Abstract
The songs of oscine passerine birds vary on many spatial scales, reflecting the actions of diverse evolutionary pressures. Here we examine the songs of Cisticola erythrops, which effectively signal species identity across a geographical area spanning 6500 km in sub-Saharan Africa. Selection for species identification should promote stability in song traits, while sexual selection and geographical segregation should promote diversity. Cisticola erythrops share syllable types across the entire range of species and structure songs similarly, but individuals sing highly variable songs through improvisational recombination of syllables. Patterns of syllable use change gradually across the range of the species and do not show distinct breaks at subspecies boundaries. The acoustic properties of the most common syllable type also change gradually with distance. The results illustrate how songs can be simultaneously species-specific and highly variable at an individual level. At a larger level, patterns of variation indicate that cultural drift has generated song diversity through an isolation by distance mechanism.
Keywords: bird song, geographical variation, cultural drift, isolation by distance, Cisticola erythrops
1. Introduction
Vocalizations of oscine passerine birds are learnt and are therefore subject to both biological and cultural evolution, making them highly labile (Slater 1989). Many species of birds show significant variation in vocal traits over their geographical ranges, often in the form of local dialects that may cause complete turnover in song type within only a few miles (Marler & Tamura 1962; Baker & Cunningham 1985). Across very large geographical scales most bird species show songs with substantial regional variation that is often attributed to learning and the success of local dialects through sexual selection (Mundinger 1982; Podos & Warren 2007). By contrast, a few species show very little variation in song over enormous distances (Martens 1996). Song similarity across ranges is to be expected if song functions primarily as a species-recognition signal, but such a signal could also be produced by species with dialects if all individuals retain just one or a few common song characteristics (Price 2008). This balance between variation and stability in song traits presents an interesting evolutionary puzzle and few studies have examined song diversity across very large geographical scales in species where song may be an important species recognition signal.
The genus Cisticola contains approximately 50 species of little brown birds with relatively similar ecology and morphology (Lynes 1930). Vocal traits, by contrast, vary substantially across this group and effectively communicate species identities (Ryan 2006). Among sympatric species, song presumably maintains species boundaries by acting as a pre-mating barrier to reproduction (Marler 1957; Ryan 2006). Here, we examine geographical variation in the song of Cisticola erythrops (red-faced cisticola), a species with a range spanning over 6500 km across sub-Saharan Africa, multiple subspecies and high song variability relative to other cisticolas (Erard et al. 1997). The geographical range of C. erythrops overlaps with those of over 30 congeneric species (Erard et al. 1997). We show how songs can be variable yet consistently signal species identity. We also examine how song structure changes with location. Species with wide geographical ranges are likely to experience cultural drift in song structure (Koetz et al. 2007). We test whether song structure changes sharply at subspecies boundaries reflecting differentiation through vicariance, or changes gradually over space reflecting isolation by distance mechanisms (Koetz et al. 2007).
2. Material and methods
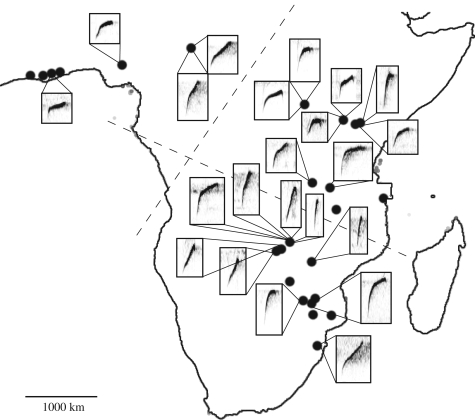
We obtained recordings of 34 individual C. erythrops from 25 recording locations across Africa (figure 2, electronic supplementary material). The latitude and longitude of recording locations were mapped with Microsoft Virtual Earth (maps.live.com).
We described distinct song types and for each individual we measured the average song duration (s) and the rate of song delivery (syllables s−1). All vocalizations containing more than two syllables separated by less than 1 s were considered songs. Variables were compared across song types with Student's t-tests. Regressions of these variables on latitude tested for geographical variation in song production. Unless otherwise stated all statistical analyses were performed using JMP v. 5 (SAS Institute Inc., Cary, NC, 1989–2002) and reported values are mean ± s.d.
Cisticola erythrops share syllable types across their range. We identified all distinct song and call syllable types in every recording. To examine species-wide syllable use patterns, we used a Mantel test implemented in PopTools (Hood 2000) to look for correlations between a matrix of geographical distance and a matrix of syllable sharing for all individuals (Mantel 1967; Hood 2000). Distances between recording sites were calculated using the Haversine formula (Sinnott 1984). We performed a similar test for correlation between a matrix of subspecies identity and syllable sharing. Subspecies identity was assigned based on recording location following Ryan (2006). We also performed a regression of syllable number on latitude to test for changes in vocalization complexity across the range.
To examine geographical variation in syllable structure, we measured properties of the most commonly recorded syllable across the species' range. This syllable is a brief tonal, ascending (and then occasionally descending again) ‘weep’ that was recorded from 22 individuals in 16 locations (figure 2). We visualized weep syllables in Raven sound analysis software v. 1.2, (Cornell Laboratory of Ornithology, NY, USA) and measured the following 10 variables: duration (s), frequency range (Hz), lowest frequency (Hz), highest frequency (Hz), frequency of maximum power (Hz), and slope of the ascending sweep, along with the duration (s), frequency range (Hz), lowest frequency (Hz) and frequency of maximum power (Hz) of the descending sweep. Measurements were made from Hanning type spectrograms with a grid size of 10.8 Hz, and a discrete Fourier transform size of 4096 samples. Principal components (PC) describing syllable shape were generated for each individual using the average values of all 10 measurements for 5–10 ‘weep’ syllables. We tested for geographical variation in syllable shape using regression of individual PC values on latitude. To assess potential subspecific call variation, we plotted PC-1 and PC-2 and examined the overlap of 95 per cent confidence ellipses around the calls of three subspecies.
Figure 2.
Geographical distribution of Cisticola erythrops recording locations (black dots) and spectrograms of ‘weep’ syllable structure (in boxes) across sub-Saharan Africa. Dashed lines separate subspecies.
3. Results
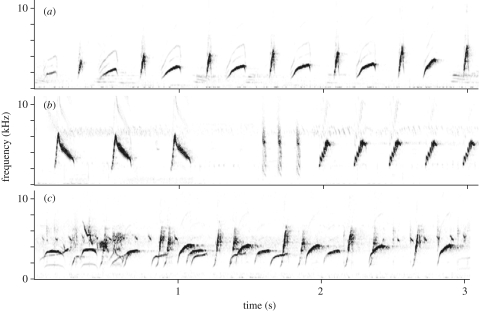
Cisticola erythrops do not sing distinct song types, but instead combine syllables to form a wide variety of vocal productions. Across the entire geographical range, C. erythrops individuals constructed mixed syllable songs (figure 1a,c) which included multiple syllable types arranged in variable orders, and repeated syllable songs (figure 1b) which included one or more repeated syllable type(s). Individuals across the entire geographical range also sang both song types in duets or choruses with conspecifics (figure 1c). Mixed syllable songs lasted an average of 8.4 ± 6.4 s (but could be up to 319 s long) and were delivered at a rate of 3.62 ± 0.7 syllables s−1. Repeated syllable songs were significantly more brief (t46 = 4.25, p < 0.0001), lasting only for 2.4 ± 1.1 s, but were delivered at a nearly identical rate (t46 = 0.092, p = 0.927) of 3.6 ± 1.1 syllables s−1. Song duration and production rate did not differ geographically (duration: F1,46 = 4.00, p = 0.051, rate: F1,46 = 1.23, p = 0.273).
Figure 1.
Three types of songs sung by Cisticola erythrops: (a) mixed syllable song; (b) repeated syllable song; (c) a chorus of mixed syllable songs.
We identified 48 syllable types produced by C. erythrops and found that individuals used up to 14 syllable types in a single singing bout. Because sampling efforts were not exhaustive, these numbers are surely underestimates of total repertoire size. Syllable repertoire sizes did not vary significantly by latitude (F1,46 = 2.82, p = 0.10). The most common syllable types, including a broadband ‘tick’ an upsweeping ‘weep’ syllable and others were produced by birds across the geographical range of the species. In fact, even uncommon syllables were sometimes produced in distant locations. For example, one syllable was recorded in only three sites, two of which were separated by 4753 km. Conversely, 23 per cent (n = 13) of bird pairs recorded in the same location shared no syllable types. Mantel tests indicated that syllable sharing correlated with geographical distance (r = −0.169, p = 0.02), but not with subspecies identity (r = 0.0583, p = 0.205).
The acoustic properties of ‘weep’ syllables varied within and between locations (figure 2). The first and second principal components generated by our analysis explained 52.5 and 21.0 per cent of the total variation in syllable shape. Regression analysis indicated that 25 per cent of the variance in PC-1 was explained by latitude (F1,20 = 6.65, p = 0.018). The three subspecies did not segregate in a plot of PC-1 versus PC-2; 95 per cent confidence ellipses overlapped heavily (electronic supplementary material).
4. Discussion
Cisticola erythrops sing variable songs, with simple or no syllable type structuring. Nevertheless, they are identifiable to species throughout their range by vocal characteristics alone. Recognition is possible because, regardless of location, individuals use particular syllables that are typical of the species and all birds sing with consistent timing. Unlike many species, C. erythrops does not appear to have vocal dialects or clusters of syllable types that vary geographically (Mundinger 1982; Tracy & Baker 1999). Our song analyses found no evidence that sexual selection has promoted dialects or has led to gradients in song complexity over the species' range (Irwin 2000; Podos & Warren 2007). Subspecies boundaries, which reflect differences in colour and size, do not align with vocal traits, suggesting that different evolutionary pressures may be operating on different phenotypic traits (Ornelas et al. 2009). Observers have long recognized that C. erythrops songs differ geographically, but no one has previously characterized the subtle and relatively continuous nature of this change across a 6500 km geographical range (Lynes 1930).
Interestingly, red-faced cisticolas can sing highly variable songs in terms of syllable type and syntax. The observed pattern of vocal variation in this species is most consistent with a scenario where males learn syllable types from other males and construct songs individually through improvisation. Improvisational singing is expected to generate low levels of geographical variation relative to dialect systems (Kroodsma 1996). Nevertheless, at large geographical scales cultural drift may generate diversity as syllable repertoires and acoustic properties change as a result of copying errors or random individual variation. Cisticola erythrops song diversity changes gradually over space and best fits an isolation by distance model of evolution. This pattern differs from that found in other species, where cultural drift has generated variation in bird song after vicariance events (Baker et al. 2006; Koetz et al. 2007).
Some features of C. erythrops songs are relatively invariant over the species' range, probably because of selection for species recognition. Similarity in songs might reflect stabilizing selection, but that scenario is at odds with the high levels of song variation shown by individual C. erythrops (Price 2008). It is possible that similarity reflects stasis following a relatively recent range expansion, but that possibility is also unlikely given existing phylogeographical studies of widespread African birds that identify multiple old areas of stability (Fjeldså & Bowie 2008). Cisticola erythrops shows a complex mixture of stasis in distinctive vocal traits, variability at the individual level, and gradual geographical variation over very large distances. Additional studies of population genetics and song recognition across the species' range would be informative in teasing apart how different evolutionary mechanisms shape bird song at these different spatial scales. Much work has been devoted to identifying vocal differences and recognition failures that restrict gene flow within species, creating potential speciation sites (Slabbekoorn & Smith 2002). As a complement to that question, it is also important to understand where and when widely separated conspecifics sing similar songs that effectively maintain species identities.
Acknowledgements
We thank the Wildlife Division of the British Library, the Macaulay Library of Natural Sounds, and the Transvaal Museum for providing song samples. This manuscript was improved by comments from Callan Cohen and two anonymous reviewers. Funding came from the Museum of Vertebrate Zoology Alexander Fund.
References
- Baker M. C., Cunningham M. A.1985The biology of bird-song dialects. Behav. Brain Sci. 8, 85–133 [Google Scholar]
- Baker M. C., Baker M. S. A., Tilghman L. M.2006Differing effects of isolation on evolution of bird songs: examples from an island-mainland comparison of three species. Biol. J. Linn. Soc. 89, 331–342 (doi:10.1111/j.1095-8312.2006.00677.x) [Google Scholar]
- Erard C., Fry C. H., Grimes L. G., Irwin M. P. S., Keith S., Lack P. C., Pearson D. J., Tye A.1997Sylviidae, old world warblers. In The birds of Africa, vol. V (eds Urban E. K., Fry C. H., Keith S.), pp. 138–215 San Diego, CA: Academic Press [Google Scholar]
- Fjeldså J., Bowie R. C. K.2008New perspectives on the origin and diversification of Africa's forest avifauna. Afr. J. Ecol. 46, 235–247 (doi:10.1111/j.1365-2028.2008.00992.x) [Google Scholar]
- Hood G. M.2000PopTools: software for the analysis of ecological models; v. 2.5.5. http://www.cse.csiro.au/poptools. [Google Scholar]
- Irwin D. E.2000Song variation in an avian ring species. Evolution 54, 998–1010 [DOI] [PubMed] [Google Scholar]
- Koetz A. H., Westcott D. A., Congdon B. C.2007Geographical variation in song frequency and structure: the effects of vicarant isolation, habitat type and body size. Anim. Behav. 74, 1573–1583 (doi:10.1016/j.anbehav.2007.03.022) [Google Scholar]
- Kroodsma D. E.1996Ecology of passerine song development. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 3–19 Ithaca, NY: Cornell University Press [Google Scholar]
- Lynes H.1930Review of the genus Cisticola. Ibis 6(Suppl. 12), 1–673 [Google Scholar]
- Mantel N.1967The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220 [PubMed] [Google Scholar]
- Marler P.1957Specific distinctiveness in the communication signals of birds. Behaviour 11, 13–39 (doi:10.1163/156853956X00066) [Google Scholar]
- Marler P., Tamura M.1962Song ‘dialects’ in three populations of white-crowned sparrows. Condor 64, 368–377 (doi:10.2307/1365545) [Google Scholar]
- Martens J.1996Vocalizations and speciation of palearctic birds. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 221–240 Ithaca, NY: Cornell University Press [Google Scholar]
- Mundinger P. C.1982Microgeographic and macrogeographic variation in the acquired vocalizations of birds. In Acoustic communication in birds, vol. 2 (eds Kroodsma D. E., Miller E. H.), pp. 147–208 New York, NY: Academic Press [Google Scholar]
- Ornelas J. F., Gonzalez C., Espinoza de los Monteros A.2009Uncorrelated evolution between vocal and plumage coloration traits in the trogons: a comparative study. J. Evol. Biol. 22, 471–484 (doi:10.1111/j.1420-9101.2008.01679.x) [DOI] [PubMed] [Google Scholar]
- Podos J., Warren P. S.2007The evolution of geographic variation in birdsong. Adv. Study Behav. 37, 403–458 (doi:10.1016/S0065-3454(07)37009-5) [Google Scholar]
- Price T.2008Speciation in birds Greenwood Village, CO: Roberts and Company [Google Scholar]
- Ryan P.2006Family Cisticolidae (cisticolas and allies). In Handbook of the birds of the world, vol. 11: old world flycatchers to old world warblers (eds del Hoyo J., Elliott A., Christie D. A.), pp. 378–492 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Sinnott R. W.1984Virtues of the Haversine. Sky Telescope 68, 159 [Google Scholar]
- Slabbekoorn H., Smith T. B.2002Bird song, ecology and speciation. Phil. Trans. R. Soc. Lond. B 357, 493–503 (doi:10.1098/rstb.2001.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater P. J. B.1989Bird song learning: causes and consequences. Ethol. Ecol. Evol. 1, 19–46 [Google Scholar]
- Tracy T. T., Baker M. C.1999Geographic variation in syllables of house finch songs. Auk 116, 666–676 [Google Scholar]