Abstract
The presence of bone growth marks reflecting annual rhythms in the cortical bone of non-avian tetrapods is now established as a general phenomenon. In contrast, ornithurines (the theropod group including modern birds and their closest relatives) usually grow rapidly in less than a year, such that no annual rhythms are expressed in bone cortices, except scarce growth marks restricted to the outer cortical layer. So far, cyclical growth in modern birds has been restricted to the Eocene Diatryma, the extant parrot Amazona amazonica and the extinct New Zealand (NZ) moa (Dinornithidae). Here we show the presence of lines of arrested growth in the long bones of the living NZ kiwi (Apteryx spp., Apterygidae). Kiwis take 5–6 years to reach full adult body size, which indicates a delayed maturity and a slow reproductive cycle. Protracted growth probably evolved convergently in moa and kiwi sometime since the Middle Miocene, owing to the severe climatic cooling in the southwest Pacific and the absence of mammalian predators.
Keywords: Apteryx, kiwi, lines of arrested growth, moa, New Zealand, skeletochronology
1. Introduction
The presence of temporary decreases and/or interruptions of growth reflecting annual rhythms in the cortical bone of non-avian tetrapods is now established as a general phenomenon (e.g. Castanet et al. 1993; Chinsamy-Turan 2005). Such bone growth marks (BGMs) are known in a few non-ornithurine birds (Chinsamy-Turan 2005; Cambra-Moo et al. 2006). In contrast, BGMs are either absent or scarce and restricted to the outer cortical layer (OCL) of bone cortices in most ornithurines including Hesperornis, Ichthyornis and neornithine birds (Chinsamy-Turan 2005). This growth pattern is due to the achievement of complete skeletal development in less than a year, which is why skeletochronological studies based on living birds remain limited and controversial (Broughton et al. 2002; Castanet 2006). Among modern birds, BGMs not associated with the OCL are presently restricted to the Eocene Diatryma, the extant psittacid Amazona amazonica (Ricqlès et al. 2001) and the extinct New Zealand (NZ) moa (Dinornithidae) (Turvey et al. 2005). Previously, BGMs were regarded as absent in extant ratites (Turvey et al. 2005). Here we show that BGMs are present in the kiwi (Apterygidae), a group of small living ratites endemic to NZ.
2. Material and methods
Four adult individuals of Apteryx including two specimens of Apteryx australis, one specimen of Apteryx haastii and one specimen of Apteryx owenii were used in the study. The sampling consisted of sections at the minimal diameter of the diaphysis for the left femur (midshaft), left tibiotarsus (one-third of shaft from distal end) and left tarsometatarsus (midshaft). Thin sections of bone embedded in polyester resin were examined in ordinary and polarized light, for all individuals. We also prepared frozen sections stained with Ehrlich's haematoxylin for one individual of A. australis. These were examined in ordinary light.
Institutional abbreviations: AMNH, American Museum of Natural History, New York; CMC, Canterbury Museum, Christchurch; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington; NMW, Naturhistorisches Museum Wien, Vienna.
3. Bone histology of apteryx
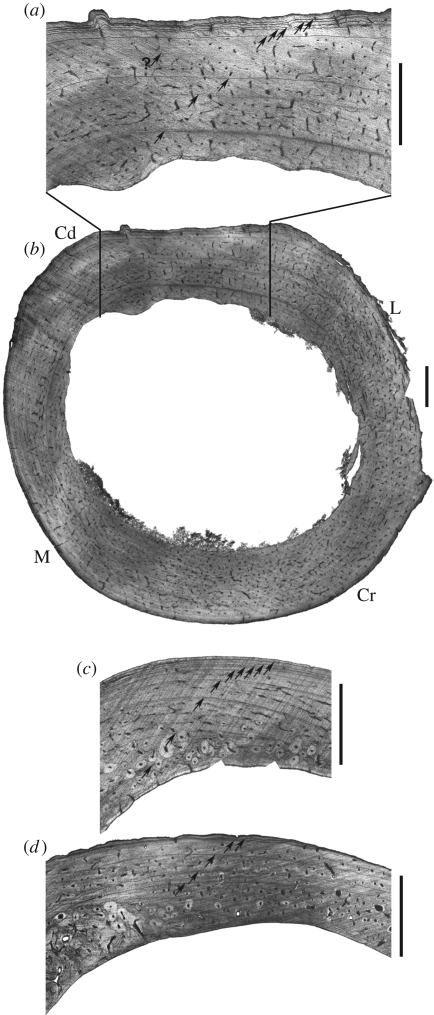
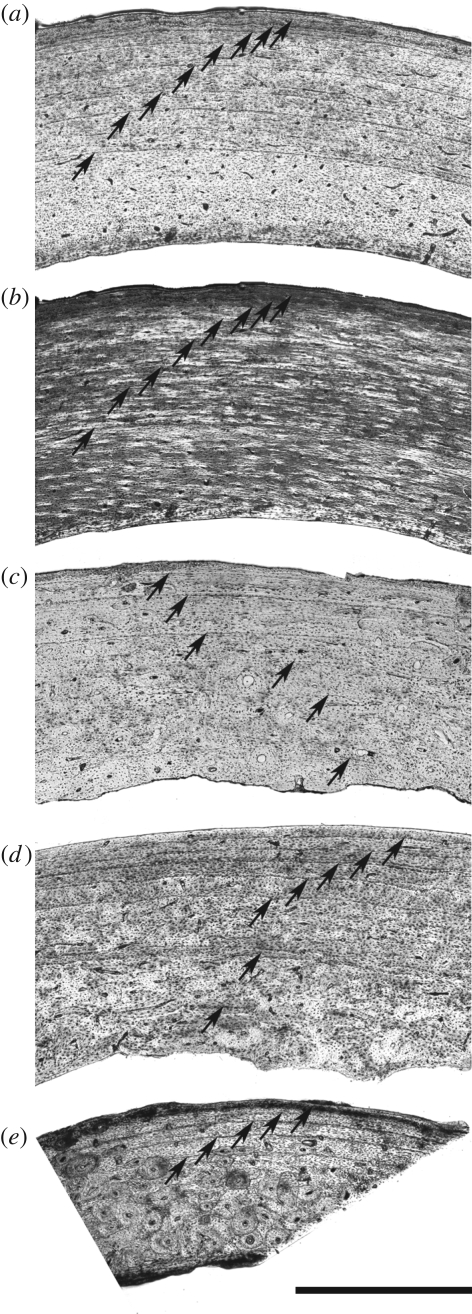
The hind limb bones of Apteryx show a parallel fibred bone matrix. The vascular density is weak and decreases from the inner part of bone cortices towards the periosteum. The vascularization mainly consists of longitudinally oriented simple primary vascular canals or primary osteons. Secondary osteons (Haversian systems) are virtually lacking in the femur of A. australis NMW3606 (figures 1a,b and 2a,b) and are limited to the inner part of femoral cortices in other individuals (figure 2c–e). They are more abundant in the tibiotarsus and the tarsometatarsus (figure 1c,d). Endosteal resorption has erased the innermost part of the cortices, which correspond to the earliest part of the ontogeny (figure 1b).
Figure 1.
Cross sections in long bone diaphyses of Apteryx australis NMW3606 stained with Ehrlich's haematoxylin (ordinary light). (a) and (b) femur; (c) tibiotarsus, craniomedial region; (d) tarsometatarsus, medial region. Cd, caudal region; Cr, cranial region; L, lateral region; M, medial region. Arrows indicate LAGs. Scale bars, 1 mm.
Figure 2.
Cross sections in cranial regions of femoral diaphyses of Apteryx in ordinary (a,c,d,e) and polarized (b) light. (a) and (b) Apteryx australis NMW3606; (c) A. australis NMNZ20995; (d) Apteryx haastii CMC32617; (e) Apteryx owenii NMNZ23214. Arrows indicate LAGs. Scale bar, 1 mm.
In all examined bones, lines of arrested growth (LAGs) are distributed in the entire thickness of cortices. We evaluated the number of erased LAGs to 1 or 2 in the femur of A. australis NMW3606 (figure 1b), based on a method for retrocalculating the age of eroded primary bone tissue (Horner & Padian 2004). We based our estimation on the assumption that radial bone growth rate is higher in young individuals than in older ones (Horner & Padian 2004). The inner preserved LAGs (1 to 4–5) are well separated, indicating significant bone growth rate during 4–5 years after the 1–2-year period of most active growth (assuming that LAGs are annually deposited, as it is the general rule in other vertebrates; Castanet et al. 1993). The outer preserved LAGs are tightened in the OCL, indicating a decrease in bone growth rate. The maximum LAG number amounts to 8 or 9 in the femur and tibiotarsus of A. australis NMW3606 (figures 1a–c and 2a,b). Taking into account the number of erased LAGs, this individual was at least 9 years old at death.
4. Discussion
It is usually stated that kiwi attain their adult size at either 18–20 months of age (Marchant & Higgins 1990; del Hoyo et al. 1992; Davis 2002; Heather & Robertson 2005) or 2–2.5 years (Heather & Robertson 2005). This period of active growth corresponds to the innermost part of bone cortices destroyed by endosteal resorption, and its end marks the achievement of adult bone length and statural adult size. Our histological study reveals that Apteryx does not reach its full adult body size until 5–6 years of age and subsequently shows a prolonged low periosteal osteogenesis for at least four more years. Radial growth of long bones persists several years after the cessation of longitudinal growth, which implies that the robustness of the skeleton and probably the body mass continue to increase over several years. One radiological study by Beale (1985, 1991) has shown that the maturation of the skeleton of the kiwi is not fully achieved until 4.5–5 years of age, which concurs with the ending of significant radial bone growth. The fact that one individual in our study was at least 9 years old at death is not surprising, given that kiwis are known to live over 17 years in the wild and over 20 years in captivity (Marchant & Higgins 1990; del Hoyo et al. 1992; Davis 2002; Heather & Robertson 2005). Another peculiarity of kiwis is that they reach sexual maturity prior to the full achievement of growth: earliest recorded breeding is between 14 months and 3 years for males and between 2 and 4 years for females (del Hoyo et al. 1992; Davis 2002; Sales 2005). Histological information is congruent with the fact that kiwis have a slow reproductive rate and a low metabolic rate when compared with other birds (Marchant & Higgins 1990; del Hoyo et al. 1992; Davis 2002; Heather & Robertson 2005; Sales 2005).
Parsimony suggests that the cyclical growth pattern found in chelonians and lepidosaurs may correspond to the plesiomorphic condition for sauropsids. This growth strategy is largely independent of thermal metabolism. It appears mainly in ectotherms, but also in endotherms (Castanet et al. 2001). Although LAGs in chelonians and lepidosaurs are most often associated with a parallel fibred bone tissue with vascularization either weak or absent, in archosaurs including many dinosaurs, they may appear within the fibrolamellar complex (Chinsamy-Turan 2005), forming what has been called the fibrolamellar-zonal complex (Castanet et al. 2001; Castanet 2006). Among birds, LAGs have been observed in a few non-ornithurines such as Enantiornithes and Patagopteryx (Chinsamy-Turan 2005; Cambra-Moo et al. 2006). Chinsamy-Turan (2005) used this evidence of cyclical growth to argue that non-ornithurine birds grew at much slower rates when compared with modern birds and were not fully endothermic. However, the occurrence of a fibrolamellar complex of fast-growing bone during most of early ontogeny in the basal bird Confuciusornis suggests that the ability to grow rapidly (and the requisite metabolism) is plesiomorphic for birds and originated from a reduction in body size with respect to the ancestral theropodian dinosaur pattern (Ricqlès et al. 2003). Regardless of these opposite interpretations, Ricqlès et al. (2003) and Chinsamy-Turan (2005) agree that basal birds had lower growth rates than most living birds. Derived skeletal growth strategy among sauropsids is found in Ornithurae including Hesperornis, Ichthyornis and neornithine birds (Chinsamy-Turan 2005). It consists of a very fast uninterrupted osteogenesis achieved in less than a year (generally a few weeks), such that no annual rhythms are usually expressed in the bones, except for scarce tightened BGMs restricted to the thin peripheral OCL. Very few modern birds depart from this growth pattern. One single LAG not associated with OCL has been found in the extinct Diatryma and the living parrot Amazona amazonica (Ricqlès et al. 2001). A prolonged osteogenesis interrupted by several LAGs is found in NZ ratites (Turvey et al. 2005; this study), including kiwi as the only example among living birds (this study).
NZ ratites most probably evolved cyclical growth in response to unusual environmental factors. In contrast to a number of recent molecular and morphological works (Bourdon et al. in press) suggesting that NZ ratites are not monophyletic, a new morphological phylogeny (Bourdon et al. in press) revives the traditional hypothesis of a moa–kiwi clade. Cyclical growth might have been apomorphically acquired by the moa–kiwi ancestor, which evolved in the absence of mammalian predators since NZ became isolated from other Gondwanan landmasses, some 82 Myr ago (Worthy & Holdaway 2002). The recent discovery of land mammals in the Middle Miocene of NZ (Worthy et al. 2006) suggests that the NZ avifauna did not evolve in the absence of mammals until the last few million years, but there is no evidence that these small mammals had any influence on ratite growth strategies. On the other hand, the onset of a temperate climate with marked seasonality in NZ only began with the severe climatic cooling that initiated in the southwest Pacific at the Middle Miocene, some 15 Myr ago (Flower & Kennett 1994). In this context, it is more likely that protracted growth evolved convergently in the ancestors of moa and kiwi sometime since the Middle Miocene, whether these two taxa form a monophyletic group or not. The conjunction of a marked seasonality and a lack of predation pressure may have favoured the development of protracted growth and slow reproductive cycle in moa and kiwi. The appearance of cyclical growth in NZ ratites indicates that the growth trajectory in ornithurines is more flexible than previously thought. We propose to explore whether protracted growth appeared in some NZ birds outside ratites.
Acknowledgements
E. Bauernfeind (NMW) kindly provided kiwi bones. This work was supported by the Collège de France (Paris) and the AMNH (F. M. Chapman Memorial Fund, Department of Ornithology). Two anonymous reviewers made useful comments on the manuscript.
References
- Beale G.1985A radiological study of the kiwi (Apteryx australis mantelli). J. R. Soc. N. Z. 15, 187–200 [Google Scholar]
- Beale G.1991The maturation of the skeleton of a kiwi (Apteryx australis mantelli)—a ten year radiological study. J. R. Soc. N. Z. 21, 219–220 [Google Scholar]
- Bourdon E., Ricqlès A. D., Cubo J.In press A new transantarctic relationship: morphological evidence for a Rheidae–Dromaiidae–Casuariidae clade (Aves, Palaeognathae, Ratitae). Zool. J. Linn. Soc [Google Scholar]
- Broughton J. M., Rampton D., Holanda K.2002A test of an osteologically based age determination technique in the double-crested cormorant Phalacrocorax auritus. Ibis 144, 143–146 (doi:10.1046/j.0019-1019.2001.00004.x) [Google Scholar]
- Cambra-Moo O., Buscalioni A. D., Cubo J., Castanet J., Loth M. M., Margerie E. D., Ricqlès A. D.2006Histological observations of enantiornithine bone (Saurischia, Aves) from the Lower Cretaceous of Las Hoyas (Spain). C. R. Palevol 5, 685–691 (doi:10.1016/j.crpv.2005.12.018) [Google Scholar]
- Castanet J.2006Time recording in bone microstructures of endothermic animals; functional relationships. C. R. Palevol 5, 629–636 (doi:10.1016/j.crpv.2005.10.006) [Google Scholar]
- Castanet J., Francillon-Vieillot H., Meunier F. J., Ricqlès A. D.1993Bone and individual aging. In Bone growth (ed. Hall B. K.), pp. 245–283 Boca Raton, FL: CRC Press [Google Scholar]
- Castanet J., Cubo J., Margerie E. D.2001Signification de l'histodiversité osseuse: le message de l'os. Biosystema 19, 133–147 [Google Scholar]
- Chinsamy-Turan A.2005The microstructure of dinosaur bone: deciphering biology with fine-scale techniques Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Davis S. J. J. F.2002Ratites and tinamous: Tinamidae, Rheidae, Dromaiidae, Casuariidae, Apterygidae, Struthionidae New York, NY: Oxford University Press [Google Scholar]
- del Hoyo J., Elliott A., Sargatal J.1992Handbook of the birds of the world. Vol. 1, ostrich to ducks Barcelona, Spain: Lynx Edicions [Google Scholar]
- Flower B. P., Kennett J. P.1994The middle Miocene climatic transition: East Antarctic ice sheet development, deep ocean circulation and global carbon cycling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 108, 537–555 (doi:10.1016/0031-0182(94)90251-8) [Google Scholar]
- Heather B. D., Robertson H. A.2005The field guide to the birds of New Zealand Auckland, New Zealand: Viking [Google Scholar]
- Horner J. R., Padian K.2004Age and growth dynamics of Tyrannosaurus rex. Proc. R. Soc. Lond. B 271, 1875–1880 (doi:10.1098/rspb.2004.2829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant S., Higgins P. J.1990Handbook of Australian, New Zealand and Antarctic Birds. Vol. 1, ratites to ducks Melbourne, Australia: Oxford University Press [Google Scholar]
- Ricqlès A. D., Padian K., Horner J. R.2001The bone histology of basal birds in phylogenetic and ontogenetic perspectives. In New perspective on the origin and evolution of birds (eds Gauthier J. A., Gall L. F.), pp. 411–426 New Haven, CT: Yale University Press [Google Scholar]
- Ricqlès A. D., Padian K., Horner J. R., Lamm E.-T., Myhrvold N.2003Osteology of Confuciusornis sanctus (Theropoda: Aves). J. Vert. Paleontol. 23, 373–386 (doi:10.1671/0272-4634(2003)023[0373:OOCSTA]2.0.CO;2) [Google Scholar]
- Sales J.2005The endangered kiwi: a review. Folia Zool. 54, 1–20 [Google Scholar]
- Turvey S. T., Green O. R., Holdaway R. N.2005Cortical growth marks reveal extended juvenile development in New Zealand moa. Nature 435, 940–943 (doi:10.1038/nature03635) [DOI] [PubMed] [Google Scholar]
- Worthy T. H., Holdaway R. N.2002The lost world of the moa: prehistoric life of New Zealand Bloomington, NZ: Indiana University Press [Google Scholar]
- Worthy T. H., Tennyson A. J. D., Archer M., Musser A. M., Hand S. J., Jones C., Douglas B. J., McNamara J. A., Beck R. M. D.2006Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proc. Natl Acad. Sci. USA 103, 19 419–19 423 (doi:10.1073/pnas.0605684103) [DOI] [PMC free article] [PubMed] [Google Scholar]