Abstract
Animal cooperation has puzzled biologists for a long time as its existence seems to contravene the basic notion of evolutionary biology that natural selection favours ‘selfish’ genes that promote only their own well-being. Evolutionary game theory has shown that cooperators can prosper in populations of selfish individuals if they occur in clusters, interacting more frequently with each other than with the selfish. Here we show that social networks of primates possess the necessary social structure to promote the emergence of cooperation. By simulating evolutionary dynamics of cooperative behaviour on interaction networks of 70 primate groups, we found that for most groups network reciprocity augmented the fixation probability for cooperation. The variation in the strength of this effect can be partly explained by the groups’ community modularity—a network measure for the groups’ heterogeneity. Thus, given selective update and partner choice mechanisms, network reciprocity has the potential to explain socially learned forms of cooperation in primate societies.
Keywords: cooperation, game theory, social networks, primates
1. Introduction
Cooperation can be defined as the joint action of two or more individuals associated with some cost c for the individual, but as an outcome the individuals can expect a benefit b. Defectors, on the other side, are individuals who refuse to contribute to the costs but benefit from the investment made by the others. In well-mixed populations where all individuals are equally likely to interact with each other, natural selection favours defection. However, if cooperators interact more frequently with each other than with defectors and share the benefits of mutual cooperation, they can receive higher fitness gains than the defectors (Axelrod 1984; Nowak & May 1992; van Baalen & Rand 1998; Le Galliard et al. 2003; Santos et al. 2006). To explain how this clustering of cooperators can be achieved, evolutionary biologists have developed a general kin selection model (Grafen 2007; Lehmann et al. 2007; Taylor et al. 2007). Within this general framework, Nowak (2007) suggested distinguishing among five different mechanisms: group selection, kin selection, direct reciprocity, indirect reciprocity and network reciprocity.
Network reciprocity is a natural generalization of spatial reciprocity. In spatial games (Axelrod 1984; Nowak & May 1992), evolutionary scenarios are usually modelled on a two-dimensional grid, where individuals occupy fixed cells in the grid and interact only within their direct neighbourhood. For network reciprocity, the neighbourhood needs to be understood not as a relation in Euclidean space, but can be any kind of social relationship between two individuals (Lieberman et al. 2005). Ohtsuki and collaborators (Ohtsuki et al. 2006) demonstrated how evolution of cooperation can be modelled on a graph representing an arbitrary social system. By simulating the evolutionary dynamics of a death–birth (DB) update rule, they showed that natural selection can favour cooperation when the benefit-to-cost ratio is larger than the average number of neighbours of each individual. They explained this phenomenon as a consequence of the increased social viscosity of the structured networks.
Primatologists observe high levels of cooperation in most primate species, including behaviours such as communal infant care, food sharing, grooming, coalition formation, communal group defence and cooperative hunting (see chapters in Kappeler & van Schaik 2005). Primate groups differ from the artificial systems investigated so far in important aspects: the group size is much smaller—usually containing less than 50 individuals—and they show distinctive structuring that is neither random nor regular or scale-free. Furthermore, primate groups are not sparse networks because animals usually interact with most other group members. Thus, with this study we want to investigate whether the effects found in simulations on highly arbitrary structured graphs can also be found in real-world social systems.
2. Material and methods
For the dataset we collected matrices of dyadic socio-positive interactions of 70 primate groups published in primatological journals or provided by colleagues. The database contains data from 30 different species, with group sizes ranging from 4 to 35 (electronic supplementary material). For each group, we constructed a graph where the vertices represent the individuals and edges between vertices represent social interactions. To acknowledge the specifics of primate groups, we depicted them as weighted graphs, where edge weights between vertices represent the frequency with which two individuals interact.
We used these graphs to simulate the evolution of a cooperative strategy using a DB update mechanism that works as follows: individuals can adopt one out of two strategies—cooperate or defect—and receive payoffs P from interactions with their connected neighbours according to a given payoff matrix:
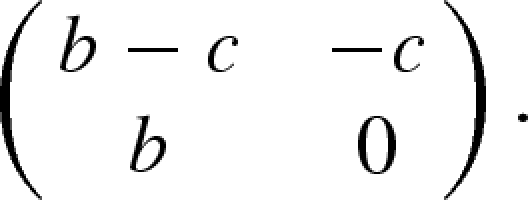 |
2.1 |
These payoffs contribute to the overall fitness of the individuals by Fi = 1 − ω + ω Pi, with ω = 0.01, indicating weak selection strength. In each round, a randomly chosen individual refines its strategy by comparing the overall fitness of its cooperating and defecting interaction partners. The probability of adopting cooperation as its new strategy is proportional to the overall fitness of its cooperating neighbours p(coop) = ∑ FNH(coop)/∑ FNH. The update process is repeated until the population reaches one of the two absorbing states—either all cooperate or all defect. This update mechanism shall be understood as an adjustment of the strategy used by a specific individual by copying its more efficient interaction partners (Nowak 2007). For each primate group, the simulation was run 1000 times for each initial condition of i = 1 to N − 1 cooperators. As the likelihood of ending up in one of the two absorbing states—all defect or all cooperate—depends on the ratio of the strategies in the initial condition, we estimated mean fixation probabilities separately for each initial condition of i = 1 to N − 1 cooperators, based on 1000 simulations per initial condition. Thereafter, we evaluated the fixation difference (FD) as the arithmetic mean of the fixation probabilities for cooperators in the structured groups minus the arithmetic mean of their fixation probabilities in the well-mixed groups (electronic supplementary material). In the same manner, we compared the fixation probabilities in structured groups with those of three differently randomized networks that were produced by: (i) randomly reshuffling edge weights but keeping the topological structure unchanged, (ii) disregarding edge weights and randomly reconnecting the edges, and (iii) randomizing edge weights and topological structure both at the same time. Randomizations were pseudo-randomizations with the condition that the resulting graph is connected. To quantify the groups’ heterogeneity, we calculated their community modularity and to control for differences in relatedness between the species we evaluated phylogenetic independent contrasts for both FD and community modularity (electronic supplementary material).
3. Results
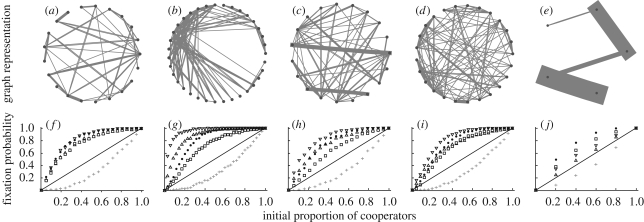
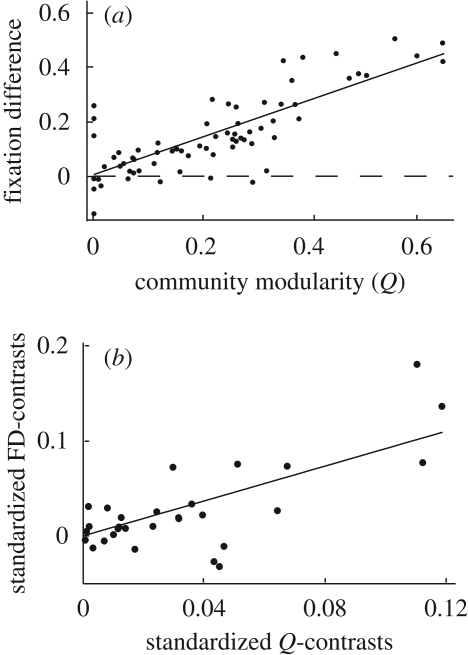
For 61 out of the 70 groups (87%, sign test, p < 0.001), FDs were positive, meaning that cooperation was more likely to reach fixation in the structured system than in a well-mixed group of the same size (figure 1). However, we found also substantial variation in the FDs and in some cases the structured groups showed even lower fixation probabilities than their mixed counterparts. For the 70 primate systems, the mean community modularity (electronic supplementary material) was 0.215 (±0.163 s.d.). A simple linear regression suggests that 60 per cent of the variance in the FD can be explained by the community modularity of the groups (ANOVA, N = 70, F1,68 = 106.4, p < 0.001, adjusted R2 = 0.605, figure 2a). For the reduced sample of 29, phylogenetic independent contrasts linear regression suggests that community modularity can still explain 52 per cent of the variance in the FD (ANOVA, N = 29, F1,28 = 31.8, p < 0.001, adjusted R2 = 0.524, figure 2b). Comparing fixation probabilities for the primate groups with those for randomized networks with equal density, we find that they were significantly higher in the primate networks than in topological random networks (sign test, N− = 18, p < 0.001) and slightly higher—although not significantly—than in networks where the topological structure was preserved but weights were randomly reshuffled (sign test, N− = 27, p = 0.072) but lower than in random networks with preserved edge weights (sign test, N+ = 16, p < 0.001). This suggests that both heterogeneity owing to the topology and heterogeneity owing to variation in the edge weights influence the fixation probability. However, FDs with randomized networks were less pronounced and more variable than FDs with the well-mixed networks (electronic supplementary figure S2).
Figure 1.
Fixation of cooperation in primate groups. (a–e) Structures of the five groups that produced the highest fixation difference (FD). (f–j) Fixation probability for cooperation on the structured system (black dots), well-mixed population of the same size (grey crosses) and random networks (type 1: squares; type 2: up-triangles; type 3: down-triangles). The solid line indicates the expectation for random drift given neutral selection.
Figure 2.
Relation between the fixation difference (FD) and community modularity. (a) FD plotted against community modularity (Q). In groups with negative FD, cooperation reached fixation less often in the structured population than in the mixed population while in groups with positive FD group structure favoured the fixation of cooperation. (b) Standardized phylogenetic independent contrasts for FD and community modularity reduce the sample to 29 independent contrasts.
4. Discussion
Overall, the results suggest that primate group structure facilitates the fixation of cooperation. This is in line with W. D. Hamilton's notion of the effect of social viscosity on the evolution of cooperation (Hamilton 1964). The primate networks were very small—on average less than 10 animals—and relatively dense but, nevertheless, we found still clear facilitation of cooperation. In some exceptional cases, FDs for cooperation were clearly even higher than for random graphs with the same density or regular structures and small world graphs of comparable density (electronic supplementary figures S2 and S3). This suggests that in these specific cases, the architecture of the networks facilitates cooperation to an extent that goes beyond a ‘sparcity effect’. Furthermore, we found that the high variance in the FDs can be partly explained by community modularity—a network measure for the groups’ heterogeneity.
Owing to the structuring of the population, where each individual interacts only with a small neighbourhood, network reciprocity can also foster cooperation in the absence of repeated interactions and book-keeping of the previous behaviour of others. As we assumed the networks to be static, the model is meant to explain the adoption of cooperative strategies within a time frame in which the social relationships will not change substantially. Depending on the specific group, this time span can vary from several weeks to a few years. Bearing in mind that the model is in essence individual based, we can interpret changes in strategy frequencies as a product of meme selection (Dawkins 1976). Because those memes can only be learned from the direct neighbourhood and at the same time fitness relevant interactions are also restricted to the same neighbourhood, this should be regarded as a kin selection process—although relatedness owing to common descent exists among memes, not among individuals adopting them.
The DB update rule is a convenient method to simulate the social adoption of strategies in groups of constant size, but it has nevertheless some drawbacks as it makes assumptions that might be difficult to meet in real life. First, it assumes that individuals accurately evaluate the fitness of their interaction partners, and second, when refining their own strategy individuals consider only the strategies used by their interaction partners. However, it has been shown elsewhere that other update mechanisms as e.g. ‘imitation updating’ (Ohtsuki et al. 2006; Ohtsuki & Nowak 2008), ‘learning from the best’ (Li et al. 2007) or ‘Q-learning’ (Wang et al. 2008) produce basically the same results. By using this rule we do not imply that primates use exactly this way of accounting, but the DB rule should be understood as a general model where both payoffs and influence on strategy selection are influenced by an individual's direct interaction partners. This model can, therefore, be used to predict the likelihood of finding cooperative behaviour if this behaviour is learned socially. Such a scenario was proposed by Pfeiffer et al. (2005) who suggested that a mechanism of ‘generalized reciprocity’ that could account for stable cooperation in animal groups. A conceptually different approach to the exchange of goods and services is the ‘biological market paradigm’ (Noë & Hammerstein 1995), which suggests that individuals base their decision of how much to give on the supply and demand of the exchanged commodities. As it seems plausible that individuals estimate supply and demand of the commodities based on their own interactions with others, we could use a continuous version of the model presented here (with a continuous variable for the exchange rate instead of the dichotomous variable for strategy choice) to determine the effect of group structure on such an exchange system.
Acknowledgements
We thank Chris Cannings, Peter Hammerstein, Ronald Noë, Karl Sigmund, Eörs Szathmáry, Tamas Szekely and three anonymous reviewers for helpful comments. Financial support: EU-NEST project GEBACO (28696).
References
- Axelrod R.1984The evolution of cooperation New York, NY: Basic Books [Google Scholar]
- Dawkins R.1976The selfish gene Oxford, UK: Oxford University Press [Google Scholar]
- Grafen A.2007An inclusive fitness analysis of altruism on a cyclical network. J. Evol. Biol. 20, 2278–2283 (doi:10.111/j.1420-9101.2007.01413.x) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Kappeler P. M., van Schaik C. P. (eds) 2005Cooperation in primates and humans Berlin, Germany: Springer [Google Scholar]
- Le Galliard J. F., Ferrière R., Dieckmann U.2003The adaptive dynamics of altruism in spatially heterogenous populations. Evolution 57, 1–17 [DOI] [PubMed] [Google Scholar]
- Lehmann L., Keller L., Sumpter J. T.2007The evolution of helping and harming on graphs: the return of the inclusive fitness effect. J. Evol. Biol. 20, 2284–2295 (doi:10.1111/j.1420-9101.2007.01414.x) [DOI] [PubMed] [Google Scholar]
- Li W., Zhang X., Hu G.2007How scale-free networks and large-scale collective cooperation emerge in complex homogeneous social systems. Phys. Rev. E 76, 045102(R) (doi:10.1103/PhysRevE.76.045102) [DOI] [PubMed] [Google Scholar]
- Lieberman E., Hauert C., Nowak M. A.2005Evolutionary dynamics on graphs. Nature 433, 312–316 (doi:10.1038/nature03204) [DOI] [PubMed] [Google Scholar]
- Noë R., Hammerstein P.1995Biological markets. Trends Ecol. Evol. 10, 336–339 (doi:10.1016/S0169-5347(00)89123-5) [DOI] [PubMed] [Google Scholar]
- Nowak M. A.2007Five rules for the evolution of cooperation. Science 314, 1560–1563 (doi:10.1126/science.1133755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., May R. M.1992Evolutionary games and spatial chaos. Nature 359, 826–829 (doi:10.1038/359826a0) [Google Scholar]
- Ohtsuki H., Nowak M. A.2008Evolutionary stability on graphs. J. Theor. Biol. 251, 698–707 (doi:10.1016/j.jtbi.2008.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki H., Hauert C., Lieberman E., Nowak M. A.2006A simple rule for the evolution of cooperation on graphs and social networks. Nature 441, 502–505 (doi:10.1038/nature04605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T., Rutte C., Killingback T., Taborsky M., Bonhoeffer S.2005Evolution of cooperation by generalized reciprocity. Proc. R. Soc. B 272, 1115–1120 (doi:10.1098/rspb.2004.2988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F. C., Rodrigues J. F., Pacheco J. M.2006Graph topology plays a determinant role in the evolution of cooperation. Proc. R. Soc. B 273, 51–55 (doi:10.1098/rspb.2005.3272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., Day T., Wild G.2007Evolution of cooperation in a finite homogenous graph. Nature 447, 469–472 (doi:10.1038/nature05784) [DOI] [PubMed] [Google Scholar]
- van Baalen M., Rand D. A.1998The unit of selection in viscous populations and the evolution of altruism. J. Theor. Biol. 193, 631–648 [DOI] [PubMed] [Google Scholar]
- Wang S., Szalay M. S., Zhang C., Csermely P.2008Learning and innovative elements of strategy adoption rules expand cooperative network topologies. PLoS ONE 3, e1917 (doi:10.1371/journal.pone.0001917) [DOI] [PMC free article] [PubMed] [Google Scholar]