Abstract
Sexual conflict is predicted to generate more rapid reproductive isolation between larger populations. While there is some empirical support for this, the data are inconsistent and, additionally, there has been criticism of some of the evidence. Here we reanalyse two experimental-evolution datasets using an isolation index widely applied in the speciation literature. We find evidence for reproductive isolation through sexual conflict in Sepsis cynipsea, but not in Drosophila melanogaster, and this occurred to a greater degree in larger populations, which is consistent with previous findings.
Keywords: sexual selection, divergence, Diptera, speciation, prezygotic isolation
1. Introduction
Sexual conflict (Parker 1979) is ubiquitous in sexually reproducing organisms and can generate substantial selection on reproductive characters (Parker 1979, 2006; Lessells 2006). As a result, sexual conflict has the potential to cause populations to diverge in their reproductive traits, and hence to cause reproductive isolation (Parker & Partridge 1998; Rice 1998; Gavrilets 2000). Interestingly, some theoretical investigations have suggested that reproductive isolation could occur faster between larger populations since they harbour more genetic variation and represent a larger mutational target, speeding the recruitment of sexually antagonistic alleles (Gavrilets 2000). This contrasts with neutral expectations where reproductive isolation is expected to be more rapid in small populations (at least over short time scales) through stochastic fixation of different reproductive alleles by genetic drift (e.g. Lande 1981).
Work with Drosophila melanogaster where levels of sexual conflict were manipulated by altering the adult sex ratio (while census population size was kept constant) found no evidence that increased sexual conflict generated more rapid reproductive isolation (Wigby & Chapman 2006). By contrast, experimental evolution of Sepsis cynipsea (a dungfly) with and without sexual conflict (monogamous versus polyandrous populations) provided powerful evidence for isolation through conflict (Martin & Hosken 2003a). This latter experiment also tested the idea that sexual conflict could generate reproductive isolation more rapidly in larger populations. In agreement with theory (Gavrilets 2000), high density, larger census-size populations showed more behavioural reproductive isolation from each other than did smaller or monogamous—reduced sexual conflict—populations (Martin & Hosken 2003a).
While the findings of these studies seem robust and clear-cut, both studies were subsequently criticized for the analyses employed (Bacigalupe et al. 2007). In fact, it was suggested that there was no adequate way to assess differential effects of sexual conflict on reproductive isolation across treatments in these studies, due to the complicated nesting structure of the experimental designs, which mixed random effects of selection and nested fixed effects of mating type (allopatry and sympatry). This assessment seems to challenge much of the speciation literature, which in essence, performs these comparisons (see Coyne & Orr 2004).
There are many ways to measure behavioural isolation (Coyne & Orr 2004) and one commonly used statistic (Powell 1997) I, can be represented as:
 |
This measure scales across (allopatric) versus within (sympatric) population measures of reproductive success (isolation), and in the context of the sexual conflict studies mentioned above, generates an index that allows reproductive isolation to be assessed across different experimental treatment levels. Here we reassess previous findings using this established index of isolation, to estimate levels of behavioural reproductive isolation in D. melanogaster and S. cynipsea. Our findings support previous conclusions.
2. Material and methods
Experimental populations of the two flies (D. melanogaster and S. cynipsea) were allowed to evolve under different levels of sexual conflict as described by Martin & Hosken (2003a) and Wigby & Chapman (2004). Briefly, for D. melanogaster, adult flies were maintained in either female biased (FB, 25 male : 75 female; low sexual conflict), equal sex ratio (ES, 50 : 50; intermediate sexual conflict) or male biased (MB, 75 : 25; high sexual conflict) populations, with three replicates of each (=nine populations in total). Fresh sugar–yeast food (e.g. Wigby & Chapman 2004) with added live yeast was supplied every 2–3 days. Adults interacted for 10 days before eggs were sampled in order to propagate the next generation. Larvae were grown at standard density and emerging adults were allowed to enclose over 2 days before they were sorted into their appropriate treatments to start the next generation (see Wigby & Chapman 2004).
After 41 generations, the selected flies were assessed for reproductive isolation. Pairs of virgin flies were placed together with males from their own treatment (FB, ES, MB), but from either sympatric (e.g. MB1×MB1) or allopatric (e.g. MB1×MB2) populations. The time to mate for each pair, and the total number of matings were recorded for 1 hour after the flies were placed together. For each cross type (sympatric or allopatric), the behaviour of 50 pairs was observed (see the dung fly experiment below and Wigby & Chapman 2006).
For S. cynipsea, flies were housed under one of three treatments, monogamy (M=relaxed sexual conflict: n=20 fly pairs/populations), low-density populations (LD: n=50 (25 males, 25 females)) and high-density populations (HD: n=500 (250 males, 250 females)), with three replicates of each treatment (=nine populations in total). All populations were maintained with excess water, pollen and sugar. Populations were supplied with small portions of fresh dung every 3 days and flies were left to interact. After approximately 12 days (=approximately 50% of laboratory lifespan), larger dung portions were provided. Flies emerging from these were separated by sex (within populations) and used to start the next generation (for further description see Martin & Hosken 2003a,b).
After 35 generations, flies were housed under standardized selection for two generations to eliminate differential maternal effects. To assess population divergence, experimental females were placed with males from the same treatment (M or LD or HD), but from either their own (‘sympatric’, e.g. M1×M1) or different populations (‘allopatric’, e.g. M1×M2) and mating behaviour was recorded. This was done by placing each female with a single male for 30 min and their behaviour assessed by counting the numbers of females to copulate. For each cross type, the behaviour of 50 females was observed (e.g. 50 M1 females with M1 males for pairings within the M1 population or 25 M1 females with 25 M2 and 25 M2 females with 25 M1 males for pairings between populations M1 and M2, and so on).
(a). Analyses
There are many ways that reproductive isolation can be assessed (e.g. Coyne & Orr 2004), but in the experiments we test here, the between-population crosses were pooled according to female origin (e.g. female M1 crossed with M2 and M3 males were pooled and treated as the allopatric cross for M1; Martin & Hosken 2003a). We avoid the complicated nesting of the full experimental design by deriving a single isolation index for each experimental unit: each female line in each ‘conflict’ treatment. Each population appears more than once in the crosses because we are interested in their isolation from each other (within conflict treatments), but this approach reduces the influence of any single cross combination while minimizing pseudoreplication by keeping our total replication at nine (=number of experimental units/species). Thus, the approach we chose was a compromise between these concerns. For each species, we first calculated the proportion of each cross type (allopatry/sympatry) that copulated in each treatment. This was done at a single time for S. cynipsea (percentage copulating within 30 min), and at 10, 17 and 40 min for D. melanogaster. Then an isolation index (I=(percentage of successful allopatric pairings−percentage of successful sympatric pairings)/percentage of successful sympatric pairings) was calculated for each population in each treatment (M, LD, HD or ES, MB, FB) and a GLM was used to compare the degree of behavioural isolation across treatments for each species. This isolation index should be zero if there is no difference between allopatric and sympatric crosses, negative if there are more sympatric than allopatric matings and positive if there are more allopatric than sympatric matings. Residuals from all these analyses were normally distributed (Kolmogorov–Smirnov tests p>0.66).
3. Results
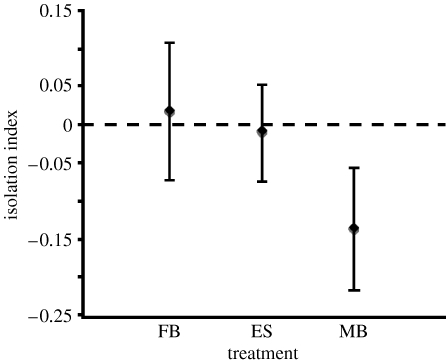
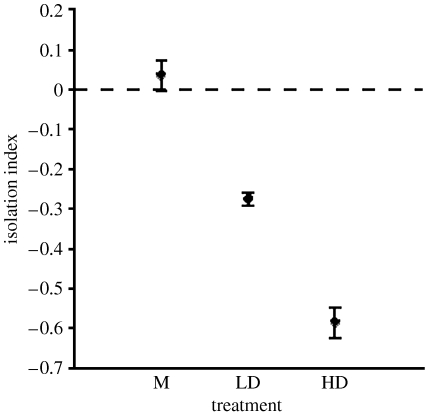
For D. melanogaster, there was no statistically significant effect of treatment on the isolation indices in any time comparison or in a multivariate analysis (all F<1.4; all p>0.3), although for all of them, the male-biased populations tended to show the highest degree of isolation (the lowest index; figure 1). For S. cynipsea, the model relating our isolation indices to treatment as the predictor was highly statistically significant (F 2,6=91.5; p=0.0001), with post hoc tests (Fisher's protected least significant difference) showing IM>ILD>IHD (IM versus ILD, p=0.0005; IM versus IHD, p=0.0005; ILD versus IHD p=0.0001; figure 2).
Figure 1.
The effects of sexual conflict levels on behavioural measures of reproductive isolation in experimental populations of D. melanogaster. (Shown here is the 40 min comparison (see text).)
Figure 2.
The effects of sexual conflict levels on behavioural measures of reproductive isolation in experimental populations of S. cynipsea. (Populations evolving with the highest levels of sexual conflict (HD) show the greatest degree of isolation.)
4. Discussion
For D. melanogaster, there was no evidence that sexual conflict generated behavioural isolation, which agrees with previous findings (Wigby & Chapman 2006), and also reflects findings in Drosophila pseudoobscura (Bacigalupe et al. 2007). However, it should be noted that in all comparisons, there was a tendency for the populations with more sexual conflict (MB) to show more isolation, although the within treatment variation was large (figure 1). Nevertheless, it is interesting that there was no strong behavioural isolation in D. melanogaster, a species characterized by sexual conflict and sexually antagonistic selection (e.g. Rice 1996; Pitnick & García-González 2002). However, much of this conflict seems to be manifest at the post-copulatory level (e.g. Chapman et al. 1995; Wigby & Chapman 2005), and it was there that micro-evolutionary responses to sex-ratio manipulation were documented in these same populations (Wigby & Chapman 2004). By contrast, there was evidence for isolation in S. cynipsea, a species that exhibits extreme pre-copulatory sexual conflict (e.g. Allen & Simmons 1996). Here the larger, HD populations showed more behavioural isolation from each other than the LD populations, and the monogamous populations showed the least isolation. Additionally, since the standard error bars of the monogamous populations crossed zero, there is no indication that sympatric or allopatric crosses diverged under M, even though drift was potentially the strongest in these populations. These results are consistent with theoretical predictions (Gavrilets 2000), although new mutations are unlikely to have underpinned the evolution we documented. Additionally, these findings confirm previous conclusions (Martin & Hosken 2003a), and together document patterns consistent with sexually antagonistic coevolution in this species. For S. cynipsea, the largest populations showing the greatest isolation were five times larger than those used in the D. melanogaster study, which could explain the different findings: there was more standing genetic variation in the S. cynipsea populations on which selection could act. However, it also seems likely that although sexual conflict is ubiquitous, its effects vary markedly across taxa, and therefore it is not surprising that we find different outcomes in different species—this is the hallmark of laboratory studies of speciation in general (Coyne & Orr 2004). To further illustrate this point, there are for example striking differences in the apparent effects of sexual conflict in two very closely related Drosophila: D. melanogaster and Drosophila simulans. In D. melanogaster, more attractive males reduce direct measures of female fitness (e.g. Pitnick & García-González 2002), while this is not the case for D. simulans (e.g. Hosken et al. 2008).
Acknowledgments
Thanks to Roger Butlin, Tom Starmer, Goran Arnqvist, Locke Rowe, Kate Lessells, Tom Tregenza, Laurene Gay and Paul Eady, and NERC and the SNF for financial support.
Footnotes
One contribution of 16 to a Special Feature on ‘Sexual conflict and sex allocation: evolutionary principles and mechanisms’.
References
- Allen G.R., Simmons L.W.1996Coercive mating, fluctuating asymmetry, and male mating success in the dung fly Sepsis cynipsea. Anim. Behav 52, 737–741doi:10.1006/anbe.1996.0218 [Google Scholar]
- Bacigalupe L.D., Crudgington H.S., Hunter F., Moore A.J., Snook R.R.2007Sexual conflict does not drive reproductive isolation in experimental populations of Drosophila pseudoobscura. J. Evol. Biol 20, 1763–1771doi:10.1111/j.1420-9101.2007.01389.x [DOI] [PubMed] [Google Scholar]
- Chapman T., Liddle L.F., Kalb J.M., Wolfner M.F., Partridge L.1995Costs of mating in Drosophila females is mediated by male accessory gland products. Nature 373, 241–244doi:10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Coyne J.A., Orr H.A.Speciation 2004Sunderland, MA:Sinauer [Google Scholar]
- Gavrilets S.2000Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–888doi:10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Hosken D.J., Taylor M.L., Hoyle K., Higgins S., Wedell N.2008Attractive males have greater success in sperm competition. Curr. Biol 18, R553–R554doi:10.1016/j.cub.2008.04.028 [DOI] [PubMed] [Google Scholar]
- Lande R.1981Models of speciation by sexual selection on polygenic characters. Proc. Natl Acad. Sci. USA 78, 3721–3725doi:10.1073/pnas.78.6.3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells C.M.2006The evolutionary outcome of sexual conflict. Phil. Trans. R. Soc. B 361, 301–317doi:10.1098/rstb.2005.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O.Y., Hosken D.J.2003aThe evolution of reproductive isolation through sexual conflict. Nature 423, 979–982doi:10.1038/nature01752 [DOI] [PubMed] [Google Scholar]
- Martin O.Y., Hosken D.J.2003bCosts and benefits of evolving under experimentally enforced polyandry or monogamy. Evolution 57, 2765–2772doi:10.1111/j.0014-3820.2003.tb01518.x [DOI] [PubMed] [Google Scholar]
- Parker G.A.1979Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects eds Blum M.S., Blum N.A.pp. 123–166 London, UK:Academic Press [Google Scholar]
- Parker G.A.2006Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235–259doi:10.1098/rstb.2005.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A., Partridge L.1998Sexual conflict and speciation. Phil. Trans. R. Soc. Lond. B 353, 261–274doi:10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S., García-González F.2002Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. Lond. B 269, 1821–1828doi:10.1098/rspb.2002.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.R.1997Progress and prospects in evolutionary biology: the Drosophila model Oxford, UK:Oxford University Press [Google Scholar]
- Rice W.R.1996Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234doi:10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rice W.R.1998Intergenomic conflict, interlocus antagonisic coevolution, and the evolution of reproductive isolation. In Endless forms: species and speciation eds Howard D.J., Berlocher S.H.pp. 261–270 Oxford, UK:Oxford University Press [Google Scholar]
- Wigby S., Chapman T.2004Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution 58, 1028–1037doi:10.1554/03-568 [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2005Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol 15, 316–321doi:10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2006No evidence that experimental manipulation of sexual conflict drives premating reproductive isolation in Drosophila melanogaster. J. Evol. Biol 19, 1033–1039doi:10.1111/j.1420-9101.2006.01107.x [DOI] [PubMed] [Google Scholar]