Abstract
Despite widespread interest in the interplay between evolutionary and developmental processes, we still know relatively little about the evolutionary history of larval development. Many clades exhibit multiple shifts from planktotrophic (feeding) to non-planktotrophic (non-feeding) larval development. An important question is whether these switches are scattered randomly through geological history or are concentrated in particular intervals of time. This issue is addressed using the Cretaceous spatangoid sea urchins, which are unusual in that larval strategy can be determined unambiguously from abundantly fossilized adult tests. Using a genus-level phylogeny, we identify five clades of non-planktotrophic taxa, each of which first appears in the fossil record in the Campanian or Maastrichtian (the final two Cretaceous stages). No examples of non-planktotrophy have been identified in any of the earlier stages of the Cretaceous. This strongly suggests that shifts to non-planktotrophic development are clustered in certain episodes of geological history, and this, in turn, implies that extrinsic factors operating at these times are responsible for driving shifts in developmental strategy.
Keywords: non-planktotrophy, planktotrophy, echinoids, spatangoids, Cretaceous
1. Introduction
Most marine invertebrates employ one of two distinct strategies for larval development. In the first of these, termed planktotrophy, the larvae feed in the water column for a period of weeks or months before they settle and metamorphose into juveniles. In the alternative strategy, termed non-planktotrophy, the larvae are produced in much smaller numbers and do not feed, but instead are supplied with nourishment in the form of an egg yolk for the much shorter period they spend either living in the water column or being brooded prior to metamorphosis.
A common theme that has been identified is for clades to exhibit multiple shifts from planktotrophic development to non-planktotrophic development. This has been inferred by mapping larval modes onto phylogenetic trees of various taxa (e.g. echinoids (Wray 1992; Smith et al. 1995); temnopleurid echinoids (Jeffery & Emlet 2003; Jeffery et al. 2003); asterinid starfish (Hart et al. 1997); Conus gastropods (Duda & Palumbi 1999); littorinid gastropods (Reid 1989); turritellid gastropods (Lieberman et al. 1993)). We now know a great deal about the developmental and genetic changes involved in these switches owing, in particular, to the large body of work on the non-planktotrophic echinoid Heliocidaris erythrogramma and the closely related Heliocidaris tuberculata, which has planktotrophic development (reviewed by Raff & Byrne 2006). However, an important, and yet much overlooked, issue is when these switches to non-planktotrophy occurred: were shifts to non-planktotrophy scattered randomly through time, or were they instead concentrated in particular intervals of geological history? The question is important as it may help to determine the factors that drive shifts to non-planktotrophy. If the switches were concentrated in particular time intervals, then this would strongly imply that extrinsic factors operating at these times are responsible for driving the switches to non-planktotrophy.
The tacit assumption among biologists appears to be that the switches were scattered randomly throughout geological history (Jeffery 1997). However, when Jeffery (1997) carried out a broad survey of all major echinoid clades, she found that the first known occurrence of non-planktotrophy in five different orders lay in the final two stages of the Cretaceous (the Campanian and Maastrichtian), with no examples of non-planktotrophic echinoids known from earlier than this interval. Despite this study, little is known about the finer scale patterns within orders and at lower taxonomic levels. The present study addresses this by focusing in detail on a single echinoid order, the Spatangoida.
2. Material and methods
(a). Choice of model organism
The Cretaceous spatangoid echinoids were chosen as the model organism for this study as they are ideal for a number of reasons: (i) they are unusual in that larval strategy can be inferred from the fossilized adult test using either morphological criteria or crystallographic analysis of the apical system (discussed subsequently); (ii) they are abundantly preserved, usually with the apical system present, providing a large volume of suitable material for analysis; (iii) their complex morphology makes it relatively straightforward to establish their phylogenetic relationships; and (iv) the group contains both planktotrophic and non-planktotrophic taxa and spans the time when non-planktotrophy is thought to have first evolved within the group.
(b). Inferring modes of larval development
Larval modes were determined for 87 fossil spatangoid specimens from museum collections that span the early history of the group (see electronic supplementary material for details). Three criteria were used for inferring the mode of larval development from adult fossil sea urchin specimens.
Identification of brood pouches (marsupia). Some non-planktotrophic taxa brood their larvae and a subset of these do so in specialized brood pouches on the test of the female; the identification of such marsupia is indicative of non-planktotrophic development (e.g. Kier 1969).
Extreme sexual dimorphism of gonopore size. Because non-planktotrophic taxa have much larger eggs (Emlet et al. 1987; Emlet 1989), the females frequently have enlarged gonopores through which to extrude these eggs. Extreme sexual dimorphism can indicate non-planktotrophic development (Emlet 1989), although gonopores can also be enlarged by dissolution (S. K. Donovan 2008, personal communication).
Crystallographic orientation of the apical plates. Larval mode can be inferred from the orientation of the crystallographic axes of the plates in the apical system (Emlet 1985, 1989). This is possible because planktotrophic larvae have skeletal calcite rods, the main function of which is to support the larval arms that are used in feeding; during metamorphosis some of the apical plates grow from the proximal ends of the rods, imparting characteristic c-axis orientations to the plates. In non-planktotrophic larvae, these rods are absent and the apical plates all form de novo at metamorphosis, producing a distinct pattern of c-axis orientations.
(c). Determining number and timing of switches
The inferred larval modes were mapped onto a novel phylogeny of the group (see electronic supplementary material for details of analysis). This allowed determination of the number and direction of switches in developmental mode within the group. This information was combined with the stratigraphic ranges of the taxa to elucidate the timing of these shifts in developmental mode.
3. Results
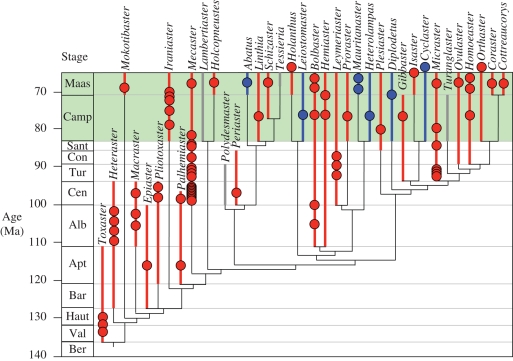
The data on larval modes inferred from morphological and crystallographic criteria were mapped onto the phylogeny of Cretaceous spatangoids, which is plotted against the geological time scale in figure 1. These results reveal that non-planktotrophic development arose independently five times within the Cretaceous spatangoids. All five non-planktotrophic clades first appear in the fossil record in either the Campanian or Maastrichtian. The known ranges of taxa exhibiting non-planktotrophy extend no further back in time than the early Campanian, and planktotrophy is the only strategy observed prior to this. The Campanian to Maastrichtian is also the interval during which the first known instance of non-planktotrophy in four other echinoid orders has been recorded; there are no reported occurrences of non-planktotrophy in echinoids from any time earlier than this (Jeffery 1997).
Figure 1.
Strict consensus tree of Cretaceous spatangoid genera plotted against the geological time scale. Planktotrophic taxa are shown in red, non-planktotrophic taxa in blue and unknown development in grey. Coloured circles represent specimens for which development has been inferred. The green band highlights the Campanian to Maastrichtian interval. Analysed specimens of Holanthus, Cyclaster and Orthaster are Danian in age (see electronic supplementary material for details of phylogenetic analysis and specimens studied).
4. Discussion
While this study identifies five shifts to non-planktotrophic development during the Late Cretaceous, Peterson (2005) recognized that switches occurred in the opposite direction at least four, and perhaps as many as eight, times between the Late Cambrian and Middle Ordovician. Taken together, these studies show that switches in developmental mode are, at least in some circumstances, concentrated within particular intervals of geological history. This pattern of coordinated shifts in independent clades strongly implies that extrinsic factors operating at the times of the shifts were responsible for driving these switches in developmental mode. The two most likely external driving mechanisms are predation (Peterson 2005) and environmental change (e.g. Jeffery 1997). While the data to quantitatively test which factor is most likely to be responsible are not yet available, it is possible to elucidate this issue qualitatively by assessing whether major changes in either proposed factor occurred at the time of the developmental switches.
Having argued that the radiation of epifaunal suspension feeders in the Late Cambrian to Middle Ordovician was most probably responsible for the coordinated switches to planktotrophy that occurred at this time, Peterson (2005) argued that benthic predation was also likely to have driven later switches to non-planktotrophy. The basis for this argument is that adaptations associated with non-planktotrophic development (e.g. positive buoyancy) also serve to protect the larva from predation. In addition, it has been shown that reducing time to metamorphosis is advantageous in environments with high levels of predation (Wray 1995).
While predation may well account for some of the selection pressure to evolve non-planktotrophy, it is unlikely to account for the coordinated switches to non-planktotrophic development that we have identified in sea urchins at the end of the Cretaceous. If benthic predation were responsible, then we would expect to see a major radiation of benthic predators coinciding with the shifts. However, while there is little literature on change in predation rate in the Late Cretaceous, it seems that any increases in predation occurred earlier in the Cretaceous than the interval in question (Kosnik 2005).
The other factor that has been suggested as a driving mechanism for switches to non-planktotrophy is environmental change (Jeffery 1997). In the Campanian and Maastrichtian, the climate was changing dramatically. Sea surface temperature was falling rapidly (e.g. Gale 2000) and, while total primary productivity was increasing (Faul et al. 2003), there is evidence that this was associated with increasing climatic seasonality (Steuber 1996; Francis & Poole 2002; Steuber et al. 2005; Dutton et al. 2007) and vigorous seasonal upwelling (Handoh et al. 2003). These changes would have led to the nutrient supply available to planktotrophic larvae becoming more abundant yet less reliable. Modern invertebrates living in areas with intermittent nutrient supply, such as strongly seasonal regions, tend to have evolved either so that their reproductive cycle coincides with the nutrient blooms, or else so that they develop with non-planktotrophic larvae and thus become independent of the nutrient supply (e.g. Picken 1980). The fact that the coordinated shifts in developmental mode that we have identified coincide with these major environmental changes (but not with a marked change in predation—the other proposed driver) identifies environmental factors as the most plausible driving mechanism for these switches to non-planktotrophic development. Thus, it seems likely that there are intrinsic links between major developmental change and major environmental change.
Acknowledgements
We thank Daniela Schmidt (Bristol) for discussion, two anonymous reviewers for helpful suggestions and Andrew Smith, Dave Lewis and Andrew Ross (NHM, London), Jann Thompson and Daniel Levin (Smithsonian Institute), Jean-Paul Saint-Martin, Jean-Michel Pacaud, Agnès Rage and Didier Merle (MNHN, Paris) and Andreas Kroh (NHMW, Vienna) for access to and loan of material. J.A.C. was funded by NERC studentship NER/S/A/2004/12698.
References
- Duda T. F., Palumbi S. R.1999Developmental shifts and species selection in gastropods. Proc. Natl Acad. Sci. USA 96, 10 272–10 277 (doi:10.1073/pnas.96.18.10272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Huber B. T., Lohmann K. C., Zinsmeister W. J.2007High-resolution stable isotope profiles of a dimitobelid belemnite: implications for paleodepth habitat and late Maastrichtian climate seasonality. Palaios 22, 642–650 [Google Scholar]
- Emlet R. B.1985Crystal axes in recent and fossil adult echinoids indicate trophic mode in larval development. Science 230, 937–940 (doi:10.1126/science.230.4728.937) [DOI] [PubMed] [Google Scholar]
- Emlet R. B.1989Apical skeletons of sea-urchins (Echinodermata, Echinoidea): 2 methods for inferring mode of larval development. Paleobiology 15, 223–254 [Google Scholar]
- Emlet R. B., McEdward L. R., Strathmann R. R.1987Echinoderm larval ecology viewed from the egg. In Echinoderm studies 2 (eds Jangoux M., Lawrence J. M.), pp. 55–136 Rotterdam, The Netherlands: Balkema [Google Scholar]
- Faul K. L., Anderson L. D., Delaney M. L.2003Late Cretaceous and Early Paleogene nutrient and paleoproductivity records from Blake Nose, western North Atlantic Ocean. Paleoceanography 18, 1–16 (doi:10.1029/2001PA000722) [Google Scholar]
- Francis J. E., Poole I.2002Cretaceous and early Tertiary climates of Antarctica: evidence from fossil wood. Palaeogeogr. Palaeoclimatol. Palaeoecol. 182, 47–64 (doi:10.1016/S0031-0182(01)00452-7) [Google Scholar]
- Gale A. S.2000The Cretaceous world. In Biotic response to global change. The last 145 million years (eds Culver S. J., Rawson P. F.), pp. 4–20 Cambridge, UK: The Natural History Museum/Cambridge University Press [Google Scholar]
- Handoh I. C., Bigg G. R., Jones E. J. W.2003Evolution of upwelling in the Atlantic Ocean basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 202, 31–58 (doi:10.1016/S0031-0182(03)00571-6) [Google Scholar]
- Hart M. W., Byrne M., Smith M. J.1997Molecular phylogenetic analysis of life-history evolution in asterinid starfish. Evolution 51, 1848–1861 (doi:10.2307/2411007) [DOI] [PubMed] [Google Scholar]
- Jeffery C. H.1997Dawn of echinoid nonplanktotrophy: coordinated shifts in development indicate environmental instability prior to the K-T boundary. Geology 25, 991–994 (doi:10.1130/0091-7613(1997)025<0991:DOENCS>2.3.CO;2) [Google Scholar]
- Jeffery C. H., Emlet R. B.2003Macroevolutionary consequences of developmental mode in temnopleurid echinoids from the Tertiary of southern Australia. Evolution 57, 1031–1048 [DOI] [PubMed] [Google Scholar]
- Jeffery C. H., Emlet R. B., Littlewood D. T. J.2003Phylogeny and evolution of developmental mode in temnopleurid echinoids. Mol. Phylogenet. Evol. 28, 99–118 (doi:10.1016/S1055-7903(03)00030-7) [DOI] [PubMed] [Google Scholar]
- Kier P. M.1969Sexual dimorphism in fossil echinoids. In Sexual dimorphism in fossil Metazoa and taxonomic implications (ed. Westerman G. E. G.), pp. 215–222 Stuttgart, Germany: Schweizerbart [Google Scholar]
- Kosnik M. A.2005Changes in Late Cretaceous–early Tertiary benthic marine assemblages: analyses from the North American coastal plain shallow shelf. Paleobiology 31, 459–479 (doi:10.1666/0094-8373(2005)031[0459:CILCTB]2.0.CO;2) [Google Scholar]
- Lieberman B. S., Allmon W. D., Eldredge N.1993Levels of selection and macroevolutionary patterns in the turritellid gastropods. Paleobiology 19, 205–215 [Google Scholar]
- Peterson K. J.2005Macroevolutionary interplay between planktic larvae and benthic predators. Geology 33, 929–932 (doi:10.1130/G21697.1) [Google Scholar]
- Picken G. B.1980Reproductive adaptations of Antarctic benthic invertebrates. Biol. J. Linn. Soc. 14, 67–75 (doi:10.1111/j.1095-8312.1980.tb00098.x) [Google Scholar]
- Raff R. A., Byrne M.2006The active evolutionary lives of echinoderm larvae. Heredity 97, 244–252 (doi:10.1038/sj.hdy.6800866) [DOI] [PubMed] [Google Scholar]
- Reid D. G.1989The comparative morphology, phylogeny and evolution of the gastropod family Littorinidae. Phil. Trans. R. Soc. Lond. B 324, 1–110 (doi:10.1098/rstb.1989.0040) [Google Scholar]
- Smith A. B., Littlewood D. T. J., Wray G. A.1995Comparing patterns of evolution: larval and adult life-history stages and ribosomal-RNA of post-Palaeozoic echinoids. Phil. Trans. R. Soc. Lond. B 349, 11–18 (doi:10.1098/rstb.1995.0085) [Google Scholar]
- Steuber T.1996Stable isotope sclerochronology of rudist bivalves: growth rates and Late Cretaceous seasonality. Geology 24, 315–318 (doi:10.1130/0091-7613(1996)024<0315:SISORB>2.3.CO;2) [Google Scholar]
- Steuber T., Rauch M., Masse J. P., Graaf J., Malkoc M.2005Low-latitude seasonality of Cretaceous temperatures in warm and cold episodes. Nature 437, 1341–1344 (doi:10.1038/nature04096) [DOI] [PubMed] [Google Scholar]
- Wray G. A.1992The evolution of larval morphology during the post-Paleozoic radiation of echinoids. Paleobiology 18, 258–287 [Google Scholar]
- Wray G. A.1995Evolution of larvae and developmental modes. In Ecology of marine invertebrate larvae (ed. McEdward L.), pp. 413–447 Boca Raton, FL: CRC Press [Google Scholar]