Abstract
Although predator control programmes rarely consider complex competitive interactions among predators, it is becoming clear that removal of larger ‘superior’ competitors often releases the ‘inferior’ ones and can precipitate trophic cascades. In contrast, our study indicates that culling hooded crows Corvus cornix appears to release a larger competitor, the common raven Corvus corax. Ravens ranged more widely, and the predation of artificial nests was significantly faster (although total predation was similar), after the removal of crows. Our study provides evidence of a novel reversal of competitive release where a larger species was freed from constraints imposed on its distribution and behaviour by a smaller species, and emphasizes the importance of considering community and ecosystem effects of predator manipulations when undertaken for conservation or game management.
Keywords: competitive release, intraguild predation, mesopredator release, predator control, territoriality
1. Introduction
Control of one species to bring about increases in another is a widespread feature of natural resource management. In particular, predator control is an important tool for conservation and game managers (Reynolds & Tapper 1996; Courchamp et al. 2003). Predators targeted for culling are usually common generalists that have adapted well to perturbed environments (Côté & Sutherland 1997). There is growing recognition of the potential for such management to affect ecosystems more broadly (Glen & Dickman 2005), often in complex and non-intuitive ways (Holt & Lawton 1994; Johnson et al. 2007; Sergio et al. 2008).
In control programmes with conservation objectives, mesopredator release (Soulé et al. 1988), whereby the removal of a top predator can free a smaller competitor (mesopredator) from some limits on population size or distribution, can have detrimental community effects (Courchamp et al. 1999). For example, the extirpation of dingoes Canis familiaris dingo can precipitate increases in feral cat Felis catus and red fox Vulpes vulpes populations and, in turn, causes the local or total extinction of marsupial species (Johnson et al. 2007).
Declines in a number of bird species have been linked to increases in corvid populations as general predator control has waned (Vickery et al. 1992; Gregory & Marchant 1996). As a consequence, targeted removal is frequently carried out with the aim of reducing predation, although such attempts rarely ultimately achieve population recovery (Côté & Sutherland 1997). Corvids themselves aggressively defend the area around their own nests against potential predators (Yom-Tov 1975; Ratcliffe 1997) and so, paradoxically, some birds nesting in proximity to corvid nests can experience enhanced nesting success (Richardson & Bolen 1999; Sergio et al. 2004). Therefore, compensatory mortality resulting from predation by birds whose movements were previously restricted by territorial defence may be one of the reasons why some corvid control programmes have been unsuccessful.
While there are numerous studies of the effects of corvid removal on prey (discussed earlier), interactions among corvids following control have rarely been examined. This is particularly true with respect to the common raven Corvus corax, which infrequently features in predation studies, though they can be important predators (Ratcliffe 1997). We studied the common raven and hooded crow Corvus cornix on Rathlin Island, UK, to investigate their interactions and how management might affect ground-nesting birds. In interactions at animal carcasses, ravens are dominant over other smaller corvids (Wilmers et al. 2003) and we anticipated they would be the superior competitor here too.
2. Material and methods
(a). Study site
Fieldwork was carried out on Rathlin Island (55°18′ N, 06°13′ W; 1525 ha). The vegetation is predominately heath and acid grassland, grazed by livestock at low densities. The island supports several introduced mammals, including an additional predator, the feral ferret Mustela furo.
(b). Corvid behaviour
In 2006 and 2007, all corvid nest sites were located by observation of pairs. The number and activity of ravens were recorded from April to July along standard routes, although the length of these within differing territories varied (see the electronic supplementary material for full details of all field methods). The distance from each raven sighting to the closest raven nest site and the closest crow nest site (identified in 2006) was determined using spider analysis in animal movement extension (Hooge & Eichenlaub 1997) for Arcview (ESRI, Redlands, CA). While ravens were unmarked and observations could not be definitively assigned to individuals, ravens remain on their territories throughout the year (Ratcliffe 1997), and so observations were assigned to a pair/family determined by the closest raven nest. Mean ranging distances were calculated for each pair for both years and compared with paired t-tests.
Observations were pooled to generate ‘raven activity ranges’ by plotting kernels using least-squares cross validation in Arcview to calculate smoothing factors. Intensity of raven activity in an area was inferred from contours at 10 per cent intervals from 90–30%. From March to mid-July 2007, crows were removed from all accessible areas of the island using Larsen traps (Anon 1994). Raven activity was compared before and after crow removal.
(c). Artificial nests
Low populations of ground-nesting birds meant that artificial nests (hereafter nests) were used to examine variation in predator activity. Nests comprised three intact and one wax-filled Japanese quail Coturnix japonica eggs. Predators were identified, if possible, by marks made on the wax eggs. Nests were not marked and were relocated using a GPS. Nests were placed in six blocks across the island as part of complementary studies of ferret predation. Ferrets were ubiquitous throughout the island and were removed from two blocks each year prior to nest placement for these studies of mammalian predation. Within each block, four nests were placed in each of six randomly located 1 ha plots. Care was taken to ensure that corvids were not present when nests were placed. Nests were placed in late April and checked on alternate days until failure (when the dummy and/or real eggs had been damaged) or up to 30 days (‘successful’).
Nest survival was examined using Mark 5.0 (White & Burnham 1999), with models of daily survival built according to a priori biologically relevant hypotheses. Block (to reflect the possibility of predators intensifying searches after finding nests), treatment type (which predators had been removed), intensity of raven activity (the density contour of the activity kernel covering the nest), habitat (one of six nominal vegetation categories surrounding the nest) and study day (as a linear effect to model exposure) were included as factors. Distance to nearest raven nest was included as a covariate. Models were compared using Akaike's information criterion (AIC) weights. Rates of sample depletion were compared with a Kolmogorov–Smirnov goodness-of-fit test.
3. Results
(a). Corvid breeding success and behaviour
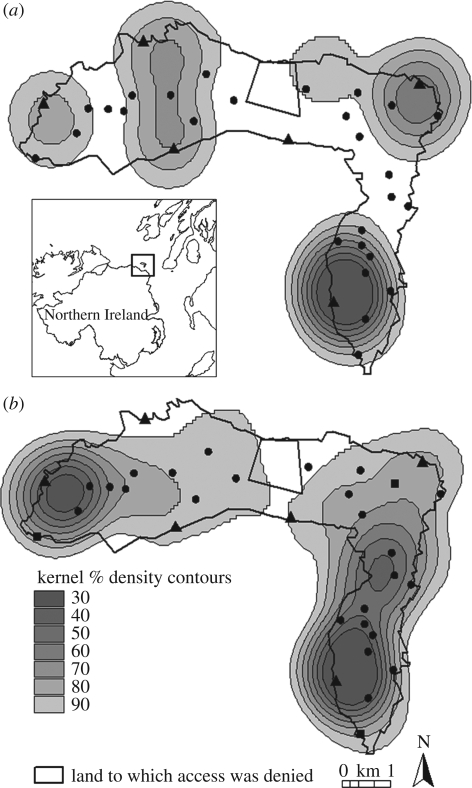
Six pairs of ravens bred in 2006 (mean fledglings ± s.d. 2.7 ± 0.8) and 2007 (2.5 ± 0.6). Twenty-seven pairs of crows bred in 2006 (2.8 ± 0.8 fledglings per successful pair), and there was a ‘floating’ population of 18 non-breeding crows. Trapping reduced the crow population from 72 adult birds in 2006 to six in 2007. There was a significant increase in the per pair mean distance of raven sightings from the closest raven nest (mean distance ± s.d. 2006: 524 ± 308 m; 2007: 796 ± 279 m; t5 = −3.51; p = 0.017), and a significant decrease in the per pair mean distance to 2006 crow nest sites (2006: 536 ± 112 m; 2007: 402 ± 58 m; t5 = −4.60, p = 0.006), while the extent of the 90 per cent contour of raven ranges increased from 15.4 to 19.8 km2 (figure 1).
Figure 1.
Density contours of raven activity based on sightings in (a) 2006 and (b) 2007. Triangles, raven nest; circles, 2006 crow nest; squares, 2007 crow nest. Inset map shows the location of Rathlin Island within the UK.
(b). Artificial nests
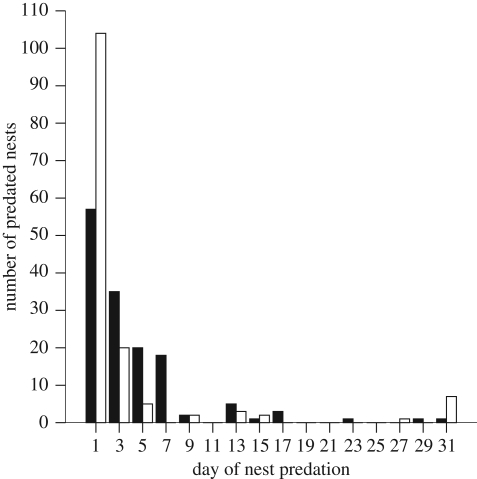
Corvids were responsible for 83 per cent (2006) and 96 per cent (2007) of definitively assignable instances of nest predation. Predation rates of nests were extremely rapid (figure 2) and were significantly faster in 2007 than in 2006 (D0.05,16,144 = 47, p < 0.001). Ferret predation was low at 17 per cent (2006) and 4 per cent (2007), suggesting opportunistic predation despite a population increase in 2007. There was no evidence suggesting that corvid predation was easier to identify than signs of other species, so it is probable that a similar proportion of nests where the predator was not definitively assigned were also predated by corvids. The best-supported model explaining variation in nest survival included raven activity, treatment type and study day (table 1), with shorter persistence of dummy nests associated with greater raven activity and with the absence of crows.
Figure 2.
Predation rates of artificial nests. One hundred and forty four nests were placed in both years. Nests present after day 29 ‘survived’ (2006, n = 1; 2007, n = 7). Filled bar, 2006 before crow removal; unfilled bar, 2007 after crow removal.
Table 1.
Most supported models of daily survival rate of artificial nests. See text for a detailed explanation of model parameters.
| model | AIC | ΔAIC | AIC weight | model likelihood | no. of parameters | deviance |
|---|---|---|---|---|---|---|
| study day, raven activity and treatment | 849.9 | 0 | 0.681 | 1 | 6 | 837.9 |
| study day, raven activity, treatment and habitat | 853.4 | 3.43 | 0.122 | 0.18 | 10 | 833.2 |
| study day and treatment | 853.5 | 3.51 | 0.118 | 0.17 | 5 | 843.4 |
| study day, treatment and habitat | 855.0 | 5.03 | 0.055 | 0.08 | 9 | 836.8 |
4. Discussion
Our study demonstrates that an ostensibly superior larger competitor (ravens) was released from constraints to its movement by the removal of a smaller competitor (crows). Although observed only over two years, raven reproductive success, the demands of feeding young, time of breeding, farming practices and weather conditions were all comparable, and standard observation points and routine precluded changes in observer behaviour as an explanation for the apparent changes in raven behaviour. Ferrets were successfully removed from similar sized areas in both years, so crow removal was the only significant change affecting the whole island between years, implying that, whether through direct competition or indirect mechanisms such as consumption of potential food resources, crow presence and activity excluded ravens from much of the area. However, crow removal occurred in only one year, so the possibility of confounding effects is not eliminated.
While range expansion by ravens appears to be consistent with a competitive release effect, this example clearly differs from others in that the larger of the two competitors is released through the removal of the smaller. In studies of interference competition among vertebrates in natural environments, larger species almost always dominate (Schoener 1983), with their presence often altering the behaviour and distribution of smaller species (Cresswell 2008; Sergio & Hiraldo 2008).
Predation pressure was high in both years, but nest losses were more rapid in 2007 when compared with 2006. As ravens are long lived, many of the same individuals were probably present throughout so, in 2007, they may have recalled finding nests in 2006, and some individuals may have expanded their ranges to search for them. However, both ravens and crows are territorial, at least for the breeding season, defending territories against conspecifics and other potential threats (Yom-Tov 1975; Ratcliffe 1997) so, even if sites were recalled, such knowledge is likely to have been restricted by crow territoriality. Release of ravens from direct aggression and/or interference competition by crows produced results comparable to those seen in examples of mesopredator release, i.e. negative impacts equal to or greater than those seen before control measures were implemented. While ravens display many complex behaviours, and inter-annual predation rates of real nests can vary, they are frequently substantial (Ratcliffe 1997). For instance, although lapwing Vanellus vanellus successfully chased off crows, they seemed incapable of preventing a determined raven attack (T. Bodey 2006–2007, personal observation).
We suggest that it is necessary to expand the horizons of competitive release in vertebrates to recognize that large predatory species can be constrained by smaller ones. This study thus provides a novel variation on the complications resulting from predator control and emphasizes the need to consider removals within a whole ecosystem context (Zavaleta et al. 2001) and ensure management is directed in ways that have maximum positive impact.
Acknowledgements
All experimental fieldwork was approved by the Queen's University Belfast ethics committee. All Larson trapping of crows was carried out in line with the regulations stipulated within the general licence provided under the Wildlife and Countryside Act 1981.
Funding was provided by QUB and EHS through the Quercus partnership, and by the RSPB. Fieldwork assistance was provided by Liam McFaul, Rosalind Kennerley, Jonathan Reeves, Rob Sheldon and Harry Stevenson. We thank the landowners of Rathlin Island for allowing access, and Will Cresswell, Steven Ewing, Fabrizio Sergio and Rob Sheldon for useful comments on previous drafts.
References
- Anon. 1994Predator control. Hampshire, UK: The Game Conservancy Ltd [Google Scholar]
- Côté I. M., Sutherland W. J.1997The effectiveness of removing predators to protect bird populations. Conserv. Biol. 11, 395–405 (doi:10.2307/1936523) [Google Scholar]
- Courchamp F., Langlais M., Sugihara G.1999Cats protecting birds: modelling the mesopredator release effect. J. Anim. Ecol. 68, 282–292 (doi:10.1046/j.1365-2656.1999.00285.x) [Google Scholar]
- Courchamp F., Chapuis J. L., Pascal M.2003Mammal invaders on islands: impact, control and control impact. Biol. Rev. 78, 347–383 (doi:10.1017/S1464793102006061) [DOI] [PubMed] [Google Scholar]
- Cresswell W.2008Non-lethal effects of predation in birds. Ibis 150, 3–17 [Google Scholar]
- Glen A. S., Dickman C. R.2005Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 80, 387–401 (doi:10.1017/S1464793105006718) [DOI] [PubMed] [Google Scholar]
- Gregory R. D., Marchant J. H.1996Population trends of jays, magpies, jackdaws and carrion crows in the United Kingdom. Bird Study 43, 28–37 (doi:10.1080/00063659609460993) [Google Scholar]
- Holt R. D., Lawton J. H.1994The ecological consequences of shared natural enemies. Annu. Rev. Ecol. Syst. 25, 495–520 (doi:10.1146/annurev.es.25.110194.002431) [Google Scholar]
- Hooge P. N., Eichenlaub B.1997Animal movement extension to Arcview Alaska Science Center, U.S. Geological Survey, USA [Google Scholar]
- Johnson C. N., Isaac J. L., Fisher D. O.2007Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B 274, 341–346 (doi:10.1098/rspb.2006.3711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe D. A.1997The raven London, UK: Poyser [Google Scholar]
- Reynolds J. C., Tapper S. C.1996Control of mammalian predators in game management and conservation. Mamm. Rev. 26, 127–155 (doi:10.1111/j.1365-2907.1996.tb00150.x) [Google Scholar]
- Richardson D. S., Bolen G. M.1999A nesting association between semi-colonial Bullock's orioles and yellow-billed magpies: evidence for the predator protection hypothesis. Behav. Ecol. Sociobiol. 46, 373–380 (doi:10.1007/s002650050632) [Google Scholar]
- Schoener T. W.1983Field experiments on interspecific competition. Am. Nat. 122, 240–285 (doi:10.1086/284133) [Google Scholar]
- Sergio F., Hiraldo F.2008Intraguild predation in raptor assemblages: a review. Ibis 150, 132–145 (doi:10.1111/j.1474-919X.2008.00786.x) [Google Scholar]
- Sergio F., Rizzolli F., Marchesi L., Pedrini P.2004The importance of interspecific interactions for breeding-site selection: peregrine falcons seek proximity to raven nests. Ecography 27, 818–826 (doi:10.1111/j.0906-7590.2004.04030.x) [Google Scholar]
- Sergio F., Caro T., Brown D., Clucas B., Hunter J., Ketchum J., McHugh K., Hiraldo F.2008Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 39, 1–19 (doi:10.1146/annurev.ecolsys.39.110707.173545) [Google Scholar]
- Soulé M. E., Bolger D. T., Alberts A. C., Wright J., Sorice M., Hill S.1988Reconstructured dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Conserv. Biol. 2, 75–92 (doi:10.1111/j.1523-1739.1988.tb00337.x) [Google Scholar]
- Vickery P. D., Hunter M. L., Wells J. V.1992Evidence of incidental nest predation and its effects on nests of threatened grassland birds. Oikos 63, 281–288 (doi:10.2307/3545389) [Google Scholar]
- White G. C., Burnham K. P.1999Program Mark: survival estimation from populations of marked animals. Bird Study 46, S120–S138 [Google Scholar]
- Wilmers C. C., Stahler D. R., Crabtree R. L., Smith D. W., Getz W. M.2003Resource dispersion and consumer dominance: scavenging at wolf- and hunter-killed carcasses in Greater Yellowstone, USA. Ecol. Lett. 6, 996–1003 (doi:10.1046/j.1461-0248.2003.00522.x) [Google Scholar]
- Yom-Tov Y.1975Synchronization of breeding and intraspecific interference in carrion crow. Auk 92, 778–785 [Google Scholar]
- Zavaleta E. S., Hobbs R. J., Mooney H. A.2001Viewing invasive species removal in a whole ecosystem context. Trends Ecol. Evol. 16, 454–459 (doi:10.1016/S0169-5347(01)02194-2) [Google Scholar]