Abstract
The ecological effects of global climate changes include shifts of species' distribution and changes in migration strategies and phenotype. Colour polymorphism, which can be envisaged as a species' evolutionary response to alternating conditions or to a wide range of habitats, may be affected by climate changes as well. The scops owl (Otus scops) shows two main colour morphs, dark- and pale-reddish, as well as intermediate morphs. We investigated temporal trends in an index of plumage colour of Italian scops owls from museum collections (1870–2007). We found a significant increase in plumage redness over the last century, which was correlated with an increase in temperature and rainfall of the years before specimen collection. However, the temporal increase in plumage redness persisted after controlling for climatic variables, suggesting that other environmental factors could be involved. Our study indicates that ongoing climate changes might have either shifted the selective balance between colour morphs, or differentially affected migration and movement patterns of colour morphs.
Keywords: climate change, colour polymorphism, evolutionary response, melanin-based coloration, morphs
1. Introduction
When confronted with climatic changes, organisms can respond by shifting their ranges in order to better match their ecological requirements, or by modifying their phenotype to adapt to changing conditions, by means of phenotypic plasticity and/or micro-evolutionary changes in phenotypic/genetic constitution (Parmesan 2006). These responses may occur over relatively short time scales, because climatic variability may exert considerable selective pressure on important fitness-related traits (Gienapp et al. 2008).
The evidence for range shifts as a way of coping with ongoing climate change is overwhelming (Parmesan 2006), and examples of phenotypic changes in relation to climatic variation abound. The latter include changes in phenology (reviewed in Parmesan 2006) as well as in morphology (Millien et al. 2006). Among phenotypic traits taken into account when studying animal responses to climate changes, intraspecific colour variation (i.e. colour polymorphism (CP); Gray & McKinnon 2007) has received little attention, except for two recent studies (Cameron & Pokryszko 2008; Lepetz et al. 2009). This is surprising, since CP is a widespread phenomenon in many animal taxa, and colour morphs can be considered as phenotypic genetic markers whose fitness effects are related to various ecologically important factors (Roulin 2004). Colour morphs may have directly evolved under both natural and sexual selection and also as an indirect response to selection exerted on genetically correlated attributes, e.g. on genes that regulate both melanogenesis and other physiological processes (Roulin 2004). Thus, CP may be appropriate for studying short- and long-term changes in gene frequencies under various sets of environmental conditions (e.g. Sinervo & Lively 1996).
A comparative study suggested that polymorphic species of owls showed a wider niche than monomorphic ones, since they frequented many different habitats, both open and closed, lived in seasonally alternating dry/wet climates and were active during both day and night (Galeotti & Rubolini 2004). Such results suggest that different colour patterns may be adaptive in different environmental conditions by providing behavioural or physiological advantages to their bearers. For example, in the Italian tawny owl (Strix aluco) populations, dark-reddish birds may suffer greater mortality in cool-dry years while being favoured in warm-wet conditions (Galeotti & Cesaris 1996). This may occur because of differences in thermoregulatory physiology among morphs (Mosher & Henny 1976). Therefore, the prevalence of dark- or pale-reddish morphs in a given population may reflect adjustments to local environment in this species, i.e. local adaptation.
If the fitness of different morphs differs between habitats, then polymorphism can be established with different equilibrium gene frequencies in different habitats or in the same habitat under different conditions. This equilibrium between morphs (and in gene frequency) may be disrupted or shifted by environmental changes, among which climate changes may play a major role through their direct and indirect effects on fitness (Lepetz et al. 2009). Differential survival of morphs because of climate variation could thus lead to an evolutionary change in the morph ratio.
Here, we examined long-term variation in the plumage colour of the scops owl (Otus scops), a small (60–135 g) nocturnal raptor of the Mediterranean region (Cramp 1998), in relation to climatic factors. Similar to many related owl species (Galeotti & Cesaris 1996), scops owls show two main colour morphs that are independent of sex and age, ‘dark-reddish’ and ‘pale-reddish’ (the latter often termed ‘grey’; e.g. Cramp 1998), with the former morph probably having more pheomelanin and eumelanin in feathers than the latter (Gasparini et al. 2009). However, intermediates are frequent (see the electronic supplementary material), and CP in this species should be considered as ‘continuous’ (see Huxley (1955) for a definition of continuous morphism and Roulin (2004) for a discussion). The trait may thus vary continuously between two extreme colour values as an outcome of codominance, multigenic control or variation in gene expression (Buckley 1987).
We used a 137 year collecting-based dataset in order to investigate temporal variation in plumage colour by taking climatic variables into account (temperature and rainfall). By analogy with related owl species showing dark- and pale-reddish morphs (Galeotti & Cesaris 1996), we predicted that increasing temperatures over the last century might have favoured dark-reddish birds over pale-reddish ones.
2. Material and methods
We collected data from 281 scops owl specimens (1870–2007) mainly from Italian natural history museums (81.1%; electronic supplementary material). For each specimen, we obtained a continuous measure of colour plumage varying from 0 (pale-reddish individuals) to 4 (dark-reddish individuals; electronic supplementary material). The frequency distribution of colour variation was clearly bimodal (figure S1, electronic supplementary material). Details of climatic data used in the analyses (regional minimum yearly and seasonal temperature anomalies (°C) and a rainfall index, recorded over a 5 yr period starting from the year before specimen collection, i.e. from year(n−1) to year(n−5)) are provided in the electronic supplementary material. In addition, we took into account the amount of Sahel wet season rainfall (Sahel rainfall index; electronic supplementary material), because the Sahelian savannahs probably represent an important wintering and passage area for the species (Cramp 1998). For the analyses, we considered 85 specimens in adult plumage, collected during the breeding season (May to September) (1875–2006), where the month, year and geographical location of the collection were reported.
3. Results
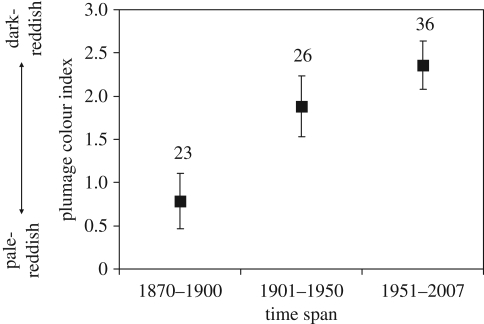
The colour index increased during the course of the study period (rs = 0.36, p = 0.001, n = 85) (figure 1). No correlations between colour index and either latitude (rs = 0.13, p = 0.22) or longitude (rs = −0.17, p = 0.15) were found. Similarly, the geographical coordinates of the specimen-collection sites did not show any temporal trend (latitude: rs = −0.01; longitude: rs = −0.17, both p-values >0.11), implying no spatio-temporal sampling bias because of shifts in collection activities in our sample of specimens.
Figure 1.
Mean (±s.e.m.) scops owl colour index over three different time periods in Italy. Sample sizes are shown.
The Akaike information criterion (AIC)-based procedure we adopted to select potential climatic predictors of CP (electronic supplementary material) identified a combination of the year of collection, minimum annual temperature of year(n−3) and summer rainfall of year(n−1) as the subset of variables best explaining colour index variation. The Spearman correlation coefficients between climatic variables and the colour index were, respectively, 0.30 and 0.26 (both p < 0.019, n = 85 and 83). The Sahel rainfall index having the lowest AIC value when added to the subset of these three variables (electronic supplementary material) was at year(n−4) and had an rs-value of −0.14 (p = 0.28, n = 60) with the colour index.
An analysis of covariance model (electronic supplementary material) indicated that the year of collection (0.010 ± 0.005 s.e.m.; F1,79 = 4.52, p = 0.037), temperature of year(n−3) (0.37 ± 0.14; F1,79 = 7.55, p = 0.007) and summer rainfall of year(n−1) (1.23 ± 0.51; F1,79 = 5.88, p = 0.018) significantly predicted colour variation (these relationships are shown graphically in figure S4 in the electronic supplementary material), while sex, longitude and latitude were removed at previous steps of the analysis (p > 0.20). The results of the final model were confirmed by a randomization test (electronic supplementary material). The additional effect of Sahel rainfall of year(n−4) in the final model (electronic supplementary material) was also not significant (F1,53 = 0.93, p = 0.34).
4. Discussion
Our study showed that plumage coloration of Italian scops owls underwent a directional change through the twentieth century, during a period of increasing temperatures all over Italy (Brunetti et al. 2006). Yearly fluctuations in plumage redness, reflecting an increase in the frequency of dark-reddish morphs, were partly explained by temperature and rainfall, with plumage redness increasing both with increasing temperatures and rainfall. However, climatic variation did not completely account for long-term phenotypic changes in this species, as the secular increase in plumage redness persisted after controlling for climatic variables.
The temporal change in plumage coloration we detected is compatible with a shift in balance of selective pressures favouring dark-reddish morphs. The spread of dark-reddish birds in the population from the early twentieth century was related to a concomitant increase in the annual minimum temperature of year(n−3) before specimen collection, a lag that is consistent with selection taking some time before displaying its effects (Ridley 2003). Such lag may reflect a decreased fecundity and survival of pale-reddish birds born in warmer years. Actually, between 1865 and 2003, the minimum annual temperature increased significantly in Italy (1.1°C per century), while the mean annual rainfall remained stable (Brunetti et al. 2006). Thus, a warmer climate might have favoured dark-reddish individuals, which may have a colour-linked susceptibility to cold (Mosher & Henny 1976; Gehlbach 1994), while concomitantly disadvantaging pale-reddish individuals. Climatic effects on morph frequencies may also be mediated by a differential parasite susceptibility of colour morphs under changing environmental conditions (Galeotti & Sacchi 2003). In both these cases, selection would be indirect, acting on physiological attributes (e.g. metabolic rate, immune response, resistance to stressors) that differ among colour morphs rather than on coloration per se. In fact, melanin-based coloration is often associated with variation in physiological and behavioural traits, stemming from the pleiotropic effects of genes regulating the synthesis of brown to black eumelanin (Ducrest et al. 2008).
However, the significant temporal trend, independent of climatic factors, suggested that other environmental variables varying over time could have simultaneously been at work to produce the morph shift towards a dark-reddish coloration. The increase in woodland extent in Italy since the early twentieth century (Piussi 2005) is a likely candidate, and could have favoured survival of dark-reddish birds because in this habitat they are more cryptic than pale-reddish ones. Several studies have documented that dark- and pale-reddish colour morphs are differently cryptic depending on habitat background and light conditions, and dark-reddish birds may be particularly cryptic in closed forests (e.g. Majerus 1998). We might thus speculate that dark-reddish scops owls in Italy have been favoured both by the increase in temperatures and by the concomitant increase in vegetation cover over the last century.
Three alternative hypotheses may account for the observed temporal change in plumage colour. First, it could be owing to differential range shifts of the different morphs. However, this should have resulted in morph-specific temporal trends in the geographical coordinates of specimen collection, which were not observed in the present sample (electronic supplementary material). Second, any change in morph frequencies might be explained by selective emigration (Lepetz et al. 2009) of pale-reddish individuals from the population or selective immigration of dark-reddish individuals, following range shifts occurring in nearby European populations. An extension of these analyses to the entire Palearctic population of scops owls could provide additional insights on this possibility. Finally, directional temporal changes in colour variation in this species might be regarded as a micro-evolutionary response to climate and habitat changes over time, with the dark-reddish morph being in the process of replacing other morphs. If the current trend persists, we may predict the extinction of pale-reddish morphs among Italian scops owls (i.e. the evolution of a monomorphic population, as occurs in females Tetrao tetrix, which are monomorphic at either end of their range; Stegmann 1932).
In conclusion, this is the first study, to our knowledge, on a bird species documenting that long-term temporal changes in morph frequencies can partially be explained by ongoing climate change.
References
- Brunetti M., Maugeri M., Monti F., Nanni T.2006Temperature and precipitation variability in Italy in the last two centuries from homogenised instrumental time series. Int. J. Climatol. 26, 345–381 (doi:10.1002/joc.1251) [Google Scholar]
- Buckley P. A.1987Mendelian genes. In Avian genetics, a population and ecological approach (eds Cooke F., Buckley P. A.), pp. 1–44 Orlando, FL: Academic Press [Google Scholar]
- Cameron R. D., Pokryszko B. M.2008Variation in Cepaea populations over 42 years: climate fluctuations destroy a topographical relationship of morph-frequencies. Biol. J. Linn. Soc. 95, 53–61 (doi:10.1111/j.1095-8312.2008.01042.x) [Google Scholar]
- Cramp S.1998The complete birds of the western Palearctic (CD-ROM). Oxford, UK: Oxford University Press [Google Scholar]
- Ducrest A.-L., Keller L., Roulin A.2008Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 23, 502–510 (doi:10.1016/j.tree.2008.06.001) [DOI] [PubMed] [Google Scholar]
- Galeotti P., Cesaris C.1996Rufous and grey colour morphs in the Italian tawny owl: geographical and environmental influences. J. Avian Biol. 27, 15–20 [Google Scholar]
- Galeotti P., Rubolini D.2004The niche variation hypothesis and the evolution of colour polymorphism in birds: a comparative study of owls, nightjars and raptors. Biol. J. Linn. Soc. 82, 237–248 (doi:10.1111/j.1095-8312.2004.00355.x) [Google Scholar]
- Galeotti P., Sacchi R.2003Differential parasitaemia in the tawny owl (Strix aluco): effects of colour morph and habitat. J. Zool. 261, 91–99 (doi:10.1017/S0952836903003960) [Google Scholar]
- Gasparini J., Bize P., Piault R., Wakamatsu K., Blount J. D., Ducrest A.-L., Roulin A.2009Strength and cost of an induced immune response are associated with a heritable melanin-based colour trait in female tawny owls. J. Anim. Ecol. 78, 608–616 (doi:10.1111/j.1365-2656.2008.01521.x) [DOI] [PubMed] [Google Scholar]
- Gehlbach F. R.1994The Eastern screech-owl: life history, ecology, and behavior in the suburbs and countryside College Station, TX: Texas A&M University Press [Google Scholar]
- Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merilä J.2008Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- Gray S. M., McKinnon J. S.2007Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22, 71–79 (doi:10.1016/j.tree.2006.10.005) [DOI] [PubMed] [Google Scholar]
- Huxley J.1955Morphism in birds. In Acta XI Congressus Internationalis Ornithologicus (eds Portman A., Sutter E.), pp. 309–328 Basel-Stuttgart, Switzerland: Birkhäuser Verlag [Google Scholar]
- Lepetz V., Massot M., Chaine A., Clobert J.2009Climate warming and the evolution of morphotypes in a reptile. Global Change Biol. 15, 454–466(doi:10.1111/j.1365-2486.2008.01761.x) [Google Scholar]
- Majerus M. E. N.1998Melanism: evolution in action. Oxford, UK: Oxford University Press [Google Scholar]
- Millien V., Lyons S. K., Olson L., Smith F. A., Wilson A. B., Yom-Tov Y.2006Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 9, 853–869 (doi:10.1111/j.1461-0248.2006.00928.x) [DOI] [PubMed] [Google Scholar]
- Mosher J. A., Henny C. J.1976Thermal adaptiveness of plumage color in Screech Owls. Auk 93, 614–619 [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Piussi P.2005Woodland recolonisation and postagricultural development in Italy. In Mountain ecosystems (eds Broll G., Keplin B.), pp. 237–251 Berlin, Heidelberg, Germany: Springer [Google Scholar]
- Ridley M.2003Evolution, 3rd edn Boston, MA: Blackwell Publishing [Google Scholar]
- Roulin A.2004The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 79, 815–848 (doi:10.1017/S1464793104006487) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Lively C. M.1996The rock–paper–scissor game and the evolution of alternative male strategies. Nature 380, 240–243 (doi:10.1038/380240a0) [Google Scholar]
- Stegmann B.1932Die geographischen Formen des Birkhuhns (Lyrurus tetrix L.). J. Ornithol. 80, 342–354 (doi:10.1007/BF01905403) [Google Scholar]