, high-energy swimming burst elicited from threatening stimuli. Because predation varies across space and time, divergent selection on locomotor abilities between predator regimes may be a major factor in morphological evolution and speciation in fishes (Webb 1984; Langerhans et al. 2007).
Theory and recent empirical work suggests that larger caudal regions (i.e. posteriorly large lateral surface area) should enhance fast-start performance (Webb 1984; Langerhans et al. 2004; Domenici et al. 2008; Tytell & Lauder 2008). This long-standing purported link between morphology and locomotion has seldom been tested in detail—no prior study has used individual-level data to test this hypothesis using high-speed video data. Moreover, it has long been suggested that greater fast-start performance enhances the probability of survival with predators, although this has very rarely been tested (Webb 1986; Katzir & Camhi 1993; Walker et al. 2005). If these links between morphology, performance and fitness are accurate, then a clear evolutionary prediction exists: fish experiencing high levels of predation from piscivorous fish will evolve larger caudal regions.
Consistent with this prediction, recent work uncovered that Bahamas mosquitofish (Gambusia hubbsi) inhabiting inland blue holes (water-filled, vertical caves) with predatory fish (Gobiomorus dormitor) exhibit larger caudal regions than populations in blue holes without piscivorous fish (Langerhans et al. 2007). Whether this post-Pleistocene radiation of mosquitofish actually involves selection on fast-start performance has not yet been tested. If enlarged caudal regions reflect an antipredator adaptation, three predictions should be upheld: (i) G. hubbsi from blue holes with piscivorous fish (H) should exhibit greater fast-start performance and greater survivorship in the presence of predators than G. hubbsi from blue holes without fish predators (L); (ii) body shape per se (not other traits covarying with the predator regime) should confer greater fast-start performance and consequently greater survivorship in the presence of predatory fish; and (iii) body shape divergence should at least partially reflect genetically based differentiation. To test the first prediction, I compare fast-start performance and survival with predators among fish from different predator regimes. I test the second prediction by measuring the morphology–performance–fitness (M–P–F) pathway and calculating selection on body shape derived solely from selection on fast-start performance—this provides the first direct test of this long-hypothesized M–P–F pathway. Finally, I use a common-garden experiment to test the third prediction.
2. Material and methods
Fish from four blue holes (2 L, 2 H) were photographed alive for morphometric analysis following Langerhans et al. (2007). Body size was estimated as centroid size; body shape was calculated by assigning each fish a score on a canonical axis describing lateral body shape variation. This axis ranges from shapes characteristic of L blue holes (small caudal region) to those characteristic of H blue holes (large caudal region) (electronic supplementary material, figure S1).
Fast-start performance trials were recorded with a high-speed digital video camera (electronic supplementary material). For each fast-start video sequence (40 ms), I measured four performance variables: dnet, 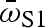 , vmax and amax · dnet is the net distance travelled by the centre of mass.
, vmax and amax · dnet is the net distance travelled by the centre of mass. 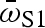 is the average rotational velocity of the head during stage 1 of the fast start (stage 1 rotation angle divided by stage 1 duration). vmax and amax are the maximum velocity and acceleration, computed using the mean-squared error quintic spline to smooth the centre-of-mass displacement data. All four variables have been previously implicated as important in evading predatory strikes (Walker et al. 2005). Differences between predator regimes in fast-start performance were tested using nested multivariate analysis of covariance, followed by mixed-model nested analysis of covariance with each performance variable. Predator regime and population nested within predator regime served as independent variables, and centroid size served as a covariate.
is the average rotational velocity of the head during stage 1 of the fast start (stage 1 rotation angle divided by stage 1 duration). vmax and amax are the maximum velocity and acceleration, computed using the mean-squared error quintic spline to smooth the centre-of-mass displacement data. All four variables have been previously implicated as important in evading predatory strikes (Walker et al. 2005). Differences between predator regimes in fast-start performance were tested using nested multivariate analysis of covariance, followed by mixed-model nested analysis of covariance with each performance variable. Predator regime and population nested within predator regime served as independent variables, and centroid size served as a covariate.
Predation trials were conducted in large experimental tanks (440 l), in which four adult G. hubbsi (one of each sex from two populations having different predator regime statuses) were exposed to one G. dormitor (electronic supplementary material). All G. hubbsi used in the experiment had previously (within 48 h) been photographed for morphometrics and had their fast-start performance measured. At the conclusion of each trial, G. hubbsi survivors were removed and identified using photographs. I used Wilcoxon signed-rank test to examine whether H fish exhibited greater survivorship than L fish.
I investigated the M–P–F pathway using a two-step process. First, effects of M (centroid size and body shape) on P (four performance variables) were examined using (multiple) regression to calculate standardized performance gradients and test significance (Arnold 1983). The effects of P on F (survival during predation trials) were examined using (multiple) regression to calculate standardized fitness gradients (Arnold 1983; Lande & Arnold 1983) and (multiple) logistic regression to test significance. I used model selection (Akaike Information Criterion, AIC; Akaike 1992) to determine the best set of independent variables for adequately predicting each dependent variable (electronic supplementary material). In all cases, I employ one-tailed p-values for tests with a priori predictions.
I conducted a common-garden experiment to test whether body shape differences between predator regimes reflected genetic differentiation (electronic supplementary material). I reared laboratory-born fish (F1 and F2) in a 120 l recirculating system and then photographed each fish for morphometric analysis. To test whether differences observed in the wild were maintained after laboratory rearing, I used a discriminant function derived from wild-caught fish to assign each laboratory-born fish to a predator regime. Significance was tested using a binomial test based on whether each fish was correctly assigned to its predator regime of origin.
3. Results
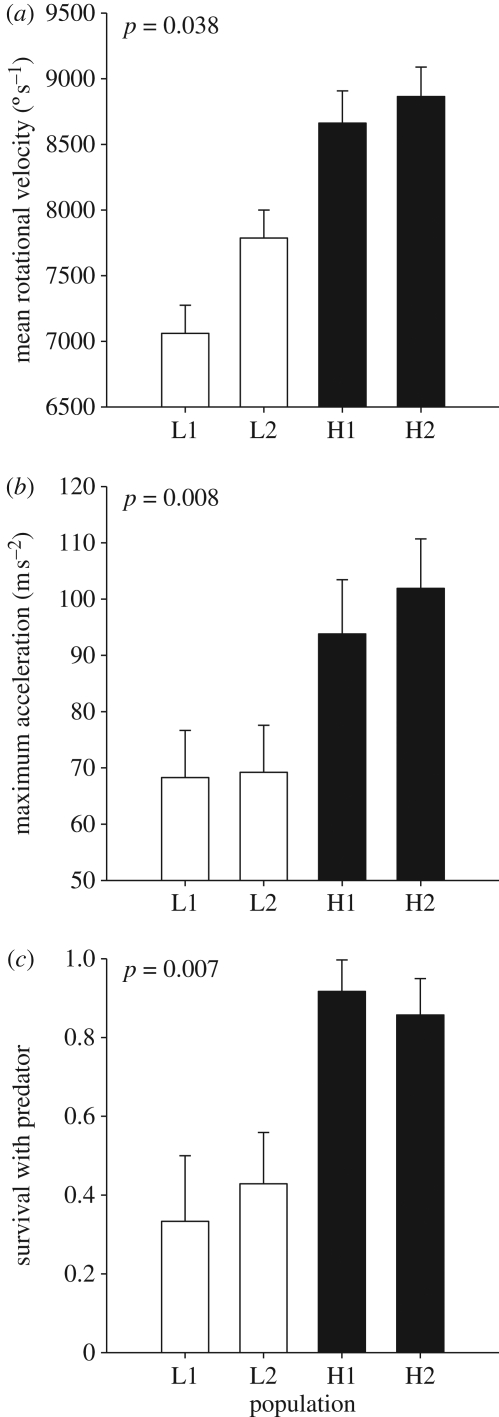
Gambusia hubbsi from H blue holes exhibited greater fast-start performance (18% higher 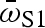 , 42% higher amax, on average) and higher survivorship in the presence of predatory fish (50% higher survival) than conspecifics from L blue holes (figure 1). No differences were observed for the other two fast-start performance variables (electronic supplementary material, table S1).
, 42% higher amax, on average) and higher survivorship in the presence of predatory fish (50% higher survival) than conspecifics from L blue holes (figure 1). No differences were observed for the other two fast-start performance variables (electronic supplementary material, table S1).
Figure 1.
Variation among G. hubbsi populations in (a) 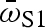 , (b) amax and (c) proportion surviving with a fish predator (least-squares means ± 1 s.e.). Open bars indicate low-predation blue holes; filled bars indicate high-predation blue holes. One-tailed significance is denoted in each graph.
, (b) amax and (c) proportion surviving with a fish predator (least-squares means ± 1 s.e.). Open bars indicate low-predation blue holes; filled bars indicate high-predation blue holes. One-tailed significance is denoted in each graph.
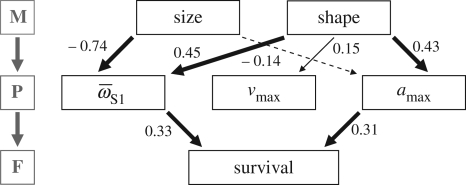
Analysis of the M–P–F pathway indicates that body shape differences between predator regimes, consequently facilitating locomotor differences, are largely responsible for differences in survivorship (figure 2; electronic supplementary material, tables S2 and S3). Three M–P relationships were strong and highly significant (all one-tailed p < 0.0001): smaller fish produced greater average rotational velocity, and fish with larger caudal regions had both greater average rotational velocity and maximum acceleration. One M–P relationship approached significance: fish with larger caudal regions tended to generate greater maximum velocity (one-tailed p = 0.09). One M–P relationship was not significant, but suggestive based on AIC: smaller fish tended to generate greater maximum acceleration (p = 0.18). Two P–F relationships were strong and highly significant: fish with greater average rotational velocity and maximum acceleration exhibited higher survival (both one-tailed p < 0.008). All relationships were consistently observed within predator regimes, indicating that correlated traits that merely covary with the predator regime cannot explain these findings (electronic supplementary material). The total selection gradient on body shape—selection resulting exclusively from its influence on survival as mediated by its effects on fast-start performance—was β = 0.28, meaning that a positive change in one standard deviation of the body shape axis is predicted to result in 28 per cent greater survival probability. Because H fish exhibit a body shape axis score 1.63 standard deviations greater, on average, than L fish, they are predicted to enjoy an approximately 46 per cent greater survival probability. Results from predation trials are remarkably close to this prediction, as H fish exhibited 50 per cent greater survivorship than L fish.
Figure 2.
M–P–F pathway for G. hubbsi in the presence of predatory fish. Path coefficients represent standardized performance (M–P) and fitness gradients (P–F). Paths selected using AIC, line thickness reflects the strength of the path, solid lines represent positive effects and dashed lines represent negative effects. Size: centroid size; shape: lateral shape axis (ranging from small to large caudal regions); other abbreviations follow the text.
After rearing in a common laboratory environment, 80 per cent of the laboratory-born fish were correctly assigned to their predator regime of origin using a discriminant function derived from wild fish (p < 0.0001; electronic supplementary material). This suggests that body shape differences between predator regimes observed in the wild at least partially reflect genetic differentiation.
4. Discussion
Fish from H blue holes, having larger caudal regions, exhibited higher fast-start performance and survival with predators than L fish, strongly suggesting adaptive differentiation; however, this does not reveal causation. A powerful approach to testing the adaptive significance of morphological traits is to examine the M–P–F pathway (Arnold 1983). Although rarely investigated, M–P–F pathways can offer a strong, functional understanding of how selection acts on morphology. Here, I found that fish with larger caudal regions produced greater fast-start performance and consequently experienced higher survivorship with predators. Differences in survivorship between predator regimes could largely be accounted for by selection on body shape, matching a priori predictions.
Fish with larger caudal regions presumably generated greater acceleration and rotational velocities during fast starts owing to higher thrust produced by the larger surface area, and higher muscle power (e.g. greater white muscle mass), respectively (Domenici et al. 2008). Higher acceleration and rotational velocity probably increased survivorship by generating more rapid turns away from danger, increasing evasion success. Interestingly, body size was under strong selection in the presence of predators (total selection, β = −0.29). However, only body shape, not size, is known to differ between predator regimes in G. hubbsi. This suggests that selection on fast-start performance might explain body shape divergence, but other factors are important for body size evolution in blue holes. Moreover, traits other than body morphology probably influence fast-start performance (e.g. median fins, muscle architecture), and traits other than fast-start performance probably influence survival with predators (e.g. behavioural avoidance of predators). Yet, results here suggest that body morphology and fast-start performance represent major targets of selection in the presence of predators, accurately predicting observed survivorship differences between fish from divergent predator regimes.
Predation is a major force of phenotypic evolution and speciation. This study suggests that natural selection via predation by piscivorous fish has driven the evolution of larger caudal regions, greater fast-start performance and higher survivorship in G. hubbsi inhabiting blue holes with predatory fish. Based on theory and recent empirical work in a congener (e.g. Langerhans in press), enlarged caudal regions are predicted to suffer endurance costs during steady swimming, and perhaps explain why fish in L blue holes—where cruising for food and mates, not bursting from predators, is commonplace—exhibit smaller caudal regions. Future work should test this ‘flip-side’ to the M–P–F pathway examined here. In any case, morphological divergence between blue holes has apparently played an important role in the process of ecological speciation. First, fish inhabiting divergent predator regimes exhibit divergent body shapes and consequently have reduced mating probabilities owing to assortative mating for body shape (Langerhans et al. 2007). Second, if L fish were to colonize H blue holes, they would probably suffer increased mortality relative to resident fish (this study). Both processes increase reproductive isolation between fish from different predator regimes relative to fish from the same predator regime (i.e. ecological speciation).
Experiments were approved by the Washington University Animal Studies Committee and the Bahamas Department of Fisheries.
Acknowledgements
I thank the Bahamas Government for permission to conduct this work, M. Blackwell, E. Joseph and Bahamas Environmental Research Center for logistical support and two anonymous reviewers for improving the manuscript. The study was funded by US EPA STAR fellowship, NSF grants DEB-0344488 and DEB-0722480, Explorers Club Exploration Fund and Society of Wetland Scientists Student Research Grant.
References
- Akaike H.1992Information theory and an extension of the maximum likelihood principle. In Breakthroughs in statistics (eds Kotz S., Johnson N.), pp. 610–624 Berlin: Springer [Google Scholar]
- Arnold S. J.1983Morphology, performance and fitness. Am. Zool. 23, 347–361 (doi:10.1093/icb/23.2.347) [Google Scholar]
- Domenici P.In pressEscape responses in fish: kinematics, performance, and behavior. In Fish locomotion: an etho-ecological perspective (eds Domenici P., Kapoor B. G.). Enfield: Science Publishers [Google Scholar]
- Domenici P., Turesson H., Brodersen J., Bronmark C.2008Predator-induced morphology enhances escape locomotion in crucian carp. Proc. R. Soc. B 275, 195–201 (doi:10.1098/rspb.2007.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir G., Camhi J. M.1993Escape response of black mollies (Poecilia shenops) to predatory dives of a pied kingfisher (Ceryle rudis). Copeia 1993, 549–553 (doi:10.2307/1447160) [Google Scholar]
- Lande R., Arnold S. J.1983The measurement of selection on correlated characters. Evolution 37, 1210–1226 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- Langerhans R. B.2006Evolutionary consequences of predation: avoidance, escape, reproduction, and diversification. In Predation in organisms: a distinct phenomenon (ed. Elewa A. M. T.), pp. 177–220 Heidelberg, Germany: Springer-Verlag [Google Scholar]
- Langerhans R. B.In pressTradeoff between steady and unsteady swimming underlies predator-driven divergence in Gambusia affinis. J. Evol. Biol (doi:10.1111/j.1420-9101.2009.01716.x) [DOI] [PubMed] [Google Scholar]
- Langerhans R. B., Gifford M. E., Joseph E. O.2007Ecological speciation in Gambusia fishes. Evolution 61, 2056–2074 (doi:10.1111/j.1558-5646.2007.00171.x) [DOI] [PubMed] [Google Scholar]
- Langerhans R. B., Layman C. A., Shokrollahi A. M., DeWitt T. J.2004Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58, 2305–2318 (doi:10.1111/j.0014-3820.2004.tb01605.x) [DOI] [PubMed] [Google Scholar]
- Tytell E. D., Lauder G. V.2008Hydrodynamics of the escape response in bluegill sunfish, Lepomis macrochirus. J. Exp. Biol. 211, 3359–3369 (doi:10.1242/jeb.020917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. A., Ghalambor C. K., Griset O. L., McKenney D., Reznick D. N.2005Do faster starts increase the probability of evading predators? Funct. Ecol. 19, 808–815 (doi:10.1111/j.1365-2435.2005.01033.x) [Google Scholar]
- Webb P. W.1984Body form, locomotion, and foraging in aquatic vertebrates. Am. Zool. 24, 107–120 (doi:10.1093/icb/24.1.107) [Google Scholar]
- Webb P. W.1986Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides). Can. J. Fish. Aquat. Sci. 43, 763–771 (doi:10.1139/CJFAS-43-4-763) [Google Scholar]