Abstract
Effects of diet on longevity are complex because acquired resources are shared among growth, reproduction and somatic maintenance. We simplify these axes by examining how dietary restriction and competitive contexts affect longevity using semelparous males of the Australian redback spider (Latrodectus hasselti). Plastic development of L. hasselti males results in trade-offs of body condition against faster development if females are present, facilitating scramble competition. In the absence of females, males develop slowly as high body condition adults, and are better equipped for mate searching. Here we focus on effects of diet and competitive context on body condition and longevity. Although male survival depended on body condition and exercise, contrary to studies in a wide range of taxa, dietary restriction did not increase longevity. However, there was an interactive effect of diet and competitive context on lifespan, because high-diet males reared in the absence of females lived longer than males reared in the presence of females. Thus males near females pay a survival cost of developing rapidly. This shows that life-history trade-offs affected by competitive context can impose longevity costs independent of the direct energy expenditure of searching, courtship, competition or reproduction.
Keywords: dietary restriction, life-history trade-offs, longevity, developmental plasticity, Latrodectus hasselti
1. Introduction
The effects of diet on adult lifespan are complex, as acquired resources are shared between regulatory processes such as somatic repair (Hughes & Reynolds 2005; Lopez-Lluch et al. 2006), and growth and reproduction (Hunt et al. 2004; Kasumovic & Andrade 2006). Further, reproductive trade-offs are diet-mediated (Mappes et al. 1996; Hunt et al. 2004) and resources allocated to current reproduction cannot be allocated for other purposes or stored for later use (Van Noordwijk & De Jong 1986). According to sexual selection theory, males that better acquire, extract and store resources than their cohorts should enjoy increased fitness (Rowe & Houle 1996) and potentially longer adult lifespans (Kokko et al. 2002). By contrast, evidence from biogerontology shows that dietary restriction (DR) generally prolongs adult lifespan in a wide range of taxa (Weindruch & Walford 1988; Masoro 2005). Prolonged lifespan under DR is sometimes associated with transient reductions in reproductive performance, possibly allowing adaptive maximization of future reproduction in organisms experiencing periodic food shortages (Shanley & Kirkwood 2000; Merry 2005; Partridge et al. 2005).
Understanding the nature of trade-offs is critical to life-history theory (Reznick et al. 2000), evolutionary ageing theory (Williams 1957) and sexual selection (Rowe & Houle 1996). However, the roles of diet and reproductive effort on lifespan are difficult to disentangle for several reasons. First, diet-restricted animals may increase the amount of food they ingest in response to the nutritional demands of reproduction (Maklakov et al. 2008). Second, animals can shift allocation patterns depending on the environmental context (Kasumovic & Andrade 2006). Third, differences in the timing and magnitude of reproductive effort between animals on different diets can vary in a non-linear fashion, making it hard to know if effects are due to diet (Hunt et al. 2004). As a result, examinations of diet-mediated effects on lifespan may be most easily examined in semelparous animals that do not feed at maturity (e.g. capital breeders, Stearns 1992). For such animals, simpler predictions about optimal life history and allocation patterns are possible as resources available at adulthood are fixed and fitness is determined in a single reproductive bout.
Male redbacks (Latrodectus hasselti) are short-lived (mean 22 days, Andrade 2003) capital breeders that can mate only once due to high rates of sexual cannibalism (Andrade 1996) and post-mating sterility (Andrade & Banta 2002b). Redback males cease capturing prey as adults (common for male web-building spiders, Foelix 1982), and they depend on resources acquired and stored as juveniles to sustain them as they search for, court and mate with females (Kasumovic et al. 2007). Male development time and body condition are adaptively plastic traits that vary in response to diet, female presence and male density (Kasumovic & Andrade 2006, 2009). In the presence of females, males mature rapidly, sacrificing condition to reach virgin females quickly, and thereby inseminate them first (Snow & Andrade 2005; Kasumovic & Andrade 2006). In the absence of females, males acquire more resources over a longer development and eclose in better condition, ensuring more resources for searching. In this study we examine longevity of male redbacks as a function of (i) diet and the developmental tactics triggered by cues of divergent competitive environments, and (ii) body condition and energetic expenditure required during mate searching and courtship.
2. Material and methods
Spiderlings from an outbred laboratory population were monitored daily and fed Drosophila melanogaster twice weekly (see Andrade & Banta 2002) and held at 25°C on a 12 : 12 h light cycle. One hundred and twelve penultimate instar males were randomly assigned to rearing in the presence or absence of females, mimicking extremes of female density in nature. Female presence was crossed with three diets: high- and mid-diet males were fed three times/week (six and three D. melanogaster, respectively), while low-diet males received one D. melanogaster each week until maturity (see Kasumovic & Andrade 2006 for details).
We noted the date of the final moult, measured (mean patella–tibia length of the first legs) and weighed (±0.01 mg) adults, and estimated condition as residuals of log10weight0.333 regressed on log10size (see Kasumovic & Andrade 2006 for justification). Males were held individually without feeding (mimicking natural cessation of prey capture, Foelix 1982) until death. Data demonstrating significant effects of female presence and diet on male body condition, size and development time are published elsewhere (Kasumovic & Andrade 2006). Here we analyse new longevity data using a two-way ANOVA to test whether diet and female presence affected adult male lifespan.
In a second experiment, we tested the assumption that body condition is positively related to longevity under elevated energy expenditure. Experimental males (n = 25) were placed on pheromone-laden webs of virgin females where they courted continuously (= energetically costly movement and vibration signalling, Stoltz et al. 2009) for 3 h. Control males (n = 25) were briefly placed on a female's web but removed before they initiated courtship. Males were weighed but not fed after trials and the date of death was recorded. We used an ANCOVA to examine whether lifespan was affected by treatment and body condition. All data were normally distributed.
3. Results
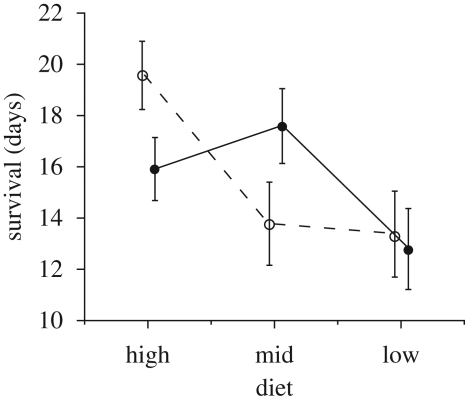
Increased food intake extended male lifespan (F2,106 = 5.16, p = 0.007, figure 1). Female presence had no general effect on male lifespan (F1,106 = 0.01, p = 0.92), but did interact significantly with diet (F2,106 = 3.47, p = 0.035), with males living longest when females were absent and food was abundant during development. Although condition at eclosion was positively correlated with male longevity (r = 0.194, p = 0.041), this effect was largely due to the diet and female presence treatments and their interactions: body condition did not add further explanatory power to the ANOVA model (F1,105 = 0.868, p = 0.354).
Figure 1.
Male survival after development on three diets in the presence (filled circles) or absence (empty circles) of females. Bars are standard errors.
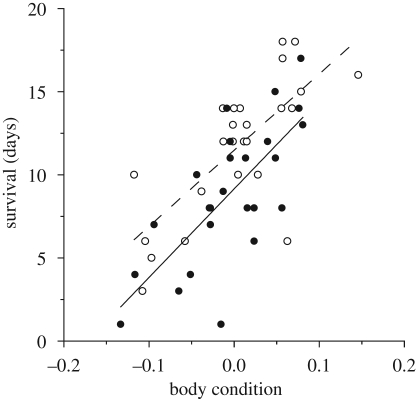
In the courtship experiment, adult body condition predicted male lifespan (F1,47 = 50.17, p < 0.0001, figure 2), and lifespan was significantly shorter if males courted (mean±s.e.: control = 11.72 ± 0.59 days, courtship = 8.88 ± 0.59 days; F1,47 = 8.12, p = 0.0065).
Figure 2.
Correlation between male survival and body condition for the control (open circles, dashed line) and exercised (closed circles, solid line) treatments.
4. Discussion
Our results demonstrate a positive association between dietary acquisition of resources before maturity and adult lifespan in male redback spiders. Males fed more as juveniles generally lived longer in the first experiment, and males in better condition lived longer and were better able to sustain the longevity costs of courtship in the second experiment. Because male L. hasselti do not feed after maturity and mate only once, our findings eliminate the potentially confounding effects of feeding to compensate for reproductive effort and the complexities of storing resources for future reproduction. Our results do not support the idea that dietary restriction (DR) prolongs adult lifespan, in contrast to a large body of research on a range of organisms (e.g. Austad 1989; Masoro 2005), but they do support suggestions that variation in DR effects may depend on factors including age, degree of DR and natural history (e.g. Kirk 1997).
Adaptive explanations of the DR effect include the potential benefit of deferring reproduction until the individual can obtain a rich food source (Partridge et al. 2005). The absence of lifespan extension by DR that we document is consistent with this adaptive explanation, because male L. hasselti mate only once in their lifetime, and do not capture prey as adults. The response of male L. hasselti and other semelparous capital breeders to dietary restriction merits further investigation because they may provide new insights into the evolution of relationships between diet, reproduction and longevity. Comparisons with female web-building spiders, which feed as adults and reproduce iteroparously, are likely to be particularly illuminating as at least one species shows prolonged lifespan under DR (Austad 1989). Many female spiders can survive for months without feeding (Forster & Kavale 1989) with little decrease in lifetime fecundity if feeding resumes (J. A. Stoltz 2008, personal communication).
Our results contrast with a recent study (Segoli et al. 2007) on semelparous males of the white widow spider (Latrodectus pallidus) in which adult males fed flies ad libitum (similar to our high-diet treatment) lived for a shorter time than those fed only three flies per week (identical to our mid-diet treatment). However, while males did not feed as adults in our design, white widow males were fed experimental diets only as adults. The ecological significance of feeding beyond maturity in widow spiders is unknown, but it is likely that feeding or the presence of prey after maturity will have dramatically different effects on individual lifespan than feeding during development.
Possibly the most intriguing finding from our study is the interaction between diet and the presence of females on male lifespan. Previously, Kasumovic & Andrade (2006) showed that males reared close to females develop faster and eclose at a smaller size and in poorer body condition, but can reach females before larger rivals (Kasumovic & Andrade 2009). We now show that variation in lifespan is also consistent with this trade-off. Our second experiment showed that increased condition of adult males results in an increased ability to tolerate longevity costs of exercise (including, presumably, mate searching). However, males reared near females forgo the benefit of acquiring body condition even when provided with ample food, resulting in shorter adult lifespan than males reared away from females. This appears to be part of a developmentally plastic trade-off between rapid development (suited to scramble competition when females are nearby, Snow & Andrade 2005; Kasumovic & Andrade 2006) and slower development to acquire condition when females are not nearby (thus living longer to search for mates, Andrade 2003). Thus, anticipated reproductive opportunities may impose reproduction-related longevity costs, even before searching, courtship or mating occur. The costs of reproduction are profound, widespread and often complex (Mappes et al. 1996; Reznick et al. 2000; Hunt et al. 2004); our results add the further complexity of developmental costs of plastic reproductive traits to these costs.
Acknowledgements
Funded by the Natural Sciences and Engineering Research Council of Canada (PDF to MMK, Discovery Grant to MCBA), the Canadian Foundation for Innovation, Ontario Research Fund and Canada Research Chairs to MCBA.
References
- Andrade M. C. B.1996Sexual selection for male sacrifice in the Australian redback spider. Science 271, 70–72 (doi:10.1126/science.271.5245.70) [Google Scholar]
- Andrade M. C. B.2003Risky mate search and male self-sacrifice in redback spiders. Behav. Ecol. 14, 531–538 (doi:10.1093/beheco/arg015) [Google Scholar]
- Andrade M. C. B., Banta E. M.2002Value of remating and functional sterility in redback spiders. Anim. Behav. 63, 857–870 (doi:10.1006/anbe.2002.2003) [Google Scholar]
- Austad S. N.1989Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp. Gerontol. 24, 83–92 (doi:10.1016/0531-5565(89)90037-5) [DOI] [PubMed] [Google Scholar]
- Foelix R.1982Biology of spiders Cambridge, MA: Harvard University Press [Google Scholar]
- Forster L. M., Kavale J.1989Effects of food deprivation on Latrodectus hasselti Thorell (Arenea: Theridiidae), the Australian redback spider. N Z J. Zool. 16, 401–408 [Google Scholar]
- Hughes K. A., Reynolds R. M.2005Evolutionary and mechanistic theories of aging. Ann. Rev. Entomol. 50, 421–445 (doi:10.1146/annurev.ento.50.071803.130409) [DOI] [PubMed] [Google Scholar]
- Hunt J., Brooks R., Jennions M. D., Smith M. J., Bentsen C. L., Bussière L. F.2004High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027 (doi:10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- Kasumovic M. M., Andrade M. C. B.2006Male development tracks rapidly shifting sexual versus natural selection pressures. Curr. Biol. 16, R242–R243 (doi:10.1016/j.cub.2006.03.017) [DOI] [PubMed] [Google Scholar]
- Kasumovic M. M., Andrade M. C. B.2009Changes in competitive context reverses sexual selection on male size. J. Evol. Biol. 22, 324–333 (doi:10.1111/j.1420-9101.2008.01648.x) [DOI] [PubMed] [Google Scholar]
- Kasumovic M. M., Bruce M. J., Herberstein M. E., Andrade M. C. B.2007Male mate choice may increase the cost of mate searching in the golden orb-web spider (Nephila plumipes). Behav. Ecol. 18, 189–195 (doi:10.1093/beheco/arl072) [Google Scholar]
- Kirk K. L.1997Life-history responses to variable environments: Starvation and reproduction in planktonic rotifers. Ecology 78, 434–441 [Google Scholar]
- Kokko H., Brooks R., McNamara J. M., Houston A. I.2002The sexual selection continuum. Proc. R. Soc. Lond. B 269, 1331–1340 (doi:10.1098/rspb.2002.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G., et al. 2006Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl Acad. Sci. USA 103, 1768–1773 (doi:10.1073/pnas.0510452103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov A. A., Simpson S. J., Zajitschek F., Hall M., Dessman J., Clissold F. J., Raubenheimer D., Bonduriansky R., Brooks R.2008Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1068 (doi:10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- Mappes J., Alatalo R. V., Kotiaho J., Parri S.1996Viability costs of condition-dependent sexual male display in a drumming wolf spider. Proc. R. Soc. Lond. B 263, 785–789 (doi:10.1098/rspb.1996.0117) [Google Scholar]
- Masoro E. J.2005Overview of caloric restriction and ageing. Mech. Ageing Dev. 126, 913–922 (doi:10.1016/j.mad.2005.03.012) [DOI] [PubMed] [Google Scholar]
- Merry B. J.2005Dietary restriction in rodents—Delayed or retarded ageing? Mech. Ageing Dev. 126, 951–959 (doi:10.1016/j.mad.2005.03.015) [DOI] [PubMed] [Google Scholar]
- Partridge L., Gems D., Withers D. J.2005Sex and death: What is the connection? Cell 120, 461–472 (doi:10.1016/j.cell.2005.01.026) [DOI] [PubMed] [Google Scholar]
- Reznick D., Nunney L., Tessier A.2000Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425 (doi:10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- Rowe L., Houle D.1996The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 (doi:10.1098/rspb.1996.0207) [Google Scholar]
- Segoli M., Lubin Y., Harari A. R.2007The effect of dietary restriction on the lifespan of males in a web-building spider. Evol. Ecol. Res. 9, 697–704 [Google Scholar]
- Shanley D. P., Kirkwood T. B. L.2000Calorie restriction and aging: A life-history analysis. Evolution 54, 740–750 [DOI] [PubMed] [Google Scholar]
- Snow L. S. E., Andrade M. C. B.2005Multiple sperm storage organs facilitate female control of paternity. Proc. R. Soc. B 272, 1139–1144 (doi:10.1098/rspb.2005.3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. C.1992The evolution of life histories Oxford, UK: Oxford University Press [Google Scholar]
- Stoltz J. A., Elias D. O., Andrade M. C. B.2009Male courtship effort determines female response to competing rivals in redback spiders. Anim. Behav. 77, 79–85 (doi:10.1016/j.anbehav.2008.09.012) [Google Scholar]
- Van Noordwijk A. J., De Jong G.1986Acquisition and allocation of resources—their influence on variation in life-history tactics. Am. Nat. 128, 137–142 (doi:10.1086/284547) [Google Scholar]
- Weindruch R., Walford R. L.1988The retardation of ageing and disease by dietary restriction Springfield, IL: Charles C Thomas [Google Scholar]
- Williams G. C.1957Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (doi:10.2307/2406060) [Google Scholar]