Abstract
Ultrasensitivity, hysteresis (a form of biochemical memory), and all-or-none (digital) responses are important signaling properties for the control of irreversible processes and are well characterized in the c-Jun N-terminal kinase (JNK) system using Xenopus oocytes. Our aim was to study these properties in the AMP-activated protein kinase (AMPK) signaling system under stress conditions that could engage a cell death program, and compare them to the JNK responses. After characterization of Xenopus AMPK, we show here that the response to antimycin (nonapoptotic) was slightly cooperative and graded (analog) in individual oocytes, whereas the response to sorbitol (which induced cytochrome c release and caspase activation) was ultrasensitive, digital in single cells, and without hysteresis, hallmarks of a monostable system. Moreover, initial graded responses of AMPK and JNK turned into digital during a critical period for the execution of the cell death program, although single cell analysis did not show complete correlation between AMPK or JNK activation and cytochrome c release. We propose a model where the life or death decision in the cell is made by integration of multiple digital signals from stress sensors.
The energy level in a cell/organism is probably the most remarkable vital constant and must be tightly regulated or cell death programs will otherwise be engaged. The AMP-activated protein kinase (AMPK)2 is an energy sensor (activated by a high AMP/ATP ratio) and a homeostatic regulator of cellular ATP levels, taking central stage in orchestrating cell metabolism (1).
Stress sensors should have, to function efficiently, ultrasensitive properties to respond to small changes in the important parameters of cell survival. In addition, all-or-none (digital) responses, at a single cell level, and sustained activation when the stimulus has disappeared (also named hysteresis) may be important properties of stress sensors in regulating cell death (2).
Mammalian AMPK is a heterotrimeric complex consisting of a catalytic α-subunit and regulatory β- and γ-subunits. AMPK is activated by AMP in two ways, both antagonized by high concentrations of ATP. Binding of AMP to the γ-subunit induces: 1) allosteric activation that can account for a 5-fold increase in the activity, and 2) phosphorylation of the α-subunit at Thr-172 (in human), which is essential for activity and causes a much marked activation (3). The combination of the two effects causes a >1000-fold increase in kinase activity (4). The protein kinases LKB1 and CaMKKα/β have been reported to phosphorylate the AMPK α-subunit at Thr-172 (1, 5). This signaling system is a clear example of multistep sensitivity, which arises when a signal molecule (AMP) affects more than one step in a cascade (allosteric activation and phosphorylation). In addition, the AMPK upstream kinases have a very low Km for AMPK (6), a phenomenon referred to as zero-order ultrasensitivity (7). In practice, the ultrasensitivity of a system is reflected in a stimulus/response curve with a very steep upstroke. These properties of the AMPK signaling system may be very important to maintain energy levels in the cell within narrow limits and can be useful for filtering noise. Although there is one report supporting that AMPK is an ultrasensitive system (6), no further research has considered its significance in the control of cell death as well as other important signaling properties associated with ultrasensitivity, such as hysteresis and all-or-none responses.
It is well known that ultrasensitive systems embedded in a positive feedback loop have the potential to exhibit bistable behavior, switching between discrete stable steady states without being able to rest in intermediate states (8, 9). The three hallmarks of a bistable system are: 1) strong ultrasensitivity, 2) digital response at the individual cell level, and 3) hysteresis. Examples of such systems are JNK and the mitogen-activated protein kinase (MAPK) cascades, implicated in oocyte maturation and maybe in apoptosis (2, 10). The hallmarks of a monostable system are the same, but without hysteresis.
Because AMPK is an energy sensor, it seems reasonable that the AMPK cascade would transmit at the individual cell level graded (analog) information about the energy status of the cell. In fact, AMPK can be considered part of a negative feedback loop because AMPK activation (by a high AMP/ATP ratio) regulates multiple steps in metabolism to restore ATP levels in the cell, which in turn down-regulates AMPK activity. However, sustained activation of AMPK by some stimuli has been considered a pro-apoptotic signal (11, 12). Because cell death is an all-or-none irreversible process, it may be more appropriate for the AMPK cascade in this situation to exhibit a digital (all-or-none) response to trigger cell death.
Here, we analyze the sensitivity, the grade of hysteresis, and the single cell response of the AMPK system under different stress conditions that could engage a cell death program. We performed experiments with Xenopus oocytes, a suitable model for discerning the character of a signaling response, as has been previously reported for JNK and MAPK cascades. Based on our results, we propose that commitment to cell death will occur after integration of multiple digital responses from stress protein kinases.
EXPERIMENTAL PROCEDURES
Stage VI oocytes were obtained as described under supplemental data and used in the experimental protocols described below.
Oocyte Lysis and Western Blot Analysis
Frozen or fresh oocytes (for cytochrome c release measurement) were lysed by pipetting up and down in 16 μl (individual oocytes) or 100 μl (pools of 10 oocytes) of ice-cold extraction buffer (0.25 m sucrose, 0.1 m NaCl, 2.5 mm MgCl2, 20 mm HEPES, pH 7.2) containing 1 mm EDTA, 1 mm EGTA, protease inhibitors (10 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin), and phosphatase inhibitors (50 mm β-glycerophosphate, 50 mm sodium fluoride, 1 mm sodium orthovanadate, 5 mm sodium pyrophosphate). Samples were clarified by centrifugation at 14,500 rpm for 5 min, and supernatants were collected and processed for immunoblotting or caspase assay as described below. The whole supernatant (in individual oocytes) or 24 μl (for the pool of oocytes) were denatured with sample buffer (50 mm Tris-HCl, pH 6.8, SDS 2%, 100 mm dithiothreitol, 10% glycerol) and subjected to 10 or 12% SDS/PAGE and transferred to Immobilon-P membranes (Millipore). Uniformity of samples loading was verified by Ponceau (Sigma) staining of the blots. Membranes were blocked for 1 h with 5% dried skimmed milk in TBST (50 mm Tris, 150 mm NaCl, 100 mm KCl, pH 7.4, and 0.1% Tween 20) and then incubated with the following polyclonal antibodies: anti-pAMPKα (Thr-172) (2531), anti-AMPKα (2532), anti-AMPKβ1/2 (4150), anti-pACC (Ser-79) (3661), anti-ACC (3662), anti-pJNK (Thr-183/Tyr-185) (9251), anti-JNK (9252), and anti-cleaved caspase-3 (Asp-175) (9661) (Cell Signaling). Polyclonal anti-LKB1 was kindly provided by Dr. J. M. Lizcano (Institut de Neurociències, Universitat Autónoma de Barcelona). Release of cytochrome c from the mitochondria to the cytosol was measured from fresh oocytes lysed and analyzed by Western blot, as previously described, using polyclonal anti-cytochrome c (sc-7159, Santa Cruz Biotechnology). Three human antibodies were tested against Xenopus AMPKγ without positive results: polyclonal anti-AMPKγ1/2/3 (FL-331) (sc-25793, Santa Cruz Biotechnology), monoclonal anti-AMPKγ1 (ab32508, Abcam), and polyclonal anti-AMPKγ2 (HPA004246, Sigma). Antibody binding was detected with horseradish peroxidase-coupled secondary antibody and the enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences).
Oocyte Injection
Oocytes were microinjected near their equator with 50 nl of neutralized AMP (40 mm) or ATP (40 and 80 mm), getting a final concentration of 2 and 4 mm, respectively. Pools of 20 oocytes were collected after 1 h, lysed, and analyzed by Western blot as previously described.
Immunoprecipitation
Oocytes were lysed by pipetting up and down in buffer containing 50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1 mm EGTA, with protease and phosphatase inhibitors as previously described. Samples were clarified by centrifugation at 14,500 rpm for 5 min, and the supernatant was collected. Extracts (100 μg of protein) were incubated with 10 μl of packed protein G-Sepharose, prebound to 0.5 μl of antibody, for 1.5 h at 4 °C on a roller mixer. Precipitates were washed twice with lysis buffer containing 0.5 m NaCl, and twice with buffer A (50 mm Tris-HCl, pH 7.5, 0.1 mm EGTA, 0.1% β mercaptoethanol), suspended in SDS sample buffer, boiled for 5 min, and subjected to electrophoresis on a 10% polyacrylamide gel.
RT-PCR
RNA was extracted from oocytes with p-aminosalicylic acid (PAS), SDS, and phenol/chloroform (13). 250 ng of total RNA were reverse-transcribed using RevertAid M-MuLV Reverse Transcriptase (Fermentas) following the manufacturer's instructions. PCR reactions were performed in a total volume of 50 μl, with 3 μl of cDNA product, 0.2 mm dNTP, 20 μm of each primer (Sigma), and 0.5 units of Taq polymerase (Roche Applied Science). The PCR products were run on a 1% agarose gel containing ethidium bromide and sequenced to confirm correspondence to the Xenopus AMPK subunits.
Assay for DEVDase Activity
Caspase-3 activity was measured in terms of assayed DEVDase activity. From each cytosolic fraction, 25 μl (corresponding to 2.5 oocytes) were assayed for DEVDase activity using the synthetic peptide Z-DEVD-AMC from EnzChek® caspase-3 assay kit (Molecular Probes). Fluorescence at 360 nm for excitation and at 460 nm for emission was measured after incubation for 30 min at 37 °C.
RESULTS
Expression and Subcellular Localization of Xenopus AMPK in the Oocyte
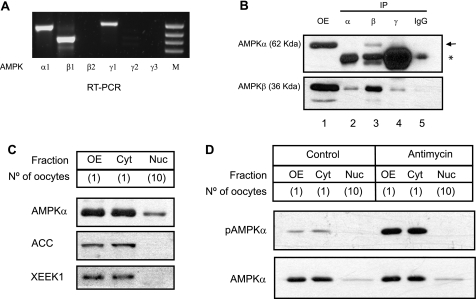
The different subunits of Xenopus AMPK conserved all the domains and the specific amino acids that have been described in mammals to be relevant for catalytic activity, complex formation, and for several regulatory roles (see supplementary data and Fig. S1, A–C). We analyzed the expression and localization of Xenopus AMPK subunits in the oocyte, our cell model of study. We detected by RT-PCR the mRNAs for α1-, β1-, and γ1-subunits in stage VI oocytes (Fig. 1A). In a second amplification from the product of the first PCR, we could also detect β2 and γ2 mRNAs (data not shown). Using the commercially available antibodies against the human AMPK α-subunit and the human AMPK β1/β2-subunits, we detected, by Western blot, proteins of the expected molecular mass (62 and 36 kDa for α- and β-subunits, respectively, Fig. 1B, lane 1). Immunoprecipitation with commercial antibodies for the mammalian α-, β-, and γ-subunits only gave positive results using the anti-human AMPK β1/2 antibody, coimmunoprecipitating the α-subunit (Fig. 1B, arrow, lane 3). Unfortunately, none of the commercial anti-AMPK γ antibodies assayed (see “Experimental Procedures”) detected the Xenopus γ protein either in the oocytes or in the cell line XL-177, derived from tadpole epithelium (data not shown).
FIGURE 1.
AMPK expression and localization in Xenopus oocytes. A, total RNA isolated from Xenopus oocytes (stage VI) was subjected to RT-PCR using specific primers for each subunit. Three AMPK subunits (α1, β1, γ1) were detected. The right lane (M) corresponds to a DNA marker. B, coimmunoprecipitation of α-subunit with β antibody. Cytosolic extracts (100 μg) were incubated with commercial antibodies (1 μl) for the α-, β-, and γ-subunits. After washing several times, α- and β-subunits were detected by Western blot. (OE, oocyte extract without immunoprecipitation; IgG, negative control; the arrow indicates the β-subunit and the asterisk an immunoglobulin band). C, subcellular localization of Xenopus AMPK. Oocyte nuclei (germinal vesicles) (Nuc) were manually dissected from mature oocytes (OE) and separated from the cytoplasm (Cyt). Various protein extracts (equivalent of one oocyte, one cytoplasm, or ten nuclei) were analyzed by Western blot with antibodies against AMPKα and the cytosolic proteins ACC and XEEK1. The oocyte number is indicated between the parentheses. D, antimycin induces phosphorylation of cytosolic AMPKα. Oocytes were treated with antimycin (1.5 μg/ml) for 2 h, then lysed, and separated in three different fractions: total extract (OE), cytosolic fraction (Cyt), or nucleus obtained by manual dissection (Nuc). Western blot was performed using an antibody against total AMPKα or its phosphorylated form (pAMPKα) at Thr-184. All the results are representative of, at least, three independent experiments.
To determine the subcellular localization of AMPK, we analyzed cytoplasmic and nuclear (germinal vesicles) fractions prepared from Xenopus oocytes. The cytosolic proteins acetyl-CoA carboxylase (ACC), which is a substrate of AMPK, and XEEK1, the ortholog of LKB1 in Xenopus (14), were used as controls of subcellular purification. As shown in Fig. 1C, the α AMPK subunit is mainly detected in the cytoplasm, with a small portion present in the nucleus (notice that we are comparing 1 cytosol with 10 nuclei). When we quantified the relative amount of α-subunit from several Western blots, we estimated that only 2.6% of the total protein was present in the nucleus (supplemental Fig. S1D). Incubation of Xenopus oocytes with antimycin induced phosphorylation of the α-subunit in the cytosol (detected with an antibody against human phospho-AMPKα Thr-172), but not in the nucleus (Fig. 1D). In conclusion, the above results demonstrate that AMPK is expressed in Xenopus oocytes and is preferentially located in the cytosol.
Xenopus AMPK Is Activated by Stress and AMP
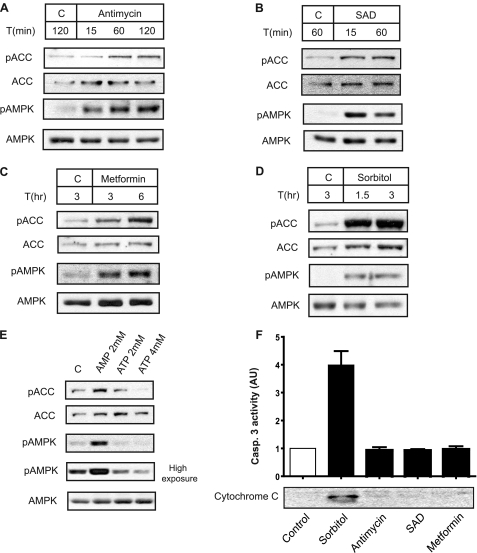
Next, we addressed whether Xenopus AMPK is activated by different stimuli, previously reported as activators of the mammalian AMPK. It is well known that AMPK activation correlates closely with the extent of phosphorylation of Thr-172 (15, 16). Another marker of AMPK activation is the phosphorylation of ACC at Ser-79, a cellular target of AMPK (17). We determined AMPK and ACC phosphorylation in oocytes treated with metformin, antimycin, sodium azide plus 2-deoxy-d-glucose (SAD), and hyperosmolar sorbitol. Metformin is reported to increase cytosolic AMP concentration (18). Antimycin and SAD are reported to decrease ATP levels in Xenopus oocytes (19). Osmotic stress has been considered to activate AMPK independent of AMP/ATP changes (20); however, in Xenopus oocytes, hyperosmotic stress induces ATP release (21). Therefore, all conditions assayed are expected to increase the AMP/ATP ratio in the oocyte. As shown in Fig. 2, all the treatments induced a rapid phosphorylation of AMPK and ACC (Fig. 2, A–D); thus indicating that Xenopus AMPK is an energy sensor. Moreover, injection of AMP in the oocyte to a final concentration of 2 mm induced activation of AMPK, whereas injection of ATP (4 mm) inhibited basal AMPK activity, measured as phosphorylation of AMPK and its substrate ACC (Fig. 2E). Importantly, from the above stimuli, hyperosmolar sorbitol induced cytochrome c release and caspase activation in the oocytes (Fig. 2F), whereas antimycin, SAD, or metformin did not, even after a long incubation. In conclusion, the above results show that Xenopus AMPK is regulated in the oocyte similar to the mammalian ortholog, making this system an ideal model in which to study AMPK signaling properties.
FIGURE 2.
AMPK activation in Xenopus oocytes by cellular stress and AMP. Oocytes (stage VI) were treated with: (A) antimycin (1.5 μg/ml), (B) SAD (sodium azide (3 mm) and 2-deoxy-d-glucose (2 mm)), (C) metformin (2 mm), and (D) sorbitol (0.5 m) at different times, as indicated at the top of the figure. AMPK activity was measured by Western blot using antibodies against pAMPK and pACC. As controls, total ACC and AMPK were visualized. All results are representative of, at least, three independent experiments. E, Xenopus AMPK activation by AMP and inhibition by ATP. 50 nl of neutralized AMP or ATP were injected in the oocytes at different concentrations, and 1 h later, whole extracts were obtained and analyzed by Western blot with antibodies against pACC, ACC, pAMPK, and AMPK. As a control, some oocytes were also injected with 50 nl of H2O (C). The final nucleotide concentration in the oocyte, after injection, is indicated in the figure. This result is representative of three independent experiments. F, sorbitol induces cytochrome c release and caspase-3 activity. Oocytes were incubated with: sorbitol (0.5 m) for 2 h; or antimycin (1.5 μg/ml), SAD (sodium azide (3 mm) and 2-deoxy-d-glucose (2 mm)) and metformin (2 mm) for 8 h, and cytochrome c release was analyzed in the cytosolic extracts by Western blot. Caspase-3 activity was determined as the concentration of fluorescent AMC formation from Z-DEVD-AMC substrate and represented as arbitrary units of caspase-3 activity; giving the value of 1 to nontreated oocytes (control). Results are represented as mean ± S.E. of three independent experiments.
Hyperosmolar Sorbitol Induces an Ultrasensitive Response of AMPK
After characterization of Xenopus AMPK in the oocyte, we used this cellular system to compare the sensitivity of response to two different stress stimuli: hyperosmolar sorbitol (apoptotic) and antimycin (nonapoptotic). Phosphorylation of Xenopus AMPK in the regulatory site (Thr-184) was well correlated with phosphorylation of ACC (a target of AMPK) (supplemental Fig. S2, A–D). Similarly, the overall activity of JNK is well correlated with dual phosphorylation at Thr-183 and Tyr-185 (22, 23). JNK is activated by hyperosmolar sorbitol (24) and shows an ultrasensitive digital and bistable response (2), serving as a control to compare the AMPK signaling properties. Therefore, we quantified the ratio pAMPK/AMPK and pJNK/JNK and referred to these values as AMPK and JNK activity, respectively.
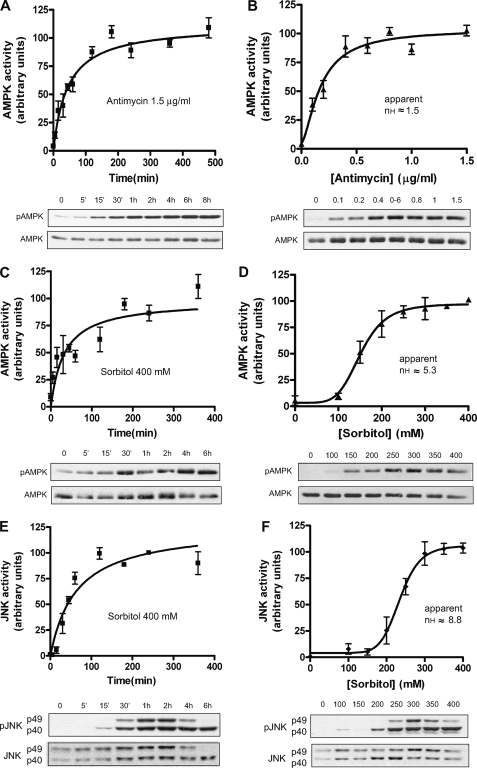
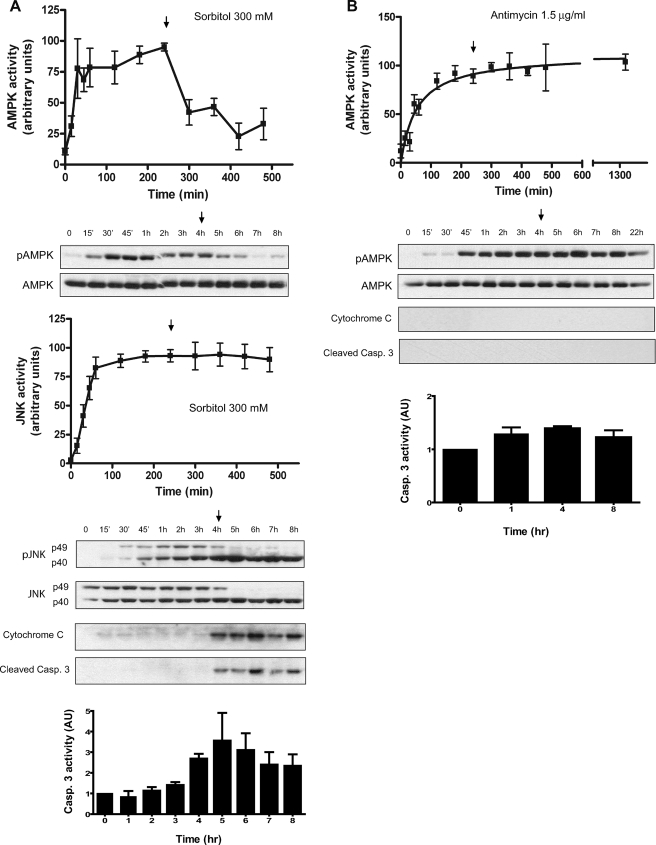
First, we examined the AMPK response to antimycin or sorbitol as a function of time. Antimycin and sorbitol increased AMPK activity to plateau levels within 2 and 3 h, respectively, remaining high through at least 6 h (Fig. 3, A and C). Antimycin did not activate JNK, but sorbitol increased the phosphorylation of JNK, reaching a plateau at 2 h and remaining high for at least 6 h (Fig. 3E). Next, we performed dose-response experiments at 4 h, a clear steady-state situation. The Hill coefficients obtained were 1.53 and 5.26 for the AMPK in response to antimycin and sorbitol, respectively, and 8.84 for JNK in response to sorbitol (Fig. 3, B, D, F).
FIGURE 3.
AMPK and JNK are ultrasensitive to hyperosmolar sorbitol. A, time course of AMPK activation to antimycin. Oocytes (stage VI) were treated with antimycin (1.5 μg/ml) and lysed at different times to analyze AMPK activity by Western blot. Results are represented as the pAMPK/AMPK ratio, giving the 100% value to the highest activity. B, dose response of AMPK to antimycin. C, time course of AMPK activity to sorbitol (400 mm). Results are expressed as indicated above. D, dose response of AMPK to sorbitol. E, time course of JNK activity to sorbitol (400 mm). Two bands (p49 and p40) were measured and summed. JNK activity is expressed as the pJNK/JNK ratio, giving the 100% value to the highest activity. F, dose response of JNK to sorbitol. Results are represented as mean ± S.E. of five independent experiments. The Hill coefficient (nH) was calculated with the SAS 9.1 informatics program and represented with the GraphPad Prism 4 program. Western blots are from representative experiments.
In conclusion, these results indicated that the response of AMPK to hyperosmolar sorbitol is ultrasensitive, whereas the response to antimycin is only slightly cooperative. As expected, JNK showed a high Hill coefficient, confirming the ultrasensitive response previously described (2).
Digital (All-or-None) Response of AMPK to Hyperosmolar Sorbitol in Individual Oocytes
The ultrasensitive responses observed at the level of a population of oocytes could be due to either individual oocytes exhibiting graded (analog) responses or to individual oocytes with all-or-none (digital) responses, as previously reported for JNK (2). Thus, we examined the character (graded or all-or-none) of the response of the AMPK system under both stress conditions: antimycin and hyperosmolar sorbitol.
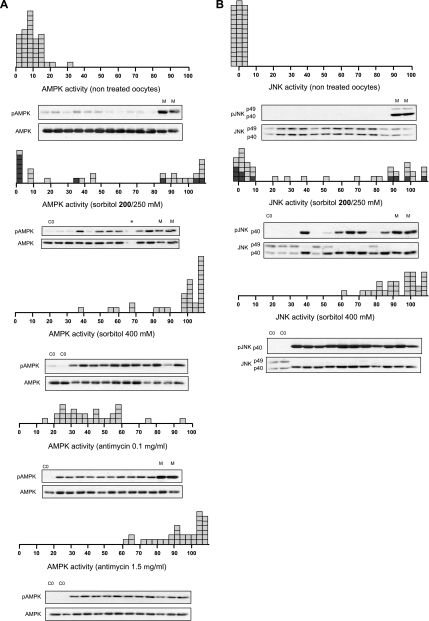
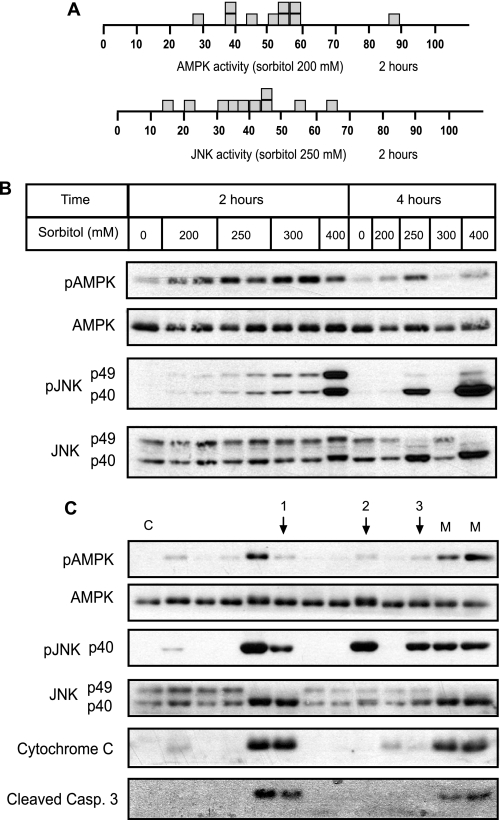
Nontreated individual oocytes have a basal AMPK activity between 0 and 20% of the maximum, whereas JNK activity was practically 0 (considering that 100% activity is the value obtained with maximum stimuli concentration) (Fig. 4, A and B). An intermediate sorbitol concentration (200–250 mm) produced a bimodal distribution of both AMPK and JNK activities, with few oocytes exhibiting intermediate levels, whereas those incubated with 400 mm sorbitol exhibited maximum activities (Fig. 4, A and B). Interestingly, in the oocytes where JNK activation was maximum, the p49 variant disappeared and was accompanied by an increase in the p40 variant, which was also phosphorylated (Fig. 4B). In contrast, the individual oocyte response to the intermediate antimycin concentration (0.1 μg/ml) exhibited intermediate levels (40%) of AMPK activity and was maximum (80–100%) at 1.5 μg/ml (Fig. 4A, bottom). Therefore, the response of AMPK to antimycin was graded at the level of individual oocytes. The digital or graded response of AMPK in individual oocytes with sorbitol and antimycin, respectively, was also observed at the level of ACC phosphorylation, a target of AMPK (supplemental Fig. S2, E–F).
FIGURE 4.
Digital response of the AMPK system to hyperosmolar sorbitol. A, AMPK activity from individual oocytes treated with sorbitol or antimycin. Oocytes were incubated for 4 h with different concentrations of sorbitol or antimycin, and AMPK activity was measured by Western blot. Results are represented as the pAMPK/AMPK ratio, taking as maximum activity (100% value), the oocytes treated with 400 mm sorbitol or 1.5 μg/ml antimycin, respectively (M). B, JNK activity in individual oocytes incubated with various concentrations of sorbitol. Oocytes were incubated for 4 h, and JNK activity was measured by Western blot. Results are expressed as the pJNK/JNK ratio, taking as maximum activity (100%) the value obtained with 400 mm sorbitol (M). Results are the pools of four independent experiments. Each box represents one individual oocyte. The Western blots show representative experiments.
In conclusion, the above results clearly show that the response of AMPK and JNK to hyperosmolar sorbitol is all-or-none in character at the level of individual oocytes, whereas the response of AMPK to antimycin is graded.
Monostability in the AMPK Signaling System to Hyperosmolar Sorbitol
All-or-none responses can arise from an ultrasensitive monostable signaling system with a high Hill coefficient or from a bistable signaling system, which should exhibit some degree of hysteresis. Because the response of JNK to sorbitol in Xenopus oocytes is bistable (2), we analyzed the degree of hysteresis in the AMPK signaling system and compared it with JNK.
When we examined the reversibility of the AMPK and JNK response in pools of oocytes incubated for 4 h and washed and returned to normal buffer, we found an important difference between both signaling systems: AMPK activity returned to basal levels 4 h after washing, whereas JNK activity remained constant during that period (Fig. 5A). Therefore, we can conclude that AMPK is an ultrasensitive monostable system. Surprisingly, we found hysteresis in the response of AMPK to antimycin (1.5 μg/ml) (Fig. 5B). It has been reported that antimycin can be bound to Complex III in a specific and irreversible manner, although no covalent linkages are involved in the binding (25). Therefore, the hysteresis observed in response to antimycin is very likely a consequence of this irreversible binding.
FIGURE 5.
Monostability in the AMPK signaling system. A, time course of AMPK and JNK activation in pools of oocytes treated with 300 mm sorbitol. After 4 h of treatment (arrow), oocytes were washed several times with modified Barth's saline (MBS) medium and maintained in MBS without sorbitol. Pools of oocytes (10) were collected at different times, and Western blot was performed to determine pAMPK, AMPK, pJNK, JNK, cytochrome c, and cleaved caspase-3. AMPK and JNK activities were expressed as the pAMPK/AMPK and pJNK/JNK ratio, respectively, giving the 100% value to the highest activity. Results are the mean ± S.E. of four independent experiments. Westerns blots are from representative experiments. Caspase-3 activity was determined as the concentration of fluorescent AMC formation from Z-DEVD-AMC substrate and represented as arbitrary units of caspase-3 activity. Results are the mean ± S.E. of three independent experiments giving the value of 1 to the nontreated (control) oocytes. B, time course of AMPK activation in pools of oocytes treated with antimycin (1.5 μg/ml). After 4 h of treatment (arrow), the oocytes were washed several times with MBS and maintained in MBS without antimycin. Pools of oocytes (10) were collected at different times and pAMPK, AMPK, cytochrome c, and cleaved caspase-3 were analyzed by Western blot. AMPK activity is expressed as the pAMPK/AMPK ratio, giving the 100% value to the highest activity. Results are the mean ± S.E. of three independent experiments. The Western blots are from a representative experiment. Caspase-3 activity was determined as described in A, and results are the mean ± S.E. of three independent experiments.
As shown at the bottom of Fig. 5A, 300 mm sorbitol induced cytochrome c release, caspase-3 cleavage, and capase-3 activation at 4 h of treatment (at 2 h in other experiments, but never earlier), which remained high even after washing the oocytes and returning them to normal buffer (see arrow in Fig. 5A). Antimycin, in contrast, did not induce cytochrome c release nor caspase activation (Fig. 5B), indicating that a sustained activation of AMPK is not sufficient to induce apoptosis in Xenopus oocytes.
We also observed in the pool of oocytes, in agreement with previous results from individual oocytes, that hyperosmolar sorbitol induced a change in the level of JNK variants at 4 or 5 h after treatment, depending on the experiment. Thus, the JNK p49 variant disappeared and was accompanied by an increase of the p40 variant, which was also phosphorylated (Fig. 5A, bottom). This change was always correlated with caspase activation, and it was protected by the broad inhibitor of caspases, Z-VAD.fmk (data not shown).
In conclusion, AMPK shows a monostable response to hyperosmolar sorbitol, in contrast to the bistable response of JNK. Moreover, a sustained activation of AMPK (obtained with antimycin treatment) is not sufficient to engage a cell death program in Xenopus oocytes.
The Digital Response of AMPK and JNK Is Time-dependent
The digital response of both AMPK and JNK to hyperosmolar stress was measured 4 h after treatment (Fig. 4). Because hyperosmolar sorbitol induced release of cytochrome c and activation of caspase-3 between 2 and 4 h, depending on the experiment (data not shown), we considered whether that period was critical for the nature of the response.
As shown in Fig. 6A, the incubation of Xenopus oocytes with intermediate concentrations of sorbitol (200 or 250 mm) for 2 h induced intermediate levels of activity for both AMPK and JNK in the individual oocytes. Fig. 6B shows a representative Western blot of AMPK and JNK response to different concentrations of sorbitol after 2 or 4 h of treatment in individual oocytes. It is clear that the pattern of activation is graded at 2 h but digital at 4 h. Therefore, we can conclude that the period of 2–4 h is important for the induction of the digital response by hyperosmolar stress. Because this critical period is characterized by cytochrome c release and caspase-3 activation, we incubated the oocytes for 4 h in the presence or absence of the broad inhibitor of caspases, Z-VAD.fmk. The digital response was observed for both AMPK and JNK, even in the presence of the caspase inhibitor (data not shown); thus, indicating that digital responses are time but not caspase-dependent.
FIGURE 6.
AMPK and JNK digital responses are time-dependent but not correlated with cytochrome c release and caspase-3 activation. A, AMPK and JNK activities in individual oocytes treated with intermediate concentrations of sorbitol (200 and 250 mm, respectively) for 2 h. Activity was measured by Western blot, as described previously, and the results expressed as the pAMPK/AMPK and pJNK/JNK ratio, taking as maximum activity the value obtained with 400 mm sorbitol. B, individual oocytes treated with different concentrations of sorbitol for 2 or 4 h. pAMPK, AMPK, pJNK, and JNK were determined by Western blot. C, oocytes treated with 200 mm sorbitol for 4 h. pAMPK, AMPK, pJNK, JNK, cytochrome c release, and cleaved caspase-3 were determined in individual oocytes by Western blot. Arrows indicate no correlation between AMPK and JNK activation or cytochrome c release.
Finally, we addressed whether AMPK and JNK responses are correlated with cytochrome c release at the level of individual oocytes. We incubated the oocytes with 200 mm sorbitol for 4 h (the time necessary to generate digital responses) and analyzed the activation of AMPK and JNK, the release of cytochrome c, and cleavage of caspase-3 by Western blot. As shown in Fig. 6C, release of cytochrome c was not always accompanied with AMPK activation (arrow 1), and JNK activation did not always induce cytochrome c release (arrows 2 and 3); thus, indicating that digital responses of both AMPK and JNK are not completely correlated with cytochrome c release. A model that may explain these results is proposed in Fig. 7.
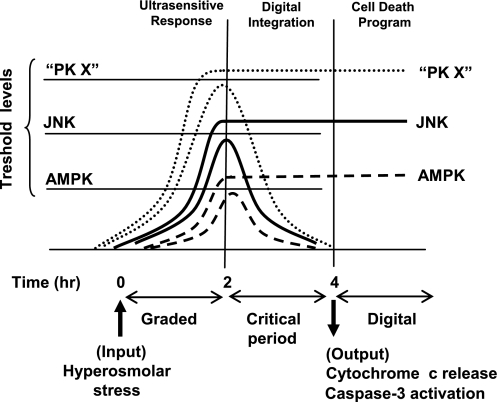
FIGURE 7.
A digital model for initiation of the cell death program. Single cell response to stress (hyperosmolar sorbitol) is ultrasensitive and graded for 2 h, and then a critical period starts where the activity achieved is compared with specific threshold levels during each protein kinase. Kinase activity under the threshold level returns to basal state, whereas activation over the threshold level remains high in the critical period. When we analyze individual oocytes at 4 h, a digital response is obtained, but at 2 h the response is analog. The cell would integrate multiple digital signals (PK X represents one or more protein kinases) for the critical period evaluating whether to trigger a cell death program. Ultrasensitivity and hysteresis are signaling properties that facilitate the digital response, but hysteresis is not present in all signaling systems (e.g. AMPK). Ultrasensitivity is an efficient system for stress sensors to control the initiation of cell death, whereas hysteresis may be important for the progression of the death program.
DISCUSSION
In this work, we first characterized Xenopus laevis AMPK and have shown that it is regulated similarly to the mammalian ortholog. Xenopus AMPK is mainly located in the cytosol and is activated by different stimuli, all previously described to increase the AMP/ATP ratio in the cell. Xenopus oocytes are an excellent model for biochemical studies at the single cell level. Here we have investigated three basic properties of the AMPK signaling system under apoptotic or non-apoptotic stimuli: 1) the sensitivity of the system, 2) the nature of this response (analog or digital), and 3) the degree of hysteresis.
It is clear that the AMPK system shows differential sensitivity depending on the stimuli: highly ultrasensitive to hyperosmolar stress but moderate to antimycin. The variability of response could be due to the activation of different signaling pathways. We have shown that hyperosmolar sorbitol induced the JNK pathway, cytochrome c release, and caspase activation, whereas antimycin did not. Therefore, antimycin and hyperosmolar sorbitol could activate the AMPK cascade by different mechanisms (AMP/ATP levels, regulation of the phosphatase or the upstream kinases, etc), thus producing the different sensitivity of response. Ultrasensitive responses can convert graded inputs into more abrupt and switch-like outputs (8, 26) and may be an efficient method to control initiation of cell death. In our model (Fig. 7), we propose that the ultrasensitive responses allow a rapid activation of sensors, which become transitory or permanent, depending on specific threshold levels for each sensor.
The AMPK response in individual oocytes was graded or all-or-none depending on the stress stimuli. Antimycin induced a graded response, whereas hyperosmolar stress, a digital response in a time-dependent manner (at 4 h it was digital, but at 2 h it was graded). What determines whether some oocytes will achieve the maximum activity or return to basal levels (that is, the individual variability of response) is not clear but could be a consequence of cell to cell variation in metabolic conditions or protein content (AMP/ATP levels, free radical formation, stress sensors, or caspase content, etc.) that would determine certain threshold levels above which the original response remains high (Fig. 7). The correlation between AMPK and JNK activation and cytochrome c release at a single cell level is not perfect, suggesting that additional factors may play a role in the initiation of cell death. We propose that commitment to cell death is taken in the critical period where graded responses turn into digital and after integration of the multiple digital responses form stress sensors (schematized as protein kinase X in Fig. 7).
Here we have shown that the ultrasensitive response of AMPK to hyperosmolar stress is monostable, not exhibiting hysteresis. A monostable system requires the continuous presence of a stimulus to remain in its “on” state, and when the stimulus is removed, the system returns to the “off” state. This means that AMPK is not embedded in a positive feedback loop. In fact, AMPK is a regulator of cellular ATP levels, forming part of a negative feedback loop to maintain energy homeostasis. The JNK response in the oocyte to hyperosmolar sorbitol was digital and showed hysteresis, as previously described (2). JNK is, thus, a bistable system, and a positive feedback loop was predicted according to the results obtained in cytoplasmic transfer experiments (2). Because it is well known that caspase activation can engage different feedback loops (27–29), JNK bistability to hyperosmolar sorbitol could be a consequence of caspase activation.
One question that naturally arises from our results is whether AMPK (and JNK) activation is important for cell fate decision (life or death). The effects of AMPK on apoptosis are complex. In some situations, AMPK appears to prevent the process (30–32), whereas in others AMPK appears to induce it (11, 12, 33). Equally, JNK can have a pro- or anti-apoptotic role depending on the stimuli or the tissue (34, 35). Several studies suggest that a sustained activation of JNK could induce apoptosis (35, 36) and that a transitory activation could be protective for the cell (36). Our study shows that sustained activation of AMPK (antimycin treatment) is not sufficient to induce apoptosis in Xenopus oocytes. We also show that AMPK and JNK activation is not completely correlated with cytochrome c release and caspase-3 activation. We suggest that the AMPK and JNK cascades initially transmit at a single cell level graded analog information about how stressful a cell environment is, and after a certain threshold level is reached, the response becomes all-or-none (digital). The life or death decision would be taken after integration of the available “digital” information from different stress sensors, generated in a critical period, and followed by cytochrome c release, caspase activation, and engagement of positive feedback loops (Fig. 7).
In conclusion, in this work we have characterized Xenopus AMPK, and we have measured important signaling properties of the system, namely sensitivity, hysteresis, and single cell response under different stress conditions. We show that the response of AMPK to hyperosmolar sorbitol, a stimulus that induces apoptosis, is ultrasensitive, digital, and monostable. We propose that integration of multiple digital responses from stress sensors will trigger the cell death program.
Supplementary Material
Acknowledgments
We thank Raúl Méndez from Centre for Genomic Regulation (CRG), José M. Lizcano and Roser Masgrau from Institut de Neurociències, Barcelona, Spain, for helpful discussions and critical reading of the manuscript. We also thank Jesús Giraldo from Institut de Neurociències for bioinformatics assistance.
This work was supported by Grant PI 05/1748 from Fondo de Investigaciones Sanitarias (FIS).

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and Figs. S1 and S2.
- AMPK
- AMP-activated protein kinase
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- ACC
- acetyl-CoA carboxylase
- MBS
- modified Barth's saline
- Z-VAD.fmk
- Z-Val-Ala-DL-Asp(OMe)-fluoromethylketone
- PAS
- p-aminosalicylic acid
- Z
- benzyloxycarbonyl.
REFERENCES
- 1.Hardie D. G. (2007) Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 2.Bagowski C. P., Ferrell J. E., Jr. (2001) Curr. Biol. 11, 1176–1182 [DOI] [PubMed] [Google Scholar]
- 3.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. (1996) J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 4.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. (2006) J. Biol. Chem. 281, 32207–32216 [DOI] [PubMed] [Google Scholar]
- 5.Witters L. A., Kemp B. E., Means A. R. (2006) Trends Biochem. Sci. 31, 13–16 [DOI] [PubMed] [Google Scholar]
- 6.Hardie D. G., Salt I. P., Hawley S. A., Davies S. P. (1999) Biochem. J. 338, 717–722 [PMC free article] [PubMed] [Google Scholar]
- 7.Goldbeter A., Koshland D. E., Jr. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 6840–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrell J. E., Jr., Machleder E. M. (1998) Science 280, 895–898 [DOI] [PubMed] [Google Scholar]
- 9.Ferrell J. E., Jr. (2002) Curr. Opin. Cell Biol. 14, 140–148 [DOI] [PubMed] [Google Scholar]
- 10.Xiong W., Ferrell J. E., Jr. (2003) Nature 426, 460–465 [DOI] [PubMed] [Google Scholar]
- 11.Okoshi R., Ozaki T., Yamamoto H., Ando K., Koida N., Ono S., Koda T., Kamijo T., Nakagawara A., Kizaki H. (2008) J. Biol. Chem. 283, 3979–3987 [DOI] [PubMed] [Google Scholar]
- 12.Meisse D., Van de Casteele M., Beauloye C., Hainault I., Kefas B. A., Rider M. H., Foufelle F., Hue L. (2002) FEBS Lett. 526, 38–42 [DOI] [PubMed] [Google Scholar]
- 13.Kirby K. S. (1965) Biochem. J. 96, 266–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su J. Y., Erikson E., Maller J. L. (1996) J. Biol. Chem. 271, 14430–14437 [DOI] [PubMed] [Google Scholar]
- 15.Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. (2007) Biochem. J. 403, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. (2006) J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
- 17.Ha J., Daniel S., Broyles S. S., Kim K. H. (1994) J. Biol. Chem. 269, 22162–22168 [PubMed] [Google Scholar]
- 18.Zhang L., He H., Balschi J. A. (2007) Am. J. Physiol. Heart Circ. Physiol. 293, H457–H466 [DOI] [PubMed] [Google Scholar]
- 19.Sehy J. V., Zhao L., Xu J., Rayala H. J., Ackerman J. J., Neil J. J. (2004) Magn. Reson. Med. 52, 239–247 [DOI] [PubMed] [Google Scholar]
- 20.Fryer L. G., Parbu-Patel A., Carling D. (2002) J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 21.Aleu J., Martin-Satué M., Navarro P., Pérez de, Lara I., Bahima L., Marsal J., Solsona C. (2003) J. Physiol. 547, 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez O. D., Nolan G. P. (2002) Nat. Biotechnol. 20, 155–162 [DOI] [PubMed] [Google Scholar]
- 23.Tran T. H., Andreka P., Rodrigues C. O., Webster K. A., Bishopric N. H. (2007) J. Biol. Chem. 282, 20340–20350 [DOI] [PubMed] [Google Scholar]
- 24.Bagowski C. P., Xiong W., Ferrell J. E., Jr. (2001) J. Biol. Chem. 276, 1459–1465 [DOI] [PubMed] [Google Scholar]
- 25.Rieske J. S., Lipton S. H., Baum H., Silman H. I. (1967) J. Biol. Chem. 242, 4888–4896 [PubMed] [Google Scholar]
- 26.Huang C. Y., Ferrell J. E., Jr. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10078–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardone M. H., Salvesen G. S., Widmann C., Johnson G., Frisch S. M. (1997) Cell 90, 315–323 [DOI] [PubMed] [Google Scholar]
- 28.Cowling V., Downward J. (2002) Cell Death Differ 9, 1046–1056 [DOI] [PubMed] [Google Scholar]
- 29.Kirsch D. G., Doseff A., Chau B. N., Lim D. S., de Souza-Pinto N. C., Hansford R., Kastan M. B., Lazebnik Y. A., Hardwick J. M. (1999) J. Biol. Chem. 274, 21155–21161 [DOI] [PubMed] [Google Scholar]
- 30.Culmsee C., Monnig J., Kemp B. E., Mattson M. P. (2001) J. Mol. Neurosci. 17, 45–58 [DOI] [PubMed] [Google Scholar]
- 31.Inoki K., Zhu T., Guan K. L. (2003) Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 32.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzatsos A., Tsichlis P. N. (2007) J. Biol. Chem. 282, 18069–18082 [DOI] [PubMed] [Google Scholar]
- 34.Davis R. J. (2000) Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 35.Weston C. R., Davis R. J. (2007) Curr. Opin. Cell Biol. 19, 142–149 [DOI] [PubMed] [Google Scholar]
- 36.Ventura J. J., Hübner A., Zhang C., Flavell R. A., Shokat K. M., Davis R. J. (2006) Mol. Cell 21, 701–710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.