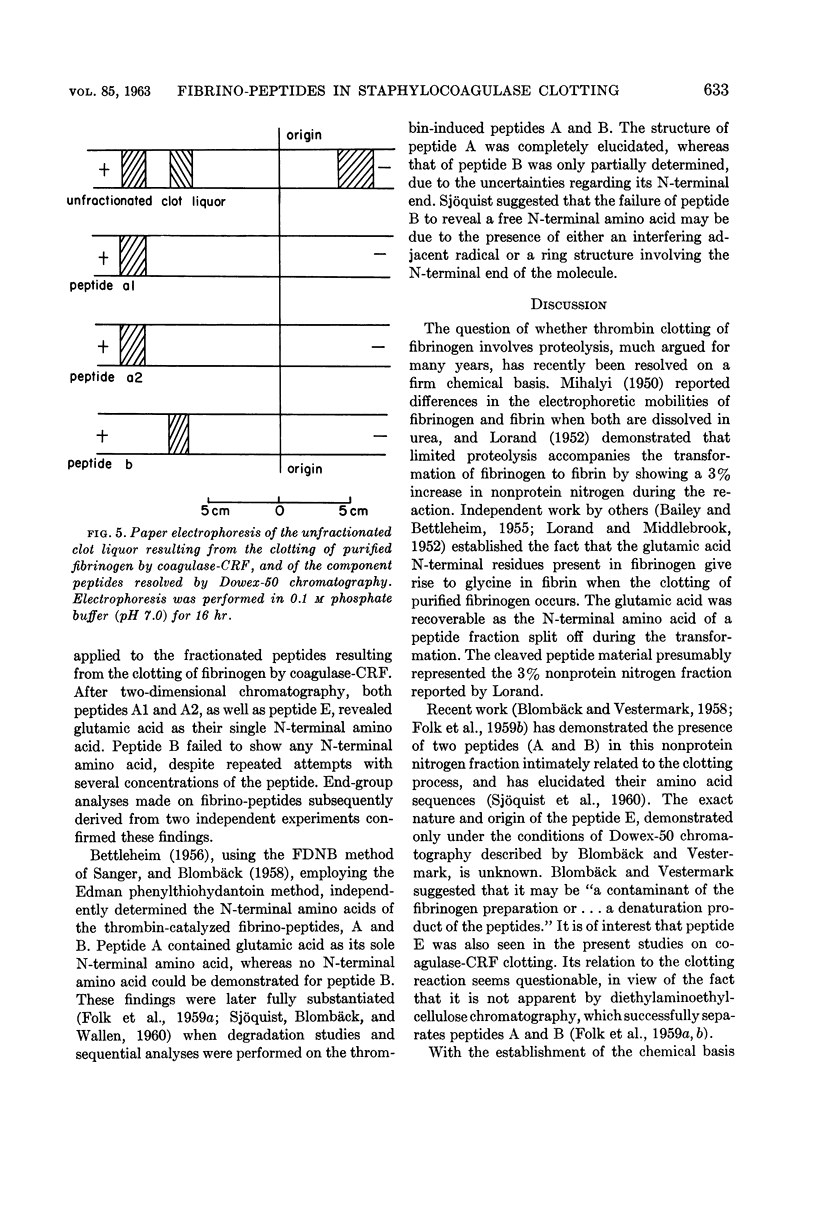
Abstract
Drummond, Margaret C. (Emory University, Atlanta, Ga.) and Morris Tager. Fibrinogen clotting and fibrino-peptide formation by staphylocoagulase and the coagulase-reacting factor. J. Bacteriol. 85:628–635. 1963.—The mechanism of fibrinogen clotting by staphylocoagulase and the coagulase-reacting factor (CRF) was studied from the standpoint of the products of the reaction, and compared with thrombin clotting of fibrinogen. Both clotting principles effect clotting with the release of nonprotein nitrogenous material, which can be resolved into three peptides by Dowex-50 chromatography. On the basis of the sedimentation constants, electrophoretic mobilities, and end-group analyses of these peptides, it appears that the products of fibrinogen clotting by coagulase-CRF and by thrombin are similar.
Full text
PDF

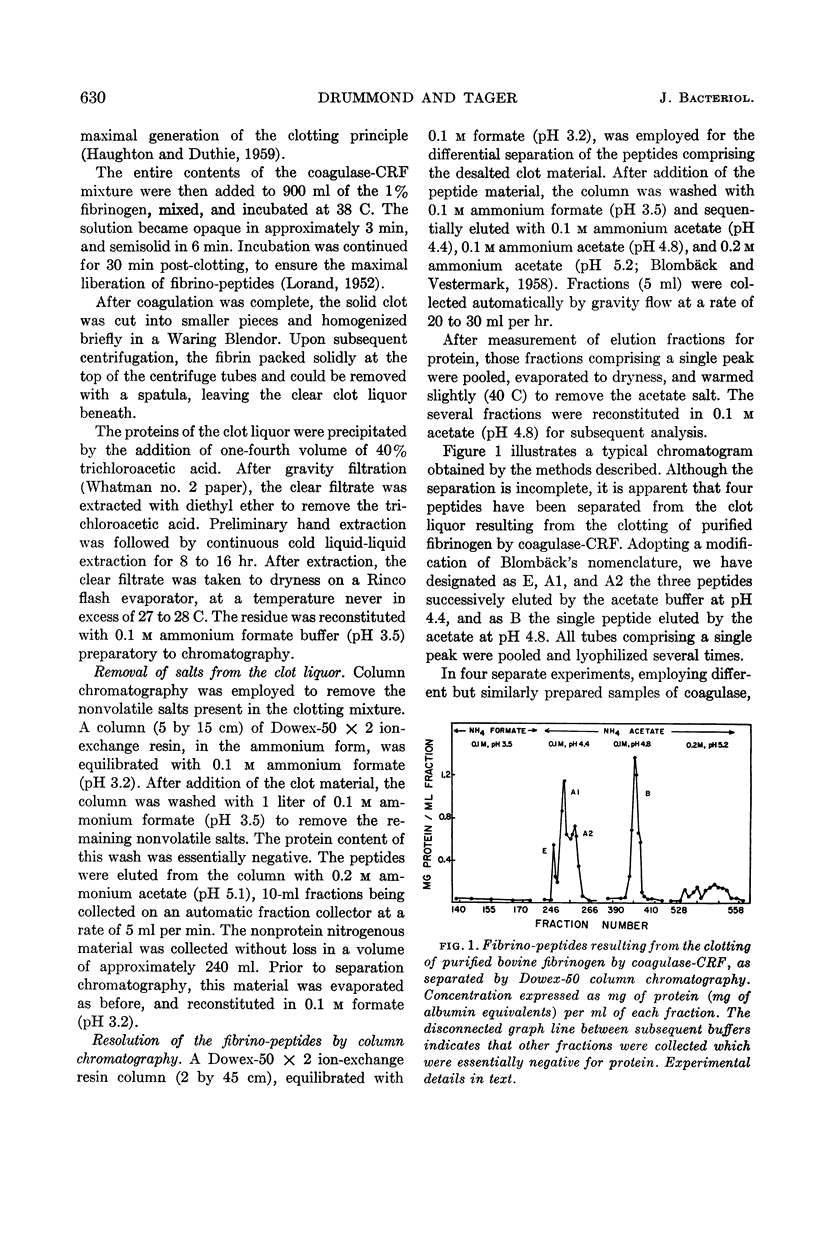
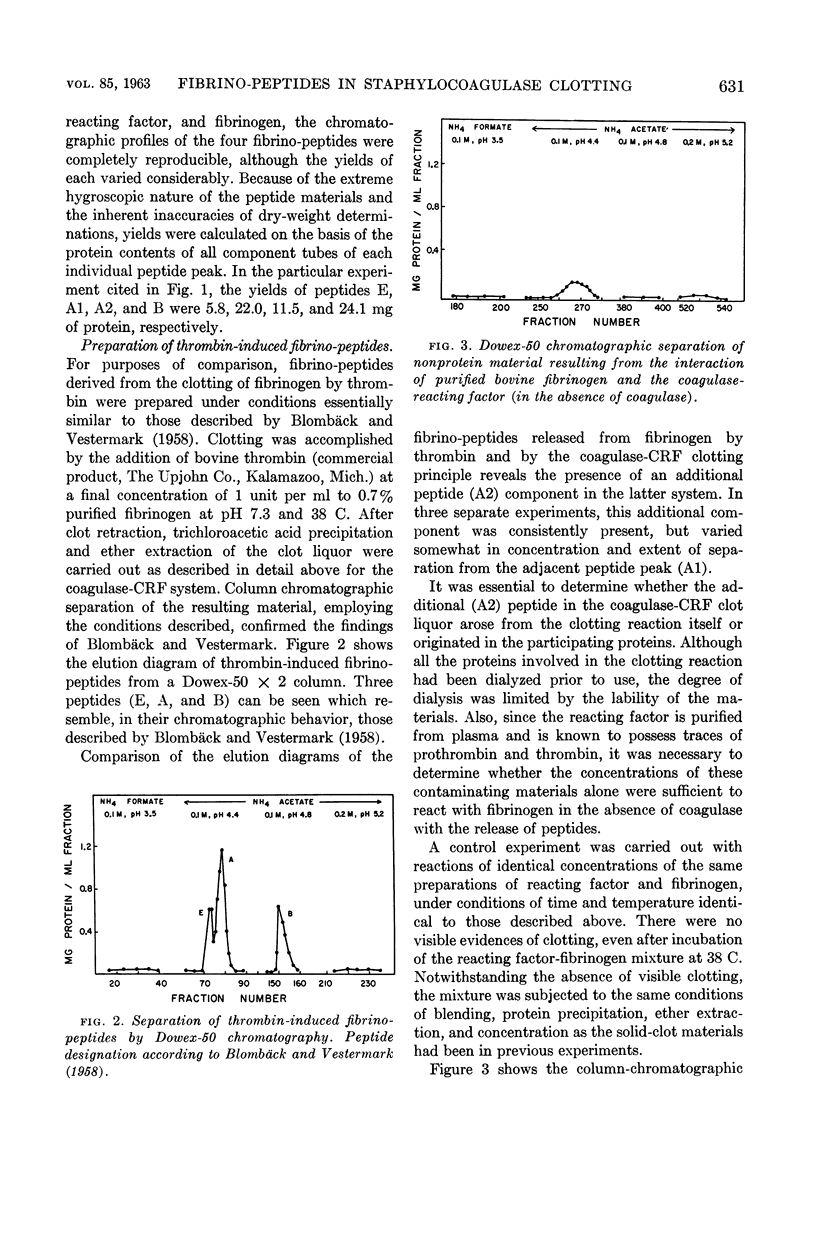
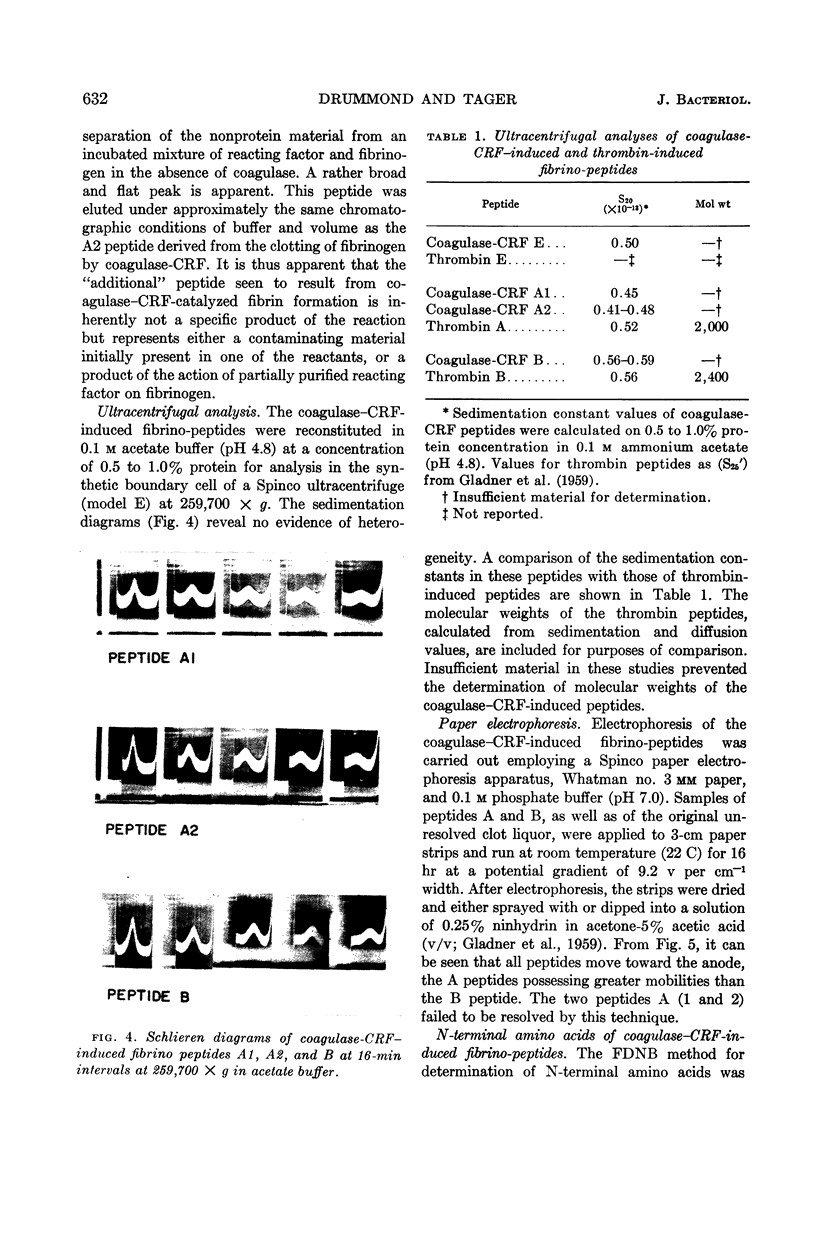


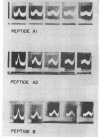
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K., BETTELHEIM F. R. The clotting of fibrinogen. I. The liberation of peptide material. Biochim Biophys Acta. 1955 Dec;18(4):495–503. doi: 10.1016/0006-3002(55)90140-2. [DOI] [PubMed] [Google Scholar]
- BETTELHEIM F. R. The clotting of fibrinogen. II. Fractionation of peptide material liberated. Biochim Biophys Acta. 1956 Jan;19(1):121–130. doi: 10.1016/0006-3002(56)90393-6. [DOI] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Enzymatic activities associated with clotting of fibrinogen by staphylocoagulase and coagulase-reacting factor and their inhibition by disopropylfluophosphate. J Bacteriol. 1962 May;83:975–980. doi: 10.1128/jb.83.5.975-980.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Enzymatic activity of staphylocoagulase. I. Characterization of an esterase associated with purified preparations. J Bacteriol. 1959 Sep;78:407–412. doi: 10.1128/jb.78.3.407-412.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Separation of acid phosphatase from tributyrinase and plasma clotting activities in partially purified staphylocoagulase. J Bacteriol. 1962 Feb;83:432–432. doi: 10.1128/jb.83.2.432-432.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLK J. E., GLADNER J. A., LAKI K. The thrombin-induced formation of co-fibrin. II. Preliminary amino acid sequence studies on peptides A and B. J Biol Chem. 1959 Jan;234(1):67–70. [PubMed] [Google Scholar]
- FOLK J. E., GLADNER J. A., LEVIN Y. Thrombin-induced formation of co-fibrin. III. Acid degradation studies and summary of sequential evidence on peptide A. J Biol Chem. 1959 Sep;234:2317–2320. [PubMed] [Google Scholar]
- GLADNER J. A., FOLK J. E., LAKI K., CARROLL W. R. Thrombin-induced formation of co-fibrin. I. Isolation, purification, and characterization of co-fibrin. J Biol Chem. 1959 Jan;234(1):62–66. [PubMed] [Google Scholar]
- HAUGHTON G., DUTHIE E. S. The activation of staphylococcal free coagulase by plasma constituents and the hydrolysis of Nalpha-toluene-p-sulphonyl-L-arginine methyl ester (TAME) by activated coagulase. Biochem J. 1959 Feb;71(2):348–355. doi: 10.1042/bj0710348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAKI K. The polymerization of proteins; the action of thrombin on fibrinogen. Arch Biochem Biophys. 1951 Jul;32(2):317–324. doi: 10.1016/0003-9861(51)90277-9. [DOI] [PubMed] [Google Scholar]
- LORAND L. Fibrino-peptide. Biochem J. 1952 Oct;52(2):200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAND L., MIDDLEBROOK W. R. The action of thrombin on fibrinogen. Biochem J. 1952 Oct;52(2):196–199. doi: 10.1042/bj0520196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGER M. Studies on the nature and the purification of the coagulase-reacting factor and its relation to prothrombin. J Exp Med. 1956 Nov 1;104(5):675–686. doi: 10.1084/jem.104.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]