Abstract
Supplementation of wild populations with captive-bred organisms is a common practice for conservation of threatened wild populations. Yet it is largely unknown whether such programmes actually help population size recovery. While a negative genetic effect of captive breeding that decreases fitness of captive-bred organisms has been detected, there is no direct evidence for a carry-over effect of captive breeding in their wild-born descendants, which would drag down the fitness of the wild population in subsequent generations. In this study, we use genetic parentage assignments to reconstruct a pedigree and estimate reproductive fitness of the wild-born descendants of captive-bred parents in a supplemented population of steelhead trout (Oncorhynchus mykiss). The estimated fitness varied among years, but overall relative reproductive fitness was only 37 per cent in wild-born fish from two captive-bred parents and 87 per cent in those from one captive-bred and one wild parent (relative to those from two wild parents). Our results suggest a significant carry-over effect of captive breeding, which has negative influence on the size of the wild population in the generation after supplementation. In this population, the population fitness could have been 8 per cent higher if there was no carry-over effect during the study period.
Keywords: captive breeding, reproductive success, parentage, steelhead trout
1. Introduction
Many conservation programmes now employ captive breeding to aid endangered and threatened species (Olney et al. 1994; Frankham et al. 2002; Allendorf & Luikart 2007). It is estimated that thousands of species will require captive breeding to prevent their extinction over the next 200 years (Allendorf & Luikart 2007). However, the effects of supplementing wild populations with captive-bred organisms (supplementation) are not clear yet.
Any negative effects of captive breeding are especially relevant for salmonid species because of the worldwide decline of native salmonid populations and the huge scale of hatchery programmes to compensate for those losses. Firstly, there is scant evidence that adding captive-bred organisms has boosted the long-term productivity of wild salmonid populations (Fraser 2008). Secondly, supplementation of declining wild populations entails risks such as disease introductions, increased competition for resources, and genetic changes in the supplemented population (Waples & Drake 2004). The genetic risk results because artificial environments can select for captive-bred individuals that are maladapted to the natural environment (hereafter ‘the wild’). For example, genetically-based loss of fitness in the wild has been well documented in the case of hatchery-reared salmonid fishes (Reisenbichler & McIntyre 1977; Reisenbichler & Rubin 1999; Araki et al. 2007a, 2008). Thus, captive-bred organisms could potentially drag down the fitness of the wild populations they are meant to support, even while temporarily boosting their numbers. Although this phenomenon has been predicted in theory (Lynch & O'Hely 2001; Ford 2002), it has never been demonstrated empirically.
In this study, we use genetic parentage assignments in a supplemented population of steelhead trout (Oncorhynchus mykiss) as follows: (i) to test whether genetic effects of captive breeding persist in the wild-born descendents of the captive-bred organisms in the wild and, if so, (ii) to estimate the change in population fitness the genetic effects potentially imposed on the wild population. In a previous study (Araki et al. 2007a,b), we examined the reproductive fitness of captive-bred fish in the wild using the same molecular technique in the same population (the Hood River, OR, USA). The results suggest that first-generation hatchery fish were reproductively less fit than wild fish, and that second-generation hatchery fish were even less fit than first-generation fish (note that here we use ‘wild’ to mean organisms born and reared in the wild, regardless of their parentage). The estimated reduction in fitness of captive-bred fish was up to 40 per cent per generation (Araki et al. 2007b). Now we ask whether their wild-born offspring, which successfully survived a full generation of selection in the wild, can leave as many adult offspring as wild fish that have not been influenced by captive breeding (figure 1). This comparison provides a unique opportunity to estimate the change in wild population size owing to any carry-over effect of captive breeding.
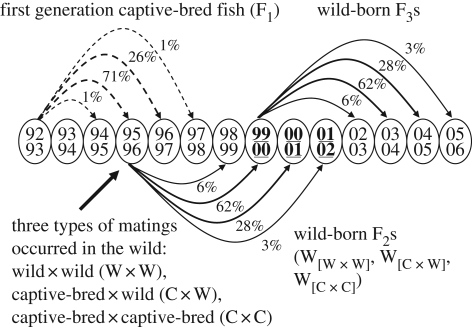
Figure 1.
Sampling design for this study. Circles, years of the fish run into the river (top) and years of their spawning (bottom). The first generation of hatchery fish (F1) were created and released in 1992. Subsequent cohorts of first-generation hatchery fish have been released every year since then, but for simplicity we here illustrate a single cohort in each generation. Solid lines, wild-born fish; dotted lines, hatchery-born fish. Percentages show the average fraction of each cohort that returned in each subsequent year, using the demographic data during 1995–2006 (shown only when 1% or higher). The first cohort of hatchery-born fish returned to spawn during 1994 to 1997. Mating among hatchery and wild fish on the spawning grounds created three types of wild-born F2s, here illustrated for 1995–1996. These wild-born F2s returned to spawn beginning in the late 1990s. We estimated the reproductive fitness of each type of F2 from the number of F3 offspring that returned as adults 3 to 6 years later in each of three run years: 1999–2000, 2000–2001 and 2001–2002 (underlined). Here we illustrate the case of year 1999–2000 as an example.
2. Material and methods
Detailed descriptions of the studied population, supplementation programme, and the parentage analysis are described in previous papers (Olsen 2003; Araki et al. 2007a,b). A summary description and sample sizes for this study are also found in the electronic supplementary material. In short, the Hood River steelhead supplementation programme has been operating since 1991. A built-in trap in a dam at the mouth of the river allows us to sample all returning adults before they are passed upriver. Wild broodstocks are used to create first-generation hatchery fish (F1s). In this study we estimated the fitness of wild-born fish (F2s) that returned during 1999–2001. They are offspring of first-generation hatchery fish and/or wild fish that had spawned in the river during 1995–1998 (figure 1).
In total, 2520 wild-born adult F2s returned to the river and reached the spawning grounds during 1999–2001. We used eight microsatellite loci and genetic parentage assignments based on exclusion for the pedigree reconstruction (see electronic supplementary material for details). We identified both parents of 779 of these F2s and categorized them as W[W×W], W[C×W] or W[C×C] (from wild×wild, captive-bred×wild, or captive-bred×captive-bred F1 crosses in the wild, respectively). We identified only one parent for each of the additional 1240 F2s (categorized as WC×-] or W[W×-], where ‘-’ refers to the missing parent). The missing parents are most probably resident forms of O. mykiss, also known as rainbow trout (Araki et al. 2007b,c, see also electronic supplementary material).
During 2001–2007, 1348 wild-born adult F3 returned to the river. We assigned them to the F2 parents using the same method as above (see electronic supplementary material, tables S1 and S2 for sample sizes). Adult-to-adult reproductive fitness of F2 was then estimated using an unbiased estimation method (electronic supplementary material). We use ‘relative reproductive success (RRS)’ to mean the ratio of the average reproductive fitness of a class of fish (e.g. W[C×W] or W[C×C]) to that of fish having exclusively wild parents (e.g. W[W×W]). The statistical significance of the difference in reproductive fitness among types of wild-born fish was tested using a one-tailed permutation test.
3. Results
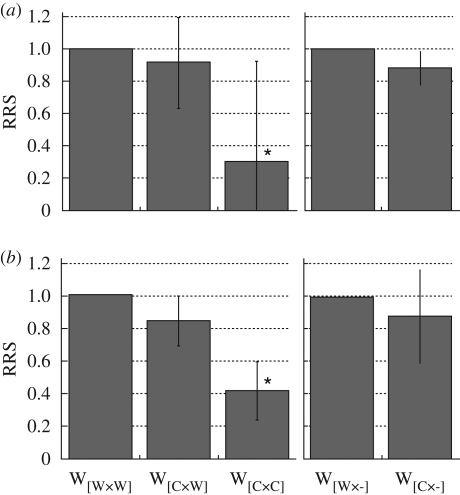
RRS of each type of F2 fish during 1999–2001 is shown in table 1. RRS varied widely among years. Over all the three years, however, fish having two captive-bred parents (W[C×C]) averaged only 37 per cent of the reproductive fitness of fish having two wild parents (W[W×W]) (table 1; figure 2, p = 0.006 by permutation test). Fish having a single captive-bred parent (W[C×W]) averaged 87.1 per cent of the fitness of W[W×W], although the difference was not significant (p = 0.414). The results from the comparison between W[C×-] and W[W×-] were remarkably similar to results from the W[C×W] versus W[W×W] comparison, with W[C×-] fish having a point estimate of 87.9 per cent of the fitness of W[W×-] (table 1; figure 2, p = 0.226). Thus, the result suggests that fish from two captive-bred parents have significantly low reproductive fitness, whereas two non-significant, but very consistent, comparisons suggest that fish having a single captive-bred parent have intermediate reproductive fitness between W[C×C] and W[W×W].
Table 1.
Relative reproductive success (RRS) of wild fish from different types of parental crosses. *p < 0.05, **p < 0.01.
| run-yeara | N[F3 assigned]b | RRSc | p-valued |
|---|---|---|---|
| F2 having two hatchery parents: W[C×C] versus W[W×W] | |||
| male | |||
| 1999 | 32 | 0.065 | 0.008** |
| 2000 | 29 | 1.268 | 0.733 |
| 2001 | 18 | 0.446 | 0.125 |
| overall malee | 0.307 | 0.026* | |
| female | |||
| 1999 | 49 | 0.297 | 0.141 |
| 2000 | 49 | 0.633 | 0.176 |
| 2001 | 25 | 0.360 | 0.053 |
| overall femalee | 0.416 | 0.039* | |
| overall both sexese | 0.370 | 0.006** | |
| F2 having one hatchery parent and one wild: W[C×W] versus W[W×W] | |||
| male | |||
| 1999 | 64 | 0.859 | 0.375 |
| 2000 | 42 | 1.247 | 0.713 |
| 2001 | 32 | 0.701 | 0.303 |
| overall malee | 0.916 | 0.540 | |
| female | |||
| 1999 | 101 | 0.984 | 0.504 |
| 2000 | 74 | 0.807 | 0.247 |
| 2001 | 38 | 0.678 | 0.202 |
| overall femalee | 0.844 | 0.288 | |
| overall both sexese | 0.871 | 0.414 | |
| F2 having one hatchery parent and one missing parent: W[C×-] versus W[W×-] | |||
| male | |||
| 1999 | 109 | 0.834 | 0.302 |
| 2000 | 79 | 0.806 | 0.279 |
| 2001 | 69 | 0.993 | 0.532 |
| overall malee | 0.884 | 0.400 | |
| female | |||
| 1999 | 146 | 1.106 | 0.693 |
| 2000 | 100 | 1.093 | 0.663 |
| 2001 | 107 | 0.603 | 0.023* |
| overall femalee | 0.877 | 0.170 | |
| overall both sexese | 0.879 | 0.226 | |
aThe year parents started arriving at the river to spawn.
bNumber of F3 assigned to F2 (parent) in each category.
cUnbiased estimate of RRS (see electronic supplementary material), which are measured relative to the reproductive success of F2 from wild-born parents (W[W×W] or W[W×-]).
dp-values by a one-tailed permutation test.
eOverall RRS was estimated using weighted geometric means. The p-values were calculated on the basis of Fisher's combined probability.
Figure 2.
Relative reproductive success (RRS) of each type of wild-born fish relative to that of fish having only wild parents (which have RRS=1.0, by definition). (a) Male F2s, (b) female F2s. Weighted geometric mean RRS among three years of samples is plotted for W[C×W] and W[C×C] relative to W[W×W] in the left panels, and for W[C×-] relative to W[W×-] in the right panels. Each point is the average over three years, and the error bar represents 1 s.d. An asterisk represents that RRS is significantly lower than 1.0 (p < 0.05).
The impact of the reduced fitness at the population level depends on the fraction of the different types of fish in the population. Using the point estimates of relative fitness above and the known frequencies of each type of breeder in each year, we estimated the change in population fitness that resulted from the heritable effect of captive breeding. Relative to a hypothetical population in which all individuals were W[W×W] or W[W×-], the average relative fitness of the Hood River population during 1999–2001 was reduced by 8 per cent (see electronic supplementary material for the detailed calculation).
4. Discussion
The F2 individuals compared in this study were all born in the same river, presumably experienced the same environment, and spawned in the river in the same year. Thus, genetic differentiation during captive breeding in the previous generation is most likely responsible for the reduced fitness of wild-born fish from hatchery parents. A strong genetic effect of captive breeding is consistent with the results of previous studies (Araki et al. 2007a, 2008). However, this study also suggests a carry-over effect of the captive breeding, which reduces the reproductive fitness of wild-born descendants in the wild and the population fitness of subsequent generations.
Figure 2 illustrates a clear pattern of the carry-over effect of captive breeding in both sexes. Nevertheless, there was substantial variation among run years in RRS (table 1). The results for females were consistent, with W[W×W] > W[W×C] > W[C×C] in all three years. But in males, that pattern held only in two years. In 2000 the point estimates for W[W×W] and W[W×W] males were actually higher than that for W[W×W] (RRS approx. 1.25). We have no good explanation for this variation in males. However, such year-to-year variation is typical for this type of estimation (Araki et al. 2007b). We speculate that environmental differences among years and/or the limited sample sizes and skewed distribution of individual reproductive success in this population (Araki et al. 2007c) are potential causes of the variation in estimated RRS. Regardless, over all three years there is a clear pattern of decline in reproductive fitness in order of W[W×W] > W[C×W] > W[C×C](figure 2).
What does this result mean for supplementation programmes? Recall that we measured reproductive fitness in wild-born F2 adults. These fish had survived the gauntlet of viability selection from egg to adulthood in the wild. Therefore, the fitness difference persisted despite a complete life cycle of natural rearing, during which time natural selection had the opportunity to eliminate less fit individuals from the population, as previously suggested (Araki et al. 2007a, 2008). We estimated that this carry-over effect reduced population fitness by 8 per cent relative to a purely wild population of the same size. Note that this result does not necessarily mean that supplementation actually decreased wild population size (relative to the case of no supplementation) because a large number of hatchery fish released into the wild will increase the total number of F2. The genetic effect, however, might be cumulative and could continue to reduce population fitness in subsequent generations. Thus, although the overall effect of supplementation on the size of this wild population over many generations is still unclear, there is reason to be concerned (Lynch & O'Hely 2001; Ford 2002). The fact that the hatchery parents in this study were themselves only one generation removed from the wild makes these results even more disturbing.
These results have important implications. Given that the genetic effect of captive breeding was not erased by a full generation in the wild, supplementation programmes could have a cumulative impact on wild populations. Furthermore, recovery will probably not be immediate after supplementation is terminated. These data might also be relevant to the question of why reintroduction attempts with salmon often fail (i.e. attempts to restock empty habitat using captive-bred founders). The mechanisms causing the observed fitness declines are currently unknown. Candidates include selection on growth rate and sexual selection (see Araki et al. 2008 for discussions). In the absence of knowledge of the mechanism, it is not clear to what extent these results extrapolate to other species. Nevertheless, the message should be clear: captive breeding for reintroduction or supplementation can have a serious, long-term downside in some taxa, and so should not be considered as a panacea for the recovery of all endangered populations.
Acknowledgements
Our study was conducted at the Department of Zoology, Oregon State University, USA (Michael Blouin's laboratory). We prove that our study, including non-lethal tissue sampling (fin-clip and scale) from captured fish, has caused no ethical issue.
We thank the staff of the Oregon Department of Fish and Wildlife (ODFW) for technical help, the OSU Center for Genome Research and Biocomputing for assistance with genetic analysis and S. Haertel-Borer, M. Ford, M. Hansen and O. Seehausen for comments. This research was funded by contracts to M.S.B. from the Bonneville Power Administration and the ODFW.
Footnotes
Present address: Eawag, Swiss Federal Institute of Aquatic Science and Technology, Center for Ecology, Evolution, and Biogeochemistry, Department of Fish Ecology and Evolution, 6047 Kastanienbaum, Switzerland.
References
- Allendorf F. W., Luikart G.2007Conservation and the genetics of populations Oxford, UK: Blackwell Publishing [Google Scholar]
- Araki H., Cooper B., Blouin M. S.2007aGenetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 318, 100–103 (doi:10.1126/science.1145621) [DOI] [PubMed] [Google Scholar]
- Araki H., Ardren W. R., Olsen E., Cooper B., Blouin M. S.2007bReproductive success of captive-bred steelhead trout in the wild: evaluation of three hatchery programs in the Hood River. Conserv. Biol. 21, 181–190 (doi:10.1111/j.1523-1739.2006.00564.x) [DOI] [PubMed] [Google Scholar]
- Araki H., Waples R. S., Ardren W. R., Cooper B., Blouin M. S.2007cEffective population size of steelhead trout: influence of variance in reproductive success, hatchery programs, and genetic compensation between life-history forms. Mol. Ecol. 16, 953–966 (doi:10.1111/j.1365-294X.2006.03206.x) [DOI] [PubMed] [Google Scholar]
- Araki H., Berejikian B. A., Ford M. J., Blouin M. S.2008Fitness of hatchery-reared salmonids in the wild. Evol. Appl. 1, 342–355 (doi:10.1111/j.1752-4571.2008.00026.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. J.2002Selection in captivity during supportive breeding may reduce fitness in the wild. Conserv. Biol. 16, 815–825 (doi:10.1046/j.1523-1739.2002.00257.x) [Google Scholar]
- Frankham R., Ballou J. D., Briscoe D. A.2002Introduction to conservation genetics Cambridge, UK: Cambridge University Press [Google Scholar]
- Fraser D. J.2008How well can captive breeding programs conserve biodiversity? A review of salmonids. Evol. Appl. 1, 535–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., O' Hely M.2001Captive breeding and the genetic fitness of natural populations. Conserv. Genet. 2, 363–378 (doi:10.1023/A:1012550620717) [Google Scholar]
- Olney P. J. S., Mace G. M., Feistner A.1994Creative conservation: interactive management of wild and captive animals London, UK: Chapman & Hall [Google Scholar]
- Olsen E. A.2003Hood River and Pelton ladder evaluation studies. Annual report 2000–2001 of the Oregon Department of Fish and Wildlife Portland, OR: Oregon Department of Fish and Wildlife [Google Scholar]
- Reisenbichler R. R., McIntyre J. D.1977Genetic differences in growth and survival of juvenile hatchery and wild steelhead trout, Salmo gairdneri. Can. J. Fish. Res. Board 34, 123–128 [Google Scholar]
- Reisenbichler R. R., Rubin S.1999Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES J. Mar. Sci. 56, 459–466 (doi:10.1006/jmsc.1999.0455) [Google Scholar]
- Waples R. S., Drake J.2004Risk/benefit considerations for marine stock enhancement: a pacific salmon perspective. In Stock enhancement and sea ranching: developments, pitfalls and opportunities (eds Leber K. M., Kitada S., Blankenship H. L., Svåsand T.), pp. 260–306 Oxford, UK: Blackwell [Google Scholar]