Abstract
Phenological responses to climate change vary among taxa and across trophic levels. This can lead to a mismatch between the life cycles of ecologically interrelated populations (e.g. predators and prey), with negative consequences for population dynamics of some of the interacting species. Here we provide, to our knowledge, the first evidence that climate change might disrupt the association between the life cycles of the common cuckoo (Cuculus canorus), a migratory brood parasitic bird, and its hosts. We investigated changes in timing of spring arrival of the cuckoo and its hosts throughout Europe over six decades, and found that short-distance, but not long-distance, migratory hosts have advanced their arrival more than the cuckoo. Hence, cuckoos may keep track of phenological changes of long-distance, but not short-distance migrant hosts, with potential consequences for breeding of both cuckoo and hosts. The mismatch to some of the important hosts may contribute to the decline of cuckoo populations and explain some of the observed local changes in parasitism rates of migratory hosts.
Keywords: brood parasitism, climate change, migration, population trends
1. Introduction
Climate change has affected the annual timing of biological events such as leafing and flowering, and animal reproduction (e.g. Parmesan & Yohe 2003). Migratory birds breeding at temperate latitudes have advanced their migration and breeding timing as a response to a generalized advancement of spring caused by climate change (Dunn 2004; Lehikoinen et al. 2004).
Phenological responses to climate change vary across species (Menzel et al. 2006; Rubolini et al. 2007), potentially owing to several reasons. First, the extent of phenotypic plasticity in specific activities (e.g. migration) in response to climatic signals can vary interspecifically. Second, response to climate change in a particular activity may be constrained by the timing of other activities. For example, anticipation of bird spring migration may be constrained by the timing of moult in wintering quarters (Gordo 2007). Moreover, differences in genetic variance in endogenous programmes governing phenophases may affect their microevolutionary change rate (Pulido 2007). Thus, phenological responses to climate change may affect ecosystem functioning by differentially influencing annual routines of ecologically interacting populations.
The ecological consequences of phenological divergence in response to climate have been mostly investigated in food webs, among primary producers and consumers or at higher trophic levels (Visser & Both 2005; Both et al. 2009). Examples of differential response to climate changes disrupting ecological relationships come from bird studies on predator–prey or competitive interactions (Both & Visser 2001; Ahola et al. 2007). Possibly as a consequence of increasing phenological mismatch with their prey, populations of some predators have declined (Both et al. 2006; Møller et al. 2008).
Differential phenological responses to climate change are similarly likely to interfere with other ecological relationships such as brood parasite–host interactions (Brooks & Hoberg 2007). Obligate brood parasites rely on their hosts to complete their life cycle (Davies 2000). Brood parasites may not be able to track host phenological changes either because of their slower rates of microevolution or because of constraints on phenological phenotypic plasticity.
We investigate how a differential phenological response to climate change by the cuckoo and its hosts may lead to a phenological mismatch. The cuckoo is an obligate brood parasite of more than 100 European passerine species (Davies 2000). Females lay a single egg in each host nest, and the chick ejects host eggs. Successful parasitism results in host clutch failure, with host parents caring for the parasite. Like several of their hosts, cuckoos are long-distance migrants wintering in sub-Saharan Africa. However, other hosts are residents or migrate over shorter distances, wintering in Europe or North Africa (Cramp 1998; Davies 2000).
Although an advancement in spring migration timing has occurred among most European birds, there is a considerable interspecific variation, which is partly explained by migratory strategy, since short-distance migrants (SDM) have advanced more than long-distance migrants (LDM) (Rubolini et al. 2007). This could be owing to, for example, more strict endogenous control of migration schedules or time constraints by competing activities (e.g. moult) during wintering in LDM (Pulido 2007).
We analyse annual rates of change in spring arrival dates of the cuckoo and 40 migratory hosts. We predicted that cuckoos have advanced migration timing less than SDM, but not LDM hosts because cuckoos are LDM, potentially resulting in increased mismatching in arrival at the breeding areas relative to SDM hosts.
2. Material and methods
We collated both published and unpublished data on changes in first arrival dates (FAD) of the cuckoo and its hosts from 20 European sites (1947–2007) (see also electronic supplementary material for tests of publication bias). The 340 time series considered started on average in 1964 (7.7 years s.d.), ended in 2002 (2.8 s.d.) and included data for 37.0 (9.28 s.d.) years on average. Changes in FAD were expressed as the slope (in d yr−1) of the linear regression of arrival date on year. FAD, rather than mean/median arrival dates (MAD), were chosen because they are the most abundant available data on bird migration phenology (Lehikoinen et al. 2004). However, FAD and MAD are positively correlated (see electronic supplementary material). We considered ‘suitable’ cuckoo host species (n = 42) or a subset of 28 ‘main’ hosts (see electronic supplementary material).
3. Results
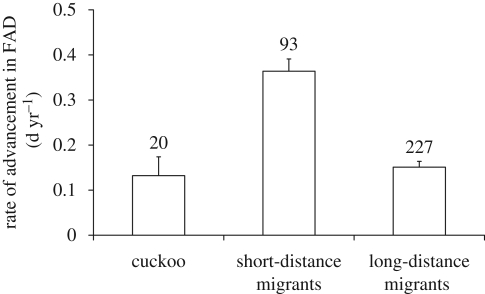
Rates of advancement of cuckoo and host FAD (figure 1) yield an estimated anticipation (in days) of 5.3 for the cuckoo, 14.6 for SDM and 6.0 for LDM, over a period of 40 years (approximately corresponding to the mean duration of the phenological time series). For each species and site, we computed the difference in change in FAD between the cuckoo and its hosts and the mean across sites was computed for each host (table S1 in the electronic supplementary material). The mean of these values was larger than 0 for SDM (one-sample t-tests; suitable hosts: mean (s.e.m.) = 0.205 d yr−1 (0.026), t15 = 7.76, p < 0.001; main hosts: 0.211 (0.026), t11 = 7.96, p < 0.001), but not for LDM (suitable hosts: −0.003 (0.025), t25 = −0.11, p = 0.913; main hosts: 0.001 (0.024), t15 = 0.06, p = 0.957). Thus, SDM, but not LDM hosts have advanced their arrival more than cuckoos. The difference in advancement relative to the cuckoo was larger for SDM than LDM hosts (independent samples t-tests; suitable hosts: 0.208 (0.038), t40 = 5.45, p < 0.001; main hosts: 0.209 (0.036), t26 = 5.84, p < 0.001).
Figure 1.
Rates of advancement in FAD (−1×change in FAD) for the cuckoo, and short- and long-distance migratory suitable host species. Sample sizes refer to the total number of time series.
A linear mixed model (see electronic supplementary material) with site and species as random effects showed a significant difference in advancement rates between the cuckoo and SDM-suitable hosts (difference between marginal means = 0.218 (0.082); post hoc test: p = 0.018) but not LDM-suitable hosts (difference = 0.012 (0.080); p = 0.879), with SDM advancing more than LDM hosts (difference = 0.205 (0.034); p < 0.001; overall effect: F2,20.7 = 18.89, p < 0.001). In this model, the rate of change in arrival dates declined with the year of start (slope = −0.013 (0.005), t = −2.69, p = 0.011) and number of years (slope = −0.008 (0.004), t = −2.14, p = 0.037) of the phenological time series. Non-significant interactions (p ≥ 0.148) with species category (cuckoo, SDM or LDM) were removed from the model. This implies that advancement in FAD was larger in recent years for all species, confirming previous findings on MAD (Rubolini et al. 2007). The same model applied to main hosts showed similar differences between cuckoo and hosts (cuckoo versus SDM hosts, p = 0.023; LDM hosts, p = 0.800; overall effect: F2,17.3 = 13.77, p < 0.001).
The above analyses gave qualitatively similar results when run on the subset of hosts whose populations have declined at least as much as the cuckoo (see electronic supplementary material). Thus, the results were not confounded by the effect of population trends on FAD estimates. In addition, when mean rates of change in FAD of hosts within sites were compared with the cuckoo rate of change, SDM hosts were again found to have advanced more than cuckoos (paired t-tests; SDM: suitable hosts: t13 = 5.32, p < 0.001; main hosts: t13 = 5.33, p < 0.001; LDM: suitable hosts: t19 = −0.19, p = 0.852; main hosts: t18 = 0.02, p = 0.982).
4. Discussion
Cuckoos are not keeping track of phenological changes in migration of all their hosts, since SDM but not LDM hosts have advanced their arrivals more than the cuckoo. This difference in the relative shift of arrival is in the predicted direction because LDM, including the cuckoo, have advanced their arrival less than SDM (Lehikoinen et al. 2004; present study).
Migration dates positively predict laying dates across species (see electronic supplementary material), and species where migration dates have advanced more have also experienced a larger anticipation of breeding (Ahola et al. 2004). In addition, cuckoo and host migration dates largely overlap (Cramp 1998). Furthermore, breeding dates of several European birds have advanced during the last decades (Dunn 2004). Thus, cuckoos may be missing an increasing proportion of breeding opportunities because of an increasing delay in their arrival relative to SDM hosts. Cuckoos might compensate for increasing mismatch by shifting to long-distance migratory hosts. For example, the reed warbler (Acrocephalus scirpaceus), a main LDM cuckoo host, has experienced a 2.4-fold increase in parasitism rate during 1972–1982 compared with the previous 32 years, while parasitism rates in five important SDM and/or resident hosts (Prunella modularis, Anthus pratensis, Carduelis cannabina, Motacilla alba, Erithacus rubecula) have approximately halved (Brooke & Davies 1987). However, such host shifts may not be general and vary geographically, according to, for example composition of hosts' community.
Cuckoos have experienced a ‘small’ decline in Europe (BirdLife International 2004). Interestingly, however, the number of host species that declined less than the cuckoo is larger than expected by chance (binomial test, suitable SDM or LDM hosts: both p < 0.022; main SDM hosts: p = 0.039; main LDM hosts: p = 0.070). If the cuckoo and its hosts are similarly sensitive to the phenomena (unrelated to parasitism) that cause a generalized decline in migratory species abundance, the larger decline in cuckoo populations may result from the additive effect of differential phenological shifts relative to some hosts.
The consequences of differential phenological shifts for cuckoo population dynamics are difficult to predict, as they depend on several factors. First, the proportion of cuckoos parasitizing resident, SDM or LDM host species is determined by host population size in combination with host-specific parasitism rates. However, host population sizes are only roughly known for several European regions and host-specific parasitism rates vary geographically (Davies 2000). Second, differential shifts may have complex effects on availability of host second clutches depending on their timing relative to the cuckoo laying period. Third, several hosts are currently undergoing changes in population size, with trends differing among species and geographically (BirdLife International 2004). Fourth, the impact of phenological change in host migration will depend on timing of host arrival relative to cuckoos. Finally, specialization of cuckoo ‘races’ (‘gentes’; Davies 2000) on host species may exacerbate the consequences of phenological mismatching by hindering the ability of cuckoos to shift to hosts to which they are less mismatched.
Phenological mismatching also has potential consequences for host population dynamics. While several hosts have low rates of parasitism, which is therefore unlikely to markedly affect their populations, parasitism rates on some hosts are more than 5 per cent and locally up to more than 50 per cent (Davies 2000). Depending on phenological shifts relative to the cuckoo, these hosts may suffer increased parasitism, with negative effects on their populations.
Microevolutionary consequences of differential phenological shifts between cuckoos and their hosts are also expected. Host shifts may select for earlier migration and breeding, particularly among the most parasitized hosts. On the other hand, variation in the relative abundance of hosts may affect cuckoo population structure, in terms of relative frequency of gentes, and may even drive to extinction the gentes that are specialized on hosts that are increasingly mismatched to the cuckoo.
In conclusion, this study provides evidence that climate change may be affecting ecological interactions and coevolutionary dynamics between brood parasites and their hosts.
References
- Ahola M., Laaksonen T., Sippola K., Eeva T., Rainio K., Lehikoinen E.2004Variation in climate warming along the migration route uncouples arrival and breeding dates. Glob. Change Biol. 10, 1610–1617 (doi:10.1111/j.1365-2486.2004.00823.x) [Google Scholar]
- Ahola M., Laaksonen T., Eeva T., Lehikoinen E.2007Climate change can alter competitive relationships between resident and migratory birds. J. Anim. Ecol. 76, 1045–1052 (doi:10.1111/j.1365-2656.2007.01294.x) [DOI] [PubMed] [Google Scholar]
- BirdLife International 2004Birds in Europe: population estimates, trends and conservation status Cambridge, UK: BirdLife International [Google Scholar]
- Both C., Visser M. E.2001Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- Both C., Bouwhuis S., Lessells C. M., Visser M. E.2006Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- Both C., Van Asch M., Bijlsma R. G., Van den Burg A. B., Visser M. E.2009Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83 (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- Brooke M., Davies N. B.1987Recent changes in host usage by cuckoos Cuculus canorus in Britain. J. Anim. Ecol. 56, 873–883 [Google Scholar]
- Brooks D. R., Hoberg E. P.2007How will global climate change affect parasite–host assemblages? Trends Parasitol. 23, 571–574 (doi:10.1016/j.pt.2007.08.016) [DOI] [PubMed] [Google Scholar]
- Cramp S.1998The complete birds of the Western Palearctic on CD-ROM Oxford, UK: Oxford University Press [Google Scholar]
- Davies N. B.2000Cuckoos, cowbirds and other cheats London, UK: T & AD Poyser [Google Scholar]
- Dunn P. O.2004Breeding dates and reproductive performance. Adv. Ecol. Res. 35, 67–85 [Google Scholar]
- Gordo O.2007Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 35, 37–58 (doi:10.3354/cr00713) [Google Scholar]
- Lehikoinen E., Sparks T. H., Zalakevicius M.2004Arrival and departures dates. Adv. Ecol. Res. 35, 1–31 (doi:10.1016/S0065-2504(04)35001-4) [Google Scholar]
- Menzel A., et al. 2006European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (doi:10.1111/j.1365-2486.2006.01193.x) [Google Scholar]
- Møller A. P., Rubolini D., Lehikoinen E.2008Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16195–16200 (doi:10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Pulido F.2007Phenotypic changes in spring arrival: evolution, phenotypic plasticity, effects of weather and condition. Clim. Res. 35, 5–23 (doi:10.3354/cr00711) [Google Scholar]
- Rubolini D., Møller A. P., Rainio K., Lehikoinen E.2007Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim. Res. 35, 135–146 (doi:10.3354/cr00720) [Google Scholar]
- Visser M. E., Both C.2005Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]