Abstract
Although it is increasingly clear that exotic invasive species affect seed-dispersal mutualisms, a synthetic examination of the effect of exotic invasive species on seed-dispersal mutualisms is lacking. Here, we review the impacts of the invasive Argentine ant (Linepithema humile) on seed dispersal. We found that sites with L. humile had 92 per cent fewer native ant seed dispersers than did sites where L. humile was absent. In addition, L. humile did not replace native seed dispersers, as rates of seed removal and seedling establishment were all lower in the presence of L. humile than in its absence. We conclude that potential shifts in plant diversity and concomitant changes in ecosystem function may be a consequence of Argentine ant invasions, as well as invasions by other ant species. Because very few studies have examined the effects of non-ant invasive species on seed-dispersal mutualisms, the prevalence of disruption of seed-dispersal mutualisms by invasive species is unclear.
Keywords: Argentine ants, exotic invasive species, seed-dispersal mutualisms, meta-analysis
1. Introduction
Seed-dispersal mutualisms influence seedling recruitment, population dynamics, species distributions, plant-community composition and gene flow (Howe & Smallwood 1982; Nathan & Muller-Landau 2000). However, the spread of exotic invasive species (invasive species hereafter) threatens seed-dispersal mutualisms, with potential consequences for native populations and communities (Traveset & Richardson 2006; Tylianakis et al. 2008).
Although a growing number of studies have examined whether invasive species disrupt seed-dispersal mutualisms, by affecting either the dispersal agent or the plant (e.g. Bond & Slingsby 1984; Kelly et al. 2006), a quantitative examination of the effect of invasive species on seed-dispersal mutualisms is lacking (Traveset & Richardson 2006). Here, we report the results of a meta-analysis aimed at determining the magnitude of the effect of the invasive Argentine ants (Linepithema humile) on seed-dispersal mutualisms. Linepithema humile has become established in Mediterranean climates globally. Importantly, some of these regions are biodiversity hot spots and harbour a number of unique and endemic plant species, which may rely on native ants to disperse their seeds. Seed dispersal by ants (i.e. myrmecochory) is particularly important in that it involves hundreds of ant species and thousands of plant species across many terrestrial ecosystems (Beattie & Hughes 2002; Rico-Gray & Oliveira 2007). Our focus is on the invasive Argentine ant, a species known to have dramatic effects on native communities and ecosystems (Holway et al. 2002). Specifically, we ask three questions: (i) do Argentine ants reduce the abundance of seed dispersers? (ii) do invasive Argentine ants disrupt seed-dispersal mutualisms by reducing the number of seeds removed? and (iii) do Argentine ants reduce seedling recruitment?
2. Material and methods
On 31 January 2009, we searched Web of Science to locate publications that included the keywords ‘disr* seed dispersal’, ‘predation seed disper*’, ‘competition seed disper*’, ‘disrup* mutualis*’ and ‘inva* or introduced or alien or exotic or non-native or non-indigenous’. We also used our knowledge of the literature and scanned the references of any relevant papers to obtain additional sources from the primary literature. To be included in our meta-analysis, a study had to be either an observational or experimental study on seed dispersal in both the presence and absence of an invasive species in the same area.
In total, we found 14 publications focusing on 31 plant species that met our criteria. Eleven out of the 14 articles focused on the impact of invasive ants on seed-dispersal mutualisms, and of these 11, 10 were concerned with the effect of L. humile and one with the effect of Solenopsis invicta. Because of the low number of publications dealing with species other than L. humile, we report only the results of a meta-analysis quantifying the effect of this species on seed-dispersal mutualisms.
Nine of the 10 publications used in the meta-analysis examined seed removal, three examined seedling establishment and four examined the abundance of seed dispersers (appendix A). From each of these publications, we extracted quantitative estimates of the mean number of seed dispersers, seeds removed and/or seedlings established in the presence and absence of the invasive species. Several publications contained information for more than one plant species or seed-dispersal agent, in which case we treated each as distinct data points in our analyses if the species were analysed separately by the author(s) of the original study. If the data were not available in the text, table or online appendix, we used GetData Graph Digitizer (v. 2.22, copyright S. Fedorov, 2002–2006) to extract data from figures in the original manuscript.
We calculated the effect of invasive species on seed-dispersal mutualisms (effect size) as the log-response ratio (ln R),
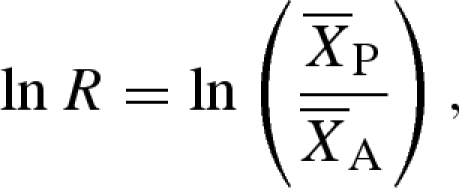 |
where 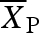 is the mean of the response variable in the presence of the invasive species and
is the mean of the response variable in the presence of the invasive species and 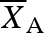 is the mean of the response in the absence of the invasive species (Hedges et al. 1999; Osenberg et al. 1999). A negative effect size indicates that the invasive species reduced the number of seed dispersers, seeds removed or seedlings established. We performed a meta-analysis using a random effect model and calculated the weighted mean effect size and 95% bootstrap CI for the three response variables using MetaWin (Rosenberg et al. 2000).
is the mean of the response in the absence of the invasive species (Hedges et al. 1999; Osenberg et al. 1999). A negative effect size indicates that the invasive species reduced the number of seed dispersers, seeds removed or seedlings established. We performed a meta-analysis using a random effect model and calculated the weighted mean effect size and 95% bootstrap CI for the three response variables using MetaWin (Rosenberg et al. 2000).
3. Results and discussion
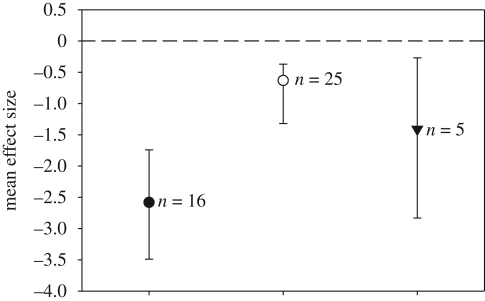
Invasive species can affect diversity, modify ecosystem function and alter interactions among native species (Mack et al. 2000; Traveset & Richardson 2006; Tylianakis et al. 2008). In our study, sites with L. humile contained 92 per cent fewer native seed dispersers than did sites without L. humile (figure 1). Moreover, it seems unlikely that L. humile replaces native seed dispersers, as overall rates of seed removal and seedling establishment were lower in the presence of invasive ants than in their absence: sites with L. humile had, on average, 47 per cent fewer seeds removed and seedling establishment was 76 per cent lower, than when L. humile was present (figure 1).
Figure 1.
Results from the meta-analysis showing the negative effects of the exotic ant L. humile on native plant-seed-dispersal mutualisms. We calculated the effect size using the log response ratio of number of seed dispersers (filled circle), seeds removed (open circle) and seedlings established (triangle). Symbols represent the mean effect size ± 95% CIs. If the CIs do not overlap the horizontal line at 0, then there is a significant negative effect of the presence of Argentine ants on that aspect of seed dispersal.
Our quantitative analysis supports previous studies that have documented the effects of invasive ants on seed-dispersal mutualisms (Holway et al. 2002; Ness & Bronstein 2004). Indeed, our study suggests that the major impact of invasive Argentine ants results from their dramatic reduction of the abundance of native seed-dispersing ants (figure 1). This is not surprising, as Argentine ants clearly alter the composition of native ant communities (Christian 2001; Carney et al. 2003; Gómez & Oliveras 2003; Gómez et al. 2003; Rowles & O'Dowd 2007).
Seed dispersal by ants has been hypothesized to benefit plants by reducing competition and predation, minimizing the effects of fire, and depositing seeds in nutrient-rich sites (Bond & Slingsby 1984; Christian 2001; Ness & Bronstein 2004). Plants that depend on ants as seed dispersers often disproportionately rely on a single ant species (Gove et al. 2007), making them especially vulnerable if the behaviour or abundance of the keystone mutualist is altered (Giladi 2006). Our meta-analysis and the studies that did not meet our criteria for inclusion in the meta-analysis suggest that invasive ants, such as L. humile and S. invicta, are typically poor seed dispersers relative to the native ants they displace. Invasive ants may find seeds more slowly or collect fewer seeds per unit of time than do natives (Bond & Slingsby 1984; Horvitz & Schemske 1986; Carney et al. 2003; Gómez et al. 2003; Ness 2004; Oliveras et al. 2007; Rowles & O'Dowd 2009). Additionally, invasive ants often bury seeds less frequently than do native ant species (Bond & Slingsby 1984; Christian 2001; Zettler et al. 2001; Gómez & Oliveras 2003; Gómez et al. 2003). These invasive ants may also act as seed predators (Horvitz & Schemske 1986; Zettler et al. 2001) or consume elaiosomes without moving seeds (Quilichini & Debussche 2000; Gómez et al. 2003; Ness 2004). However, it is important to note that native ants may also consume seeds and the relative importance of seed predation among native ants may influence the ultimate impact of invasive ants.
Body size in ants plays a crucial role in seed dispersal (Ness & Bronstein 2004), an important fact, as invasive ants are sometimes smaller than the native species that they exclude (McGlynn 1999; Holway et al. 2002). As a result, invasive ants frequently fail to disperse large elaisome-bearing seeds while readily dispersing small native and exotic seeds (Ness 2004; Witt et al. 2004; Rowles & O'Dowd 2009). However, the ultimate impact of Argentine ants on seed dispersal in a community may be mediated by seed size. For example, Rowles & O'Dowd (2009) showed that Argentine ants removed large as well as small diaspores but this was influenced by elaiosome mass. Additionally, when they do remove seeds, dispersal distances by invasive ants are often shorter than those of native species (Bond & Slingsby 1984; Horvitz & Schemske 1986; Ness 2004; Rowles & O'Dowd 2009). Finally, not only do invasive ants disrupt seed-dispersal mutualisms between native species but they also successfully disperse seeds of exotic plants (Rowles & O'Dowd 2009).
As with any meta-analysis, our results could be biased by the failure to publish studies that show no effects of invasive species. In addition, there may be a research bias, whereby researchers tend to study sites or species (such as L. humile) where the effects are likely to be found. We would encourage publication of any results showing non-negative effects of invasive ants on seed-dispersal mutualisms.
Finally, we would like to highlight the fact that we found few studies on species other than L. humile. In our opinion, this paucity of studies suggests a clear need for ecologists to examine the effects of invasive species on seed-dispersal mutualisms. We also suggest that long-term studies are needed to understand the population-level consequences of the disruption of seed-dispersal mutualisms by invasive species. Nevertheless, our results at least suggest that Argentine ants have strong negative effects on the dispersal of seeds and the establishment of seedlings, but it is unclear how these effects influence population dynamics of native plant species or the structure of native plant communities.
Acknowledgements
We thank J. K. Bailey, R. R. Dunn, and T. J. Zelikova and two anonymous reviewers for providing advice that greatly improved this manuscript.
Appendix A
Table 1.
Results of the search of peer-reviewed studies that quantified the effects of the invasive Argentine ant on seed-dispersal mutualisms. (For each study, the identity of stage(s) examined, native seed dispersers and plants are shown.)
| references | stage(s) examined | native seed dispersers | plant species |
|---|---|---|---|
| Bond & Slingsby (1984) | seeds removed–seedlings | Anoplolepis custodiens, Pheidole capensis | Mimetes cucullatus (Proteaceae) |
| Carney et al. (2003) | seed dispersers–seeds removed | Dorymyrmex insanus, Messor sp., Pogonomyrmex subnitidus | Dendromecon rigida (Papaveraceae) |
| Christian (2001) | seed dispersers–seed removed–seedlings | Anoplolepis custodiens, Meranoplus peringueyi, Pheidole capensis, Tetramorium quadrispinosum | Leucospermum conocarpodendron (Proteaceae), Leucospermum truncatulum (Proteaceae), Mimetes cucullatus (Proteaceae), Serruria phylicoides (Proteaceae), Spatalla racemosa (Proteaceae), Serruria inconspicua (Proteaceae) |
| Gómez et al. (2003) | seed dispersers–seeds removed | Aphaenogaster subterranea, Cataglyphis piliscapus, Messor barbarus, Messor bouvieri, Myrmica sabuleti, Pheidole pallidula, Tapinoma nigerrimum, Tetramorium semilaeve | Rhamnus alaternus (Rhamnaceae) |
| Gómez & Oliveras (2003) | seed dispersers–seeds removed | Aphaenogaster subterranea, Crematogaster scutellaris, Formica cunicularia, Messor bouvieri, Pheidole pallidula, Tapinoma nigerrimum, Tetramorium semilaeve | Euphorbia characias (Euphorbiaceae), Euphorbia biumbellata (Euphorbiaceae), Genista linifolia (Fabaceae), Genista triflora (Fabaceae), Genista monspessulana (Fabaceae), Sarothamnus arboreus catalaunicus (Fabaceae) |
| Oliveras et al. (2005) | seedlings | Aphaenogaster subterranea, Crematogaster scutellaris, Formica cunicularia, Messor bouvieri, Pheidole pallidula, Tapinoma nigerrimum, Tetramorium semilaeve | Euphorbia characias (Euphorbiaceae) |
| Oliveras et al. (2007) | seeds removed | Messor bouvieri | Calicotome spinosa (Papilonaceae), Psoralea bituminosa (Papilonaceae), Spartium junceum (Papilonaceae) |
| Quilichini & Debussche (2000) | seeds removed | Aphaenogaster spinosa, Tapinoma nigerrimum, Tetramorium semilaeve | Anchus crispa (Boraginaceae) |
| Rowles & O'Dowd (2009) | seeds removed | Pheidole sp., Rhytidoponera victoriae | Acacia sophorae (Mimosaceae), Acacia retinodes (Mimosaceae) |
| Witt et al. (2004) | seeds removed | Anoplolepis custodiens, Anoplolepis stingroveri, Ocymyrmex cilliei, Pheidole capensis, Tetramorium quadrispinosum | Agathosma ovata (Rutaceae), Leucospermum cordifolium (Proteaceae), Paranomus reflexus (Proteaceae), Phylica pubescens (Rhamnaceae), Podalyria calyptrate (Fabaceae), Polygala myrtifolia (Polygalaceae) |
References
- Beattie A. J., Hughes L.2002Ant–plant interactions. In Plant–animal interactions: an evolutionary approach (eds Herrera C. M., Pellmyr O.) pp. 212–235 Berlin: Springer [Google Scholar]
- Bond W., Slingsby P.1984Collapse of an ant-plant mutualism: the Argentine ant (Iridomyrmex humilis) and myrmecochorous Proteaceae. Ecology 65, 1031–1037 (doi:10.2307/1938311) [Google Scholar]
- Carney S. E., Byerley M. B., Holway D. A.2003Invasive Argentine ants (Linepithema humile) do not replace native ants as seed dispersers of Dendromecon rigida (Papaveraceae) in California, USA. Oecologia 135, 576–582 [DOI] [PubMed] [Google Scholar]
- Christian C. E.2001Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 412, 635–639 (doi:10.1038/35098093) [DOI] [PubMed] [Google Scholar]
- Giladi I.2006Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112, 481–492 (doi:10.1111/j.0030-1299.2006.14258.x) [Google Scholar]
- Gómez C., Oliveras J.2003Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecologica 24, 47–53 (doi:10.1016/S1146-609X(03)00042-0) [Google Scholar]
- Gómez C., Pons P., Bas J. M.2003Effects of the Argentine ant Linepithema humile on seed dispersal and seedling emergence of Rhamnus alaternus. Ecography 26, 532–538 (doi:10.1034/j.1600-0587.2003.03484.x) [Google Scholar]
- Gove A. D., Majer J. D., Dunn R. R.2007A keystone ant species promotes seed dispersal in a ‘diffuse’ mutualism. Oecologia 153, 687–697 (doi:10.1007/s00442-007-0756-5) [DOI] [PubMed] [Google Scholar]
- Hedges L. V., Gurevitch J., Curtis P. S.1999The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 [Google Scholar]
- Holway D. A., Lach L., Suarez A. V., Tsutsui N. D., Case T. J.2002The causes and consequences of ant invasions. Ann. Rev. Ecol. Syst. 33, 181–233 (doi:10.1146/annurev.ecolsys.33.010802.150444) [Google Scholar]
- Horvitz C. C., Schemske D. W.1986Seed dispersal of a neotropical myrmecochore: variation in removal rates and dispersal distance. Biotropica 18, 319–323 (doi:10.2307/2388575) [Google Scholar]
- Howe H. F., Smallwood J.1982Ecology of seed dispersal. Ann. Rev. Ecol. Syst. 13, 201–228 (doi:10.1146/annurev.es.13.110182.001221) [Google Scholar]
- Kelly D., Robertson A. W., Ladley J. J., Anderson S. H., MacKenzie R. J.2006Relative (un)importance of introduced animals as pollinators and dispersers of native plants. In Biological invasions in New Zealand (eds Allen R. B., Lee W. G.), pp. 227–245 Berlin: Springer [Google Scholar]
- Mack R. N., Simberloff D., Lonsdale W. M., Evans H., Clout M., Bazzaz F. A.2000Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [Google Scholar]
- McGlynn T. P.1999Non-native ants are smaller than related native ants. Am. Nat. 6, 690–699 (doi:10.1086/303270) [DOI] [PubMed] [Google Scholar]
- Nathan R., Muller-Landau H. C.2000Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285 (doi:10.1016/S0169-5347(00)01874-7) [DOI] [PubMed] [Google Scholar]
- Ness J. H.2004Forest edges and fire ants alter the seed shadow of an ant-dispersed plant. Oecologia 138, 448–454 (doi:10.1007/s00442-003-1440-z) [DOI] [PubMed] [Google Scholar]
- Ness J. H., Bronstein J. L.2004The effects of invasive ants on prospective ant mutualists. Biol. Invasions 6, 445–461 (doi:10.1023/B:BINV.0000041556.88920.dd) [Google Scholar]
- Oliveras J., Bas J. M., Gómez C.2005Long-term consequences of the alteration of the seed dispersal process of Euphorbia characias due to the Argentine ant invasion. Ecography 28, 662–672 (doi:10.1111/j.2005.0906-7590.04250.x) [Google Scholar]
- Oliveras J., Bas J. M., Gómez C.2007A shift in seed harvesting by ants following Argentine ant invasion. Vie et Milieu, Life Environ. 57, 75–81 [Google Scholar]
- Osenberg C. W., Sarnelle O., Cooper S. D., Holt R. D.1999Resolving ecological questions through meta-analysis: goals, metrics, and models. Ecology 80, 1105–1117 [Google Scholar]
- Quilichini A., Debussche M.2000Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae). Acta Oecologica 21, 303–313 (doi:10.1016/S1146-609X(00)01089-4) [Google Scholar]
- Rico-Gray V., Oliveira P. S.2007The ecology and evolution of ant–plant interactions Chicago, IL: University of Chicago Press [Google Scholar]
- Rosenberg M. S., Adams D. C., Gurevitch J.2000MetaWin: statistical software for meta-analysis, v. 2.0 Sunderland, MA: Sinauer Associates [Google Scholar]
- Rowles A. D., O'Dowd D. J.2007Interference competition by Argentine ants displaces native ants: implications for biotic resistance to invasion. Biol. Invasions 9, 73–85 (doi:10.1007/s10530-006-9009-5) [Google Scholar]
- Rowles A. D., O'Dowd D. J.2009New mutualism for old: indirect disruption and direct facilitation of seed dispersal following Argentine ant invasion. Oecologia 158, 709–716 (doi:10.1007/s00442-008-1171-2) [DOI] [PubMed] [Google Scholar]
- Traveset A., Richardson D. M.2006Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 21, 208–216 (doi:10.1016/j.tree.2006.01.006) [DOI] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A.2008Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- Witt A. B. R., Geertsema H., Giliomee J. H.2004The impact of an invasive ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae), on the dispersal of the elaisome-bearing seeds of six plant species. Afr. Entomol. 12, 223–230 [Google Scholar]
- Zettler J. A., Spira T. P., Allen C. R.2001Ant-seed mutualisms: can red imported fire ants sour the relationship? Biol. Conserv. 101, 249–253 (doi:10.1016/S0006-3207(01)00074-X) [Google Scholar]