Abstract
The homologous neuropeptides vasotocin (VT) and vasopressin (VP) influence agonistic behaviours across many taxa, but peptide–behaviour relationships are complex and purportedly species-specific. Putative species-specific effects in songbirds are confounded with context, however, such that territorial species have been tested only in resident–intruder paradigms and gregarious species have been tested only in a mate competition paradigm. Using the territorial violet-eared waxbill (Estrildidae: Uraeginthus granatina), we now show that a V1a receptor antagonist reduces male aggression during mate competition (as in gregarious finches), but does not affect resident–intruder aggression in dominant males. However, the V1a antagonist disinhibits aggression in less aggressive (typically subordinate) males. These results are consistent with recent data on the activation of different VT cell groups during positive and negative social interactions. Thus, VT influences aggression similarly across territorial and gregarious species, but in context- and phenotype-specific ways that probably reflect the differential activation of discrete VT cell groups.
Keywords: vasotocin, vasopressin, aggression, songbird
1. Introduction
The homologous neuropeptides vasotocin (VT; non-mammals) and vasopressin (VP; mammals) influence a wide diversity of social behaviours and have received substantial attention from behavioural biologists, due in large part to the dramatic plasticity of VT/VP systems both within individuals (e.g. based on hormonal and environmental regulation) and across species (e.g. based on evolutionary lability in VT/VP receptor distributions) (De Vries & Panzica 2006; Goodson 2008). Notably, behavioural effects often vary across species or phenotypes (Semsar & Godwin 2004), making generalizations difficult.
Much of the focus on VT/VP and aggression has been placed on the lateral septum, which receives most of its VT/VP input from the medial bed nucleus of the stria terminalis (BSTm). Intraseptal VT infusions inhibit resident–intruder aggression in both territorial sparrows and waxbills, but in the gregarious zebra finch (Estrildidae: Taeniopygia guttata), intraseptal and intraventricular infusions of VT facilitate aggression in the context of mate competition (Goodson 2008).
These studies controlled for many aspects of behaviour and ecology, and have therefore been taken as evidence that VT exerts different effects on aggression in gregarious and territorial species (Goodson 2008). However, the purported species-specific effects of VT in songbirds are confounded with differences in context (resident–intruder versus mate competition), and the relative importance of species-typical social structure versus social context has not yet been resolved. The present experiments were conducted to directly address this issue.
2. Material and methods
(a). Subjects and housing
Captive-bred, male violet-eared waxbills (Uraeginthus granatina; n = 25) were individually housed in cages 61 cm W × 36 cm D × 43 cm H on a 14L:10D photoperiod and provided finch mix and water ad libitum. Experiments were conducted under non-breeding conditions and subjects were separated from female partners for a minimum of two weeks prior to testing. Violet-eared waxbills are highly aggressive year-round. Twenty-five adult male zebra finches were employed for drug validation and were housed in same-sex cages. Experiments were performed in compliance with federal and institutional guidelines for the ethical treatment of animals.
(b). Antagonist validation
Drug validation was conducted in zebra finches, given that V1a antagonist effects are known for zebra finches but not waxbills (Goodson et al. 2004). We used the compound JNJ-17308616 (Johnson & Johnson; Raritan, NJ, USA), which has a 140-fold selective affinity for the rat V1a receptor over the V2 receptor, readily crosses the blood–brain barrier and exerts selective effects on emotional behaviours at doses up to 60 mg kg−1 without non-selective peripheral effects (manufacturer's data). To establish efficacy in finches, we injected 0.05 ml vehicle or 60 mg kg−1 JNJ-17308616 in 0.05 ml saline into the inguinal leg fold. Sixty minutes later we conducted 4 min tests for (i) mate competition aggression, in which the subject and a stimulus male are exposed to a female through a wire barrier and (ii) courtship singing. Tests were conducted using a within-subjects design with at least 2 days between tests. Consistent with previous findings, the antagonist significantly reduced mate competition aggression (36.3 ± 9.0 displacements with saline versus 27.8 ± 7.7 with antagonist; p = 0.026, paired t-test), but did not influence directed singing (38.8 ± 7.4 songs with saline versus 43.3 ± 8.5 with antagonist; p > 0.05), indicating a lack of pronounced peripheral effects. The same regimen was therefore used for waxbill experiments.
(c). Behavioural tests
Male waxbills were screened for aggression and only males who dominated intruders in their own home cage were used as subjects in our initial experiments. Resident–intruder aggression: a stimulus male was introduced into the subject's cage and observations were conducted for 7 min or until 40 displacements were observed, in which case behaviours were prorated (see the electronic supplementary material). We recorded displacements, threats, pecks and agonistic ‘murder calls’. Three of the nine males displayed exhibited no aggression in this experiment and were replaced for the next two experiments. Data from these three males are excluded from the analysis, which does not alter the results. Mate competition aggression: violet-eared waxbills are often intolerant of opposite-sex birds, so in order to determine whether a given pairing would be accepted, we introduced a female into each subject's cage for 5 min in the morning. In the afternoon, friendly pairs were reintroduced, but in a novel cage, in an effort to reduce territoriality. After 2 min of male–female interaction, a stimulus male was introduced to the cage and observations were conducted for 7 min. Male–male aggression in a novel cage: in order to determine whether the antagonist may differentially influence aggression in the home cage and a novel cage, males were tested for territorial aggression following 2 min in a novel cage. Two days were allowed between trials for each experiment, and two weeks between experiments. Treatment order was counterbalanced and observations were conducted blind to treatment.
As shown below, the V1a antagonist did not influence resident–intruder aggression in dominant male waxbills. However, recent findings in song sparrows demonstrate that VT neuronal activation in the paraventricular nucleus of the hypothalamus (PVN) is negatively correlated with aggression (Goodson & Kabelik in press), suggesting that endogenous VT secretion is dampened in more aggressive males relative to less aggressive males. We therefore conducted additional resident–intruder tests using six subordinate male waxbills that were frequently used as stimuli for the dominant males.
3. Results
(a). Endogenous VT exerts context-dependent effects on aggression in dominant male violet-eared waxbills
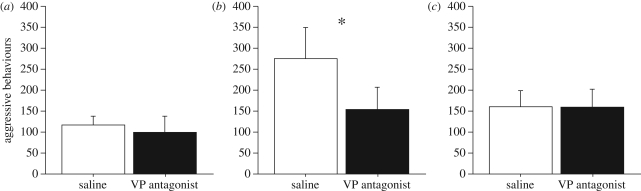
As shown in figure 1a, resident–intruder aggression in the home cage did not differ as a function of treatment (saline versus antagonist; tied z-value = −0.734, p = 0.463, Wilcoxon signed-rank test). Aggression in the presence of a female (mate competition) was very high and was significantly reduced by the V1a antagonist (figure 1b; tied z-value = −2.429, p = 0.015), whereas no effect was observed for resident–intruder aggression in a novel cage (figure 1c; tied z-value = −0.280, p = 0.779).
Figure 1.
Total numbers of aggressive behaviours (mean ± s.e.m.) exhibited by male violet-eared waxbills in the context of (a) resident–intruder tests, (b) mate competition in a novel cage and (c) male–male interaction in a novel cage. Subjects were tested in a within-subjects design following injections of saline or JNJ-17308616, a novel V1a antagonist that crosses the blood–brain barrier. Tests were conducted for 7 min. n = 6 (a) and 9 (b,c), *p = 0.015, Wilcoxon signed-rank test.
(b). V1a antagonism disinhibits aggression in typically subordinate male violet-eared waxbills
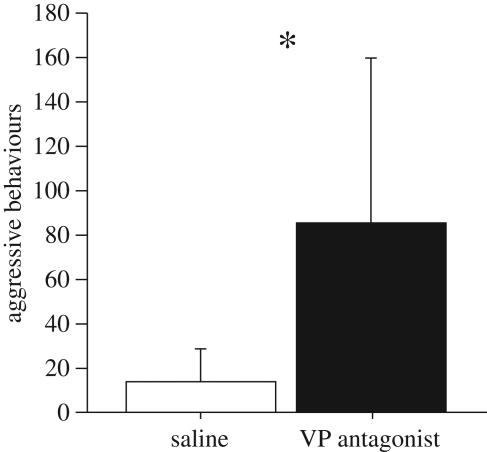
As predicted, injections of the V1a antagonist in typically subordinant males produced a significant facilitation of aggression over the control levels (figure 2; tied z-value = −2.023, p = 0.043).
Figure 2.
Total numbers of aggressive behaviours (mean ± s.e.m.) exhibited in resident–intruder tests by male violet-eared waxbills that were typically subordinate. Subjects were tested in a within-subjects design following injections of saline or V1a antagonist (JNJ-17308616). Tests were conducted for 7 min. n = 6; *p = 0.043, Wilcoxon signed-rank test.
4. Discussion
VT facilitates mate competition aggression in colonial songbirds and inhibits resident–intruder aggression in territorial songbirds (Goodson 2008). Although this difference was originally interpreted in relation to social structure, we now demonstrate that endogenous VT produces both effects in the same species, although effects on resident–intruder aggression are restricted to less aggressive males. Mate competition produced a near doubling of aggression compared with male–male tests in a novel cage, indicating that the presence of a female was highly salient. Notably, the antagonist eliminated only the portion of aggression that is attributable to mate competition, leaving normal territorial levels intact. This finding provides further support for the conclusion that non-sexual territoriality in dominant males is not influenced by endogenous VT.
Although multiple factors may underlie such contextual and phenotypic variation in peptide–behaviour relationships, one important factor appears to be that different VT cell groups are activated in different contexts. VT neurons of the BSTm respond to ‘positive’ stimuli that are associated with affiliation, inclusive of mate competition, but not to stimuli that normally elicit aversion or territorial aggression (Goodson & Wang 2006). In contrast, VT/VP neurons of the PVN respond to stressful events (Wotjak et al. 1996; Goodson & Evans 2004) and exhibit activity that correlates negatively with territorial aggression (Goodson & Kabelik in press). We find no evidence for aggression-promoting VT neurons in the anterior hypothalamus of birds (Goodson & Kabelik in press), as found in hamsters (Delville et al. 1996; Ferris et al. 1997).
The negative correlation between aggression and VT-Fos colocalization in the PVN (Goodson & Kabelik in press) suggests the hypotheses that (i) endogenous release of VT from the PVN inhibits territorial aggression and (ii) dominant males achieve high levels of aggression by reducing VT release. Combined, these hypotheses yield the prediction that a VT receptor antagonist should exert phenotype-dependent effects on behaviour—facilitating aggression in less aggressive, subordinate males (i.e. in males that express higher levels of VT neuronal activity), while having little or no effect on aggressive males, based on their lower levels of VT activity. Both of these hypotheses receive good support from the present results: the V1a antagonist had no effect on resident–intruder aggression in aggressive males, but produced a significant increase in aggression in males that were typically subordinate. Importantly, even in very aggressive males, exogenous VT inhibits aggression (Goodson 1998), indicating that the phenotype-specific antagonist effects obtained here reflect phenotype differences in VT release, not phenotype differences in V1a-like receptor distributions.
Phenotypic differences in VT/VP neurons of the PVN are similarly observed for a variety of mammals and fish (homologous VT neurons in fish are located in the parvocellular preoptic area; Moore & Lowry 1998), and in patterns that are strongly consistent with our findings here. For instance, in several species of fish, subordinate/non-territorial male phenotypes exhibit significantly higher levels of VT mRNA or greater VT immunoreactivity relative to dominant/territorial males (Grober et al. 2002; Miranda et al. 2003; Larson et al. 2006; Lema 2006; Greenwood et al. 2008). Parvocellular preoptic neurons also inhibit the social approach in fish via projections to the brainstem and a complex feedback loop through the periphery (Thompson et al. 2008) and subordinate male mice exhibit significantly higher levels of VP-Fos colocalization in the PVN than do dominant males (J. M. Ho, G. E. Demas & J. L. Goodson, unpublished observation). The present results may therefore be informative for a wide range of taxa.
Acknowledgements
Experiments were performed in compliance with federal and institutional guidelines for the ethical treatment of animals.
Funding for this work was provided by NIMH grant RO1 MH 62656 to J.L.G.
References
- De Vries G. J., Panzica G. C.2006Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138, 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y., Mansour K. M., Ferris C. F.1996Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol. Behav. 59, 813–816 (doi:10.1016/0031-9384(95)02166-3) [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Melloni R. H., Koppel G., Perry K. W., Fuller R. W., Delville Y.1997Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 17, 4331–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J. L.1998Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina). Gen. Comp. Endocrinol. 111, 233–244 (doi:10.1006/gcen.1998.7112) [DOI] [PubMed] [Google Scholar]
- Goodson J. L.2008Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 170, 3–15 (doi:10.1016/S0079-6123(08)00401-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J. L., Evans A. K.2004Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm. Behav. 46, 371–381 (doi:10.1016/j.yhbeh.2004.02.008) [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Wang Y.2006Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl Acad. Sci. USA 103, 17 013–17 017 (doi:10.1073/pnas.0606278103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J. L., Kabelik D.In press Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroendcocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J. L., Lindberg L., Johnson P.2004Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm. Behav. 45, 136–143 (doi:10.1016/j.yhbeh.2003.08.006) [DOI] [PubMed] [Google Scholar]
- Greenwood A. K., Wark A. R., Fernald R. D., Hofmann H. A.2008Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. R. Soc. B 275, 2393–2402 (doi:10.1098/rspb.2008.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober M. S., George A. A., Watkins K. K., Carneiro L. A., Oliveira R. F.2002Forebrain AVT and courtship in a fish with male alternative reproductive tactics. Brain Res. Bull. 57, 423–425 (doi:10.1016/S0361-9230(01)00704-3) [DOI] [PubMed] [Google Scholar]
- Larson E. T., O'Malley D. M., Melloni R. H.2006Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav. Brain Res. 167, 94–102 (doi:10.1016/j.bbr.2005.08.020) [DOI] [PubMed] [Google Scholar]
- Lema S. C.2006Population divergence in plasticity of the AVT system and its association with aggressive behaviors in a Death Valley pupfish. Horm. Behav. 50, 183–193 (doi:10.1016/j.yhbeh.2006.02.010) [DOI] [PubMed] [Google Scholar]
- Miranda J. A., Oliveira R. F., Carneiro L. A., Santos R. S., Grober M. S.2003Neurochemical correlates of male polymorphism and alternative reproductive tactics in the Azorean rock-pool blenny Parablennius parvicornis. Gen. Comp. Endocrinol. 132, 183–189 (doi:10.1016/S0016-6480(03)00063-7) [DOI] [PubMed] [Google Scholar]
- Moore F. L., Lowry C. A.1998Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 119, 251–260 (doi:10.1016/S0742-8413(98)00014-0) [DOI] [PubMed] [Google Scholar]
- Semsar K., Godwin J.2004Multiple mechanisms of phenotype development in the bluehead wrasse. Horm. Behav. 45, 345–353 (doi:10.1016/j.yhbeh.2004.01.003) [DOI] [PubMed] [Google Scholar]
- Thompson R. R., Walton J. C., Bhalla R., George K. C., Beth E. H.2008A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur. J. Neurosci. 27, 2285–2293 (doi:10.1111/j.1460-9568.2008.06210.x) [DOI] [PubMed] [Google Scholar]
- Wotjak C. T., Kubota M., Liebsch G., Montkowski A., Holsboer F., Neumann I., Landgraf R.1996Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J. Neurosci. 16, 7725–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]