Abstract
Kinetoplastids encode a single nuclear tryptophanyl tRNA that contains a CCA anticodon able to decode the UGG codons used in cytoplasmic protein synthesis but cannot decode the mitochondrial UGA codons. Following mitochondrial import, this problem is circumvented in Trypanosoma brucei by specifically editing the tRNATrp anticodon to UCA, which can now decode the predominant mitochondrial UGA tryptophan codons. This tRNA also undergoes an unusual thiolation at position 33 of the anticodon loop, the only known modification at U33 in any tRNA. In other organisms, tRNA thiolation is mediated by the cysteine desulfurase, Nfs1 (IscS). However, T. brucei encodes two Nfs homologues, one cytoplasmic and the other mitochondrial. We show by a combination of RNA interference and Northern and Western analyses that the mitochondria-targeted TbNfs, and not TbNfs-like protein, is essential for thiolation of both cytosolic and mitochondrial tRNAs. Given the exclusive mitochondrial localization of TbNfs, how it mediates thiolation in the cytoplasm remains unclear. Furthermore, thiolation specifically affects thiolated tRNA stability in the cytoplasm but more surprisingly acts as a negative determinant for the essential C to U editing in T. brucei. This provides a first line of evidence for mitochondrial C to U editing regulation in this system.
Protists of the genera Trypanosoma and Leishmania are kinetoplastid flagellates that belong to the eukaryotic supergroup Excavata and are causative agents of numerous serious diseases afflicting humans and animals mostly in tropical areas. Moreover, because they are amenable to various techniques of forward and reverse genetics, trypanosomes and related parasites qualify as model organisms representing this large and diverse assembly of unicellular eukaryotes.
The single mitochondrion of trypanosomes exists in the form of a reticulated network that undergoes massive changes during the life cycle and is known to contain one of the largest and most complex organellar genomes (1). Indeed, the study of this mitochondrion has produced a plethora of exciting discoveries that have shaped our views of modern cellular biology. For example, transcripts of the majority of protein-coding genes residing in the mitochondrial genome, termed kinetoplast DNA (kDNA), undergo extensive RNA editing via multiple insertions and/or deletions of uridine (2). Despite its large size, the kDNA encodes only over a dozen protein-coding genes and two ribosomal RNAs, whereas most of its coding capacity is devoted to small guide RNA genes that are involved in editing of the mRNAs. Interestingly, the kDNA does not contain any tRNA genes (3). Therefore, tRNAs are transcribed in the nucleus and imported from the cytosol into the organelle.
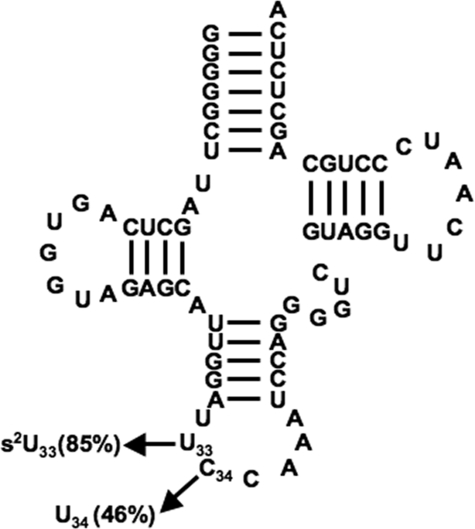
As in most eukaryotes (with the exception of plants), in kinetoplastids such as Leishmania tarentolae the mitochondrial genetic code is not universal, and the frequently occurring UGA codon has been reassigned from a stop codon in the nuclear genes to tryptophan in the organelle (4). Knowledge of an active tRNA import system led us to question how UGA is decoded, provided that the only tRNATrp found in the nuclear genome has a CCA anticodon that could not decode UGA as tryptophan in mitochondria. We previously showed that in L. tarentolae C34 in the first position of the anticodon of tRNATrp undergoes C to U editing, following import into the mitochondrion (5). Mass spectrometry analysis further showed that at least two versions of tRNATrp co-exist in the mitochondrion of L. tarentolae as follows: an unedited unthiolated version and an edited tRNA with a thiolation (s2U) at position 33. A similar situation exists in the mitochondrion of related Trypanosoma brucei, where ∼46% of the mitochondrial tRNATrp is edited (6) (Fig. 1). However, it differs from Leishmania by having ∼85% of tRNATrp thiolated (6) (Fig. 1). We have proposed that the edited and unedited molecules are not redundant in translation and are strictly assigned to the decoding of the UGA and UGG codons, respectively. To date, however, it has remained unclear how this editing balance is maintained, partly because neither the tRNA editing enzyme nor any of the anticodon loop-specific modification enzymes have been identified in these organisms (7).
FIGURE 1.
Secondary structure of tRNATrp from T. brucei. Cloverleaf structure is shown with the confirmed post-transcriptional events indicated. Frequency of each modification present in the steady-state population (in %) is indicated (modified from Ref. 6).
Because thiolation (s2U33) is so far unique to the mitochondrial tRNATrp in L. tarentolae and T. brucei, we have taken a genetic approach to explore the possibility of s2U as an editing determinant in the latter species, which is amenable to RNA interference (RNAi).2 In all organisms studied so far, the conserved cysteine desulfurase Nfs, homologue of bacterial IscS, functions as a sulfur donor as it transfers the sulfur atom of free cysteine to the scaffold protein upon which the Fe-S cluster is formed (8). This desulfurase is also involved in thiamine biosynthesis and post-transcriptional thiolation of tRNAs (9, 10).
Recently, the sequence of the yeast desulfurase gene, Nfs1, was used to search the T. brucei genomic data base leading to the identification of two T. brucei homologues (11). One of these proteins, Nfs-like (previously Nfs 1, IscS1), was predicted to be in the cytoplasm, whereas mitochondrial localization was experimentally verified for the second protein, termed Nfs (previously IscS2) (11). Currently, little is known about how tRNAs are thiolated in T. brucei and what, if any, role either of these Nfs proteins play in thiolation. In this study, we have down-regulated expression of either the Nfs-like or Nfs protein in clonal cell lines of T. brucei procyclics to assess their role in tRNA thiolation. We show the following: (i) Nfs, but not the Nfs-like protein, is responsible for tRNA thiolation in both cytoplasm and mitochondrion; (ii) s2U formation plays a crucial role in tRNA stability; and (iii) in the specific case of tRNATrp, thiolation acts as a negative determinant for C to U editing, helping explain how the levels of the two isoacceptors are maintained in the mitochondria of these cells.
EXPERIMENTAL PROCEDURES
Vectors, Cell Culture, and Fractionation
A 415-bp-long N-terminal region of the T. brucei Nfs-like gene was cloned into the pZJMβ vector and transfected into the 29-13 cells. Clonal cell lines were obtained by limiting dilution, and all growth curves were performed as described elsewhere (12). The procyclic cell line with inducible down-regulation of Nfs was described previously (11). Total and/or mitochondrial RNA was purified using guanidinium extraction. Mitochondria were isolated from 2 liters of noninduced and RNAi-induced cells at 1–2 × 107 cells/ml using hypotonic lysis and Renografin density gradients (13). Alternatively, RNA and/or protein fractions were prepared by digitonin solubilization (11).
The cell line containing the HA3-tagged Nfs-like protein was generated by PCR amplification of the gene from genomic DNA; the resulting product was digested with HindIII and XbaI and cloned into the pJH54 vector. The linearized pJH54 + Nfs-like construct was electroporated into the 29-13 procyclics, and cells were selected by growth in the SDM-79 medium containing 2.5 μg/ml phleomycin.
Antibody Generation and Western Blot Analysis
The 5′ end of the Nfs gene was PCR-amplified and cloned into the pET-100 expression system (Invitrogen). The His6-tagged protein was overexpressed and purified by the metal-chelate affinity chromatography as specified by the manufacturer (Novagen). Polyclonal antibodies were prepared by immunizing rats and chickens at 2-week intervals (Cocalico Biologicals).
Cell lysates corresponding to ∼5 × 106 cells/lane were separated on 15% SDS-polyacrylamide gel, blotted, and probed with chicken polyclonal antibodies against Nfs (at titer 1:500), and signals were visualized using the ECL substrate per the manufacturer's instructions (Pierce). Nfs-like lysates were separated and blotted as described above, but the membranes were treated with anti-HA3 tag mouse monoclonal antibody, followed by donkey anti-mouse antibody. The polyclonal rabbit antibodies against cytosolic enolase (1:150,000; kindly provided by P. A. M. Michels) and mitochondrial RNA-binding protein 1 and/or 2 (MRP1 and -2) (1:1,000) were used as described previously (12). Western blot bands were quantified with the Quantity One software (Bio-Rad).
Detection of RNA, Thiolation, and Editing Levels
Detection of Nfs-like mRNA was carried out either by reverse transcription-PCR or Northern blot analysis using formaldehyde gel electrophoresis on total RNA isolated from noninduced cells as well as in cells in which RNAi was induced for 2 and 4 days. The Nfs-like and Nfs probes were labeled by random priming (Invitrogen).
Poisoned primer extension analysis was carried out as described previously (5). Thiolation of different tRNAs was analyzed by electrophoresis of RNA samples in 7 m urea, 10 mm [(N-acryloylamino)-phenyl] mercuric chloride (APM) acrylamide gel, and blotted as described elsewhere (14). Results were visualized using a PhosphorImager (GE Healthcare) and quantitated with the ImageQuant software (GE Healthcare). Percent thiolation was calculated by dividing the signal from the thiolated species by the total of thiolated and nonthiolated tRNAs. To determine ratios of total tRNA in each sample, the volume of the whole lane was used and compared between RNA isolated from the noninduced and RNAi-induced cells. Ratios were then standardized using tRNAs that do not get thiolated in vivo.
RESULTS
T. brucei Has Two Homologues of the Nfs Proteins with Different Cellular Localization
In the T. brucei genome, there are two proteins similar to the Escherichia coli cysteine desulfurase IscS. One of these proteins, Nfs, is a typical desulfurase involved in Fe-S cluster formation and is required for proper mitochondrial function, thus essential for the procyclic stage (11).
The function and localization of the other putative desulfurase (here referred to as Nfs-like, Tb09.211.3850) has not been experimentally tested thus far. However, several conserved sequence motifs (supplemental Fig. 1A) support its role as a desulfurase. The two proteins (Nfs-like and Nfs) are 28% identical and 42% similar to one another. Nfs shares 51% identity and 66% similarity with E. coli IscS, whereas Nfs-like is more divergent (28% identical and 42% similar) (supplemental Fig. 1B). However, phylogenetic analysis strongly supported the affiliation of Nfs-like with eukaryotic selenocysteine lyases (data not shown). Nonetheless, we decided to test whether either of these two Nfs proteins is involved in tRNA thiolation. We have used the Nfs knockdown cell lines described previously (11), and in light of this study, we have prepared inducible Nfs-like RNAi knockdowns.
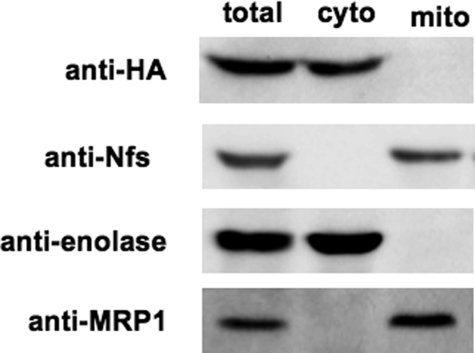
To confirm the mitochondrial localization of Nfs, and also to monitor its down-regulation in RNAi-induced cells, we generated chicken polyclonal antibodies against the overexpressed N-terminal half of the T. brucei protein. Intracellular localization of the Nfs protein was assayed on lysates obtained from parental 29-13 cells after digitonin fractionation. All Nfs protein detected by the antibody was confined to the mitochondrion (Fig. 2). Because specific antibodies against the Nfs-like protein are not available, we have prepared a cell line expressing the Nfs-like protein tagged at its C terminus by an HA3 tag. Using this approach, we were able to confirm that in the procyclic cells, Nfs-like protein has exclusive extramitochondrial localization (Fig. 2). Purity of the mitochondrial and cytosolic fractions was verified using antibodies detecting compartment-specific marker proteins, enolase (cytosolic) and mitochondrial RNA-binding protein 1 (MRP1) (mitochondrial) (Fig. 2).
FIGURE 2.
Nfs is present in the mitochondrion. Immunoblot analysis of cells containing constitutively expressed HA3-tagged Nfs-like (Nfs-like HA) is shown. Cytosolic and mitochondrial fractions were obtained by fractionation using digitonin. Upon resolution by SDS-PAGE, the lysates were blotted and immunoprobed with anti-Nfs and anti-HA3 tag antibodies revealing the mitochondrial and cytosolic localization of Nfs and Nfs-like, respectively. Anti-enolase and anti-MRP1 antibodies were used as cytosolic and mitochondrial markers, respectively. Total, cyto, and mito refer to total protein fractions isolated from total cells, cytoplasmic fractions, or mitochondrial fractions, respectively.
Nfs Is Essential for Thiolation of Cytosolic tRNAs
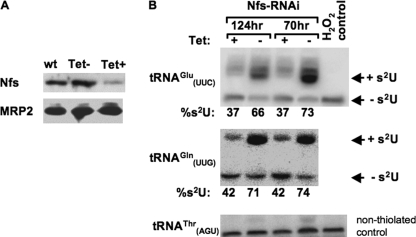
We began our studies by silencing the T. brucei Nfs protein. When RNAi of Nfs was induced by the addition of tetracycline to the medium, the cells grew at a reduced rate and virtually stopped dividing by day 5 (supplemental Fig. 2A). The elimination of Nfs mRNA was confirmed by Northern blot analysis of total RNA isolated from noninduced cells and cells induced for 5 days using an Nfs-specific probe (supplemental Fig. 2B). Western blot analysis with anti-Nfs antibodies also confirmed that most of the target protein was eliminated at day 3 following RNAi induction (Fig. 3A).
FIGURE 3.
Nfs is an essential enzyme involved in tRNA thiolation in the cytoplasm. RNAi analysis of the mitochondria-localized T. brucei Nfs is shown. A, Nfs protein level was analyzed by Western blot analysis in extracts from wild type (wt), noninduced cells, and cells after 3 days of RNAi induction. Mitochondrial MRP2 protein was used as a loading control. Tet, tetracycline. B, total RNA isolated 70 and 124 h post-induction was electrophoretically separated on APM gels and analyzed by Northern hybridization. Probes for two tRNAs (tRNAGlu and tRNAGln) that are thiolated at the wobble position in the cytosol were used for analysis. Bands corresponding to thiolated tRNA and nonthiolated tRNA are indicated by +s2U and −s2U, respectively. H2O2 is used as a control to show complete oxidation of thiolation, eliminating the mobility shift. tRNAThr is used as a loading control. The H2O2 control lane was damaged in the membrane before blotting for tRNAGln, and a similar control lane for this tRNA is shown in Fig. 4C. Percent thiolation (%) was calculated by dividing the signal from the shifted band by the total signal of shifted (thiolated) and nonshifted and multiplied by 100.
To assess effects on tRNA thiolation, RNA was collected from cells grown for 70 and 124 h in the presence of tetracycline and separated by APM gels (see “Experimental Procedures”). Under these conditions, sulfur-containing tRNAs migrate slower than their unthiolated counterparts (15). Northern blots of APM gels were then probed for two cytosolic tRNAs known to be thiolated at the wobble position, tRNAGluUUC and tRNAGlnUUG (16). Both cytosolic tRNAs showed a slower migrating band resulting from the presence of thiolation. This was confirmed by the disappearance of the slower migrating band upon oxidation with hydrogen peroxide. When the T. brucei Nfs protein was silenced, the level of thiolation in both tRNAs was decreased by almost 50% (Fig. 3B). For tRNAGlu the level decreased from 70 ± 4.9 to 37 ± 0.7% in the RNAi-induced cells as compared with the noninduced control. Similar results were seen with the tRNAGln, where in the noninduced cells the level of thiolation was 73 ± 2.1 as compared with only 42% in the RNAi-induced cells. Thus, Nfs is essential for thiolation of cytosolic tRNAs.
Nfs-like Protein Is Not Essential for Thiolation of Cytosolic tRNAs
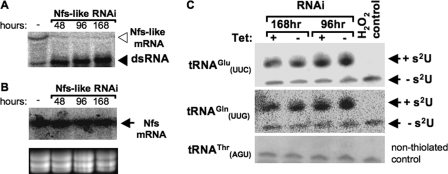
A portion of the Nfs-like gene was also used for RNAi in the T. brucei procyclics. As shown in Fig. 4A, Nfs-like mRNA was virtually eliminated 2 days after RNAi induction, but this did not result in any obvious growth phenotype (supplemental Fig. 3A). Screening of the same RNA samples with the Nfs probe confirmed the lack of off-target RNAi on the Nfs message (Fig. 4B). Moreover, silencing was confirmed using semi-quantitative reverse transcription-PCR, where a reduced level of the Nfs-like PCR product can be seen when compared with the Nfs control (supplemental Fig. 3B). When total RNA from these cells was analyzed by APM/Northern blot and probed for the same two cytosolic tRNAs (tRNAGluUUC and tRNAGlnUUG), there was no difference in the level of thiolation (Fig. 4C). The percentage of thiolation of the tRNAGlu was 66 ± 0.7 and 66 ± 4.9 in the noninduced and Nfs-like RNAi-induced cells, respectively, whereas for tRNAGln thiolation was 61 ± 2.1 and 59 ± 2.1% in the noninduced and RNAi-induced cells, respectively. Therefore, the Nfs-like protein does not play an essential role in tRNA thiolation in the cytoplasm.
FIGURE 4.
Nfs-like protein is not essential for thiolation of cytosolic tRNAs. RNAi analysis of extramitochondrial T. brucei Nfs-like is shown. A, Nfs-like mRNA levels were analyzed by blotting total RNA extracted from noninduced cells (−), and cells were harvested 48, 96, and 168 h after RNAi induction against Nfs-like. The position of the targeted mRNA and the double-stranded RNA (dsRNA) synthesized following induction are indicated with open and closed arrowheads, respectively. B, Northern blot shows that Nfs mRNA levels remain unaltered in the noninduced and RNAi-induced cells described in A. Arrow points to the Nfs mRNA. As a control, the gel was stained with ethidium bromide to visualize rRNA bands. C, total RNA isolated 96 and 168 h post-RNAi induction from the Nfs knockdowns was separated electrophoretically on APM gels. Two tRNAs (tRNAGlu and tRNAGln) that are thiolated at the wobble position in the cytosol were analyzed by Northern hybridization. Bands corresponding to thiolated tRNA and nonthiolated tRNA are indicated. H2O2 is used as a control to show complete oxidation of thiolation, eliminating the mobility shift. tRNAThr is used as a loading control.
Lack of Thiolation Leads to Selective Decay of Thiolated tRNAs
Contrary to what their phylogenetic conservation suggests, most modifications are not essential for survival (for recent review see Ref. 17). However, there is increasing evidence for synthetic lethality between modifications occurring in the backbone of tRNAs and other tRNA-specific factors (18–21). Recently, it has been shown that modifications occurring in the tRNA backbone can affect the structural and biochemical stability of various tRNAs and lead to rapid decay, mediated by the nuclear enzyme Rat1 and the cytosolic enzyme Xrn1 (22). Rapid decay, however, is triggered only when more than one of several nonessential modifications is missing (20).
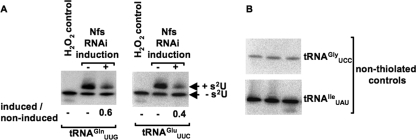
We found that when thiolation was reduced by RNAi knockdown of Nfs, the ratio of the total hybridization signal from the induced and noninduced (i.e. tRNA levels) in each lane was 0.6 ± 0.0 for tRNAGln and 0.4 ± 0.1 for tRNAGlu. No change was observed when the same membranes were probed for cytosolic tRNAThrAGU, a tRNA species that does not get thiolated in vivo (Fig. 3B and Fig. 4C). On the contrary, samples from the Nfs-like knockdown cells were nearly identical regardless of RNAi induction, 0.9 ± 0.0 for tRNAGln and 1.0 ± 0.1 for tRNAGlu (Fig. 3B and Fig. 4C). Once normalized (to the levels of tRNAThrAGU), the tRNA levels in the Nfs RNAi-induced cells were consistently reduced by about 40%. Thus, it appears that the s2U modification present in these two cytosolic tRNAs affects their steady-state levels, suggesting an effect on stability. However, the observed effect could also be due to specific decrease of the rates of transcription of the thiolated tRNAs. To investigate this possibility, two nonthiolated tRNAs (tRNAGlyUCC and tRNAIleUAU) were analyzed by Northern blot (Fig. 5B). When these tRNA controls are used to normalize the noninduced versus RNAi-induced ratio, still a 40–50% reduction (50 ± 2% for tRNAGln and 40% for tRNAGlu) is observed when thiolation is reduced by RNAi (Fig. 5) (similar results were obtained when rRNA was used for normalization; data not shown). The levels of tRNAs that do not naturally contain the wobble thiolation remained the same in all tested cell lines regardless of their genomic location. Importantly, the levels of tRNAIleUAU, found in the same gene cluster as tRNAGlnUUG (23), also remained constant suggesting that the observed decrease in the normally thiolated species, when hypomodified, is because of decreased stability leading to their decay.
FIGURE 5.
Effect on tRNA steady-state levels is not because of decreased transcription. A, RNA isolated from noninduced and RNAi-induced Nfs cells was run on an APM gel followed by Northern blot analysis. Bands corresponding to thiolated tRNA and nonthiolated tRNAs are indicated. H2O2 is used as a control to show complete oxidation of thiolation, eliminating the mobility shift. The ratio of total tRNA isolated from RNAi-induced (+) to noninduced (−) cells after standardizing, with nonthiolated tRNAs (nonthiolated controls) (B), is shown below each panel, where a ratio of 1 would indicate no change, and less than 1 indicates lower steady-state levels of a particular tRNA.
Thiolation Is a Negative Determinant for tRNATrp Editing in Mitochondria
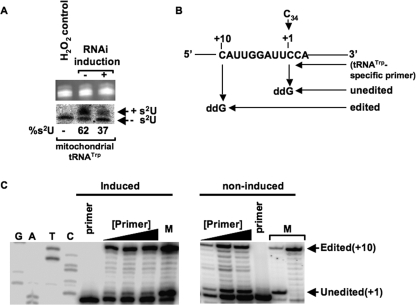
To analyze the consequences of Nfs silencing on mitochondrial tRNATrp, intact mitochondria were isolated from cells grown in the absence and presence of tetracycline. Mitochondrial RNA was then purified and analyzed by APM/Northern blot as described above. In cells with silenced Nfs, thiolation of tRNATrp was decreased from 62 to 37% when comparing the noninduced to the RNAi-induced samples (Fig. 6A). Therefore, not only is Nfs indispensable for the thiolation of cytosolic tRNAs, but it is also essential for the thiolation of tRNATrp in the mitochondria following import. Significantly, lack of thiolation did not lead to instability of tRNATrp, and the ratios of the total signal between noninduced and RNAi-induced samples is close to 1 in mitochondria.
FIGURE 6.
Knockdown of Nfs increases editing of mitochondrial tRNATrp. A, mitochondrial RNA isolated from 2 × 109 cells from noninduced and RNAi-induced cells was separated electrophoretically on an APM gel. The tRNA portion of the ethidium bromide-stained APM gel (top) was used as a loading control. Northern blot analysis probing for tRNATrp is shown at bottom. Bands corresponding to thiolated tRNA and nonthiolated tRNAs are indicated. H2O2 is used as a control to show complete oxidation of thiolation, eliminating the mobility shift. Percent thiolation of the tRNA is shown below the panel and calculated as described previously. B, schematic of the poisoned primer extension assay to measure editing levels. C, poisoned primer extension analysis followed by PAGE. Left panel shows a nonspecific sequencing ladder for orientation and the primer extension using mitochondrial RNA from Nfs knockdown cells. Right panel shows control reactions with mitochondrial RNA from noninduced RNAi cells. Bands representing stops from the edited and unedited are shown to the right. M denotes a marker lane where the primer band, unedited band, and edited band are shown, and in this lane the products were generated by performing poisoned primer extension on tRNATrp in vitro transcripts containing either a C34 (unedited) or a U34 (edited). These control reactions using transcripts were carried out simultaneously but in a separate primer extension reaction. Primer corresponds to lanes where primer alone was loaded. Black triangles denote increasing concentrations of primer used in the assay to ensure linearity during quantitation.
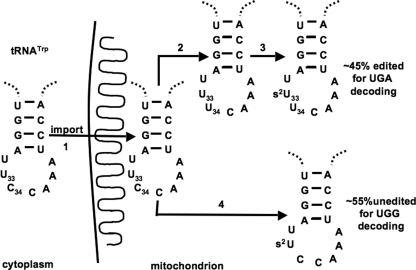
Previously, we showed that in L. tarentolae tRNATrp undergoes C to U editing in the first position of the anticodon, and importantly, only the edited tRNA species containing U34 was thiolated at position 33 after mitochondrial import (14). This suggests that the nucleus-encoded tRNATrp, while in transit through the cytoplasm, is not a substrate for the cytosolic thiolation machinery. We then proposed that thiolation serves a role as an editing determinant. In T. brucei, however, the situation is different in that thiolation levels are much higher than editing levels, indicating that thiolation occurs in both the edited and unedited tRNATrp (6). We decided to explore further the function of thiolation in the T. brucei system. We analyzed the editing levels of mitochondrial tRNATrp by poisoned primer extension (5), where cDNA synthesis was performed in the presence of ddG. This would yield a stop at +10 nucleotides away from the primer for the edited tRNA, whereas the unedited tRNA would yield a stop at +1 (Fig. 6B). Remarkably, although thiolation is significantly reduced in the Nfs knockdown cells, it also resulted in a massive increase of editing levels. In the RNAi-induced cells, edited tRNAs levels reached 90.7 ± 0.6%, whereas in the control cells only 50.7 ± 3.2% of tRNATrp were edited (Fig. 6C). The levels of editing in the noninduced cells are consistent with previously reported levels for the wild type cells of L. tarentolae and T. brucei (5, 6). These data demonstrate that U33 thiolation is not required for C34 to U34 editing in T. brucei, but more importantly, it serves as a negative determinant for editing. In this realm, thiolation and editing might in fact compete for the unedited, nonthiolated tRNA substrates (Fig. 7).
FIGURE 7.
Model for the interrelation between editing and thiolation in T. brucei mitochondria. In the model, tRNATrp can take two competing pathways in the mitochondrion. Both pathways are believed necessary to ensure accurate and efficient reading of both tryptophan codons. Following mitochondrial import (1), the tRNA undergoes C to U editing at position 34 (2), followed by thiolation at U33 (3). Alternatively the tRNA is first thiolated, which acts as a negative determinant for editing and yields the thiolated, nonedited species shown and maintains the levels of edited to nonedited tRNAs in mitochondria. Although many other modifications occur in the homologous tRNA tRNATrp from L. tarentolae, only the thiolation and editing shown here have been confirmed in T. brucei.
DISCUSSION
Besides their function in iron-sulfur cluster assembly, cysteine desulfurases are known to serve as sulfur donors in post-transcriptional formation of 2-thiouridine (s2U) and 4-thiouridine (s4U) in tRNAs and may also participate in other sulfur-containing tRNA modifications, such as ms2i6A and s2C (8, 24–26). A recent study showed that only the mitochondrial Nfs1p is involved in thiolation of tRNAs in both cytosol and mitochondria (27). Moreover, numerous components of the cytosolic Fe-S cluster assembly pathway are apparently needed for proper modification of the cytosolic tRNAs (8).
Here, we confirm these observations by showing that down-regulation of expression of the Nfs desulfurase in T. brucei disrupts thiolation of both mitochondrial and cytosolic tRNAs. T. brucei Nfs is thus the orthologue of yeast Nfs1. The important difference from the yeast (28) and human cells (29), however, is that with two different specific antibodies against the T. brucei Nfs we did not detect any Nfs protein outside of the mitochondrion (11) (Fig. 2). Therefore, it is not clear how Nfs mediates thiolation of cytosolic tRNAs in these organisms. Because TbNfs is translated in the cytoplasm and then imported into the mitochondria, it is possible that a small fraction of the cytoplasm-localized enzyme is responsible for cytoplasmic thiolation. In this scenario, the lack of detection of TbNfs in cytoplasmic extracts may reflect the fact that these small amounts escape our Western detection. There is also the remote possibility that a small but sufficient fraction of TbNfs comes out of the mitochondria and mediates thiolation. Unfortunately, given the transitory nature of TbNfs in the cytoplasm and the lack of a mitochondrial transformation system in T. brucei, it is not currently possible to clearly address these possibilities.
Further analysis also revealed that reduction of thiolation by RNAi led to decreased steady-state levels of tRNAs, but only of tRNAs that naturally undergo s2U thiolation in their anticodon (tRNAGlnUUG and tRNAGluUUC). Two alternative explanations exist that may explain such a decrease as follows: either overall transcriptional rates of tRNAs drop following the down-regulation of Nfs or, alternatively, the lack of thiolation leads to a specific decay of the hypo-thiolated species. The first possibility implies that the steady-state levels of many tRNAs, regardless of whether they undergo thiolation or not, would decrease. However, we showed that probing the same Northern blot membranes for tRNAs that do not naturally undergo thiolation led to no change in their steady-state levels, when total RNA from the noninduced and RNAi-induced cells are compared. We thus suggest that the most plausible explanation for the observed reduced levels of tRNAGlnUUG and tRNAGluUUC is that besides serving roles in translational efficiency thiolation also contributes to specific tRNA stability of naturally thiolated species. Notably, a knock-out of Nfs1 in Saccharomyces cerevisiae has no effect on the steady-state levels of the homologous tRNAs (30), indicating that this pathway is so far specific for T. brucei. In addition, rapid decay occurs in other tRNAs in a modification-specific manner, but this decay mechanism is only obvious if two or more modification enzymes are mutated simultaneously (20). In these cases, only by affecting synthetic interactions the role of seemingly nonessential modifications was revealed and led to synthetic lethality. To our knowledge, the decay of tRNAs in a thiolation-specific manner represents the only example of a single modification affecting tRNA stability. One instance of a lack of m1A leading to decay exists with tRNAMet; however, this is a special case where only the specific tRNA decreased and the levels of other m1A-containing tRNAs remained unchanged (31).
Our previous finding that ∼50% of the mitochondrial tRNATrp undergoes a specific C to U editing at the first position of the anticodon (5) following import raised the immediate question as to how the levels of editing are regulated. We were particularly interested in the thiolation of U33, which so far has only been described in the mitochondrial tRNATrp of Trypanosoma and Leishmania, whereas in 97% of all sequenced tRNAs this nucleotide remains unmodified regardless of the organism (14). As we show here, this thiolation also depends on the presence of the Nfs protein.
To explain editing specificity, we have previously proposed an interdependence model, which suggests that modifications and editing of multiple sites act in concert to achieve the degree of substrate specificity required by different systems (7, 14). The situation of tRNATrp in T. brucei is particularly suitable to test this hypothesis, because two dramatically different modifications occur on neighboring nucleotides, namely the thiolation of U33 and C to U editing of C34. Based on several lines of evidence (14), we suggest that the thiolation of U33 was an essential prerequisite for C34 editing and is thus important for cell viability (7). In this model, U33 thiolation, at the same position required to form the classical U-turn structure of the anticodon, may lead to an alteration of the tRNA anticodon loop structure, which is in turn necessary for the recognition by the so far unidentified C to U editing enzyme. Data presented herein show that thiolation and C to U editing of the tRNATrp are interrelated events, however, as it turns out in an unanticipated manner. The down-regulation of tRNA thiolation by RNAi of Nfs in T. brucei increased the editing levels to close to ∼90%, suggesting that in T. brucei thiolation is a negative determinant for tRNA editing. This situation is different from that of L. tarentolae where only the edited species is thiolated. These findings suggest a species-specific difference in how editing and thiolation are controlled in these two closely related organisms. Although seemingly unusual, species-specific differences between Leishmania and Trypanosoma have been well documented. For example, although RNAi works perfectly in T. brucei, the machinery is missing in Leishmania species (including L. tarentolae). These findings also help explain how the ∼50% editing level is maintained, raising the possibility of competition of the thiolation and editing machineries for the unedited nonthiolated tRNAs (Fig. 7). A corollary of this proposal is that once the tRNATrp has been thiolated, it either becomes less accessible to the editing enzyme or, alternatively, thiolation directly affects editing by a more direct but still unknown mechanism.
Why then do the levels of C to U editing in tRNATrp need to be maintained? As is apparent from supplemental Table I, out of a total of 106 predicted codons in the mitochondrial mRNAs decoded by tRNATrp, ∼70% require the edited version of this molecule. We posit that the large number of anticodon loop modifications in tRNATrp lead to a rather rigid anticodon loop structure, which in turn affects the ability of the tRNA to wobble. Therefore, the machinery performing modifications of the target tRNA has to operate so that an optimal balance between unedited and edited tRNATrp is established and maintained. This balance allows decoding of the mitochondrial UGG and UGA codons by the unedited and edited species, respectively, and to some extent reflects the ratio by which both codons are utilized. However, removal of thiolation not only increases editing but also may remove the wobble restriction. Perhaps, this could be used by the parasites for the differential expression of certain genes that may contain different numbers of UGG and UGA codons. Currently, however, in the absence of an in vitro mitochondrial translation and/or transformation system, these will remain open questions. Trypanosomes are known to massively up- and down-regulate mitochondrial metabolism during their development in tset-se fly and human blood, respectively (32). It is thus possible that these adaptable parasites may exploit, in a yet unexplained and unanticipated manner, the interrelation between tRNA editing and modification to regulate mitochondrial function in these very different environments. The complete pathway for either cytosolic or mitochondrial tRNA thiolation in T. brucei is not completely clear, and the extent to which thiolation is exploited for regulation will for now remain an open question.
Supplementary Material
Acknowledgments
We thank Chad Rappleye, Venkat Gopalan, Jane Jackman, Anita Hopper, and all members of the Lukeš and Alfonzo laboratories for useful comments and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM084065 from NIGMS (to J. D. A.). This work was also supported by Grant MCB-0620707 from the National Science Foundation (to J. D. A.), Grant 204/06/1558 from the Grant Agency of the Czech Republic, and Grants LC07032 and 2B06129 from the Czech Ministry of Education (to J. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table I and Figs. 1–3.
- RNAi
- RNA interference
- APM
- [(N-acryloylamino)-phenyl] mercuric chloride
- HA
- hemagglutinin.
REFERENCES
- 1.Liu B., Liu Y., Motyka S. A., Agbo E. E., Englund P. T. (2005) Trends Parasitol. 21, 363–369 [DOI] [PubMed] [Google Scholar]
- 2.Lukeš J., Hashimi H., Zíková A. (2005) Curr. Genet. 48, 277–299 [DOI] [PubMed] [Google Scholar]
- 3.Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. (1989) Nucleic Acids Res. 17, 5427–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Cruz V. F., Neckelmann N., Simpson L. (1984) J. Biol. Chem. 259, 15136–15147 [PubMed] [Google Scholar]
- 5.Alfonzo J. D., Blanc V., Estévez A. M., Rubio M. A., Simpson L. (1999) EMBO J. 18, 7056–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charrière F., Helgadóttir S., Horn E. K., Söll D., Schneider A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6847–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio M. A., Alfonzo J. D. (2005) in Topics in Current Genetics ( Grosjean H. ed) pp. 71–86, Springer-Verlag, Berlin-Heidelberg [Google Scholar]
- 8.Lill R., Mühlenhoff U. (2008) Annu. Rev. Biochem. 77, 669–700 [DOI] [PubMed] [Google Scholar]
- 9.Lauhon C. T. (2002) J. Bacteriol. 184, 6820–6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson K., Lundgren H. K., Hagervall T. G., Björk G. R. (2002) J. Bacteriol. 184, 6830–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Šmíd O., Horáková E., Vilímová V., Hrdý I., Cammack R., Horváth A., Lukeš J., Tachezy J. (2006) J. Biol. Chem. 281, 28679–28686 [DOI] [PubMed] [Google Scholar]
- 12.Vondrušková E., van den Burg J., Zíková A., Ernst N. L., Stuart K., Benne R., Lukeš J. (2005) J. Biol. Chem. 280, 2429–2438 [DOI] [PubMed] [Google Scholar]
- 13.Kapushoc S. T., Alfonzo J. D., Rubio M. A., Simpson L. (2000) J. Biol. Chem. 275, 37907–37914 [DOI] [PubMed] [Google Scholar]
- 14.Crain P. F., Alfonzo J. D., Rozenski J., Kapushoc S. T., McCloskey J. A., Simpson L. (2002) RNA 8, 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igloi G. L. (1988) Biochemistry 27, 3842–3849 [DOI] [PubMed] [Google Scholar]
- 16.Kaneko T., Suzuki T., Kapushoc S. T., Rubio M. A., Ghazvini J., Watanabe K., Simpson L., Suzuki T. (2003) EMBO J. 22, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agris P. F., Vendeix F. A., Graham W. D. (2007) J. Mol. Biol. 366, 1–13 [DOI] [PubMed] [Google Scholar]
- 18.Copela L. A., Chakshusmathi G., Sherrer R. L., Wolin S. L. (2006) RNA 12, 644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosshans H., Lecointe F., Grosjean H., Hurt E., Simos G. (2001) J. Biol. Chem. 276, 46333–46339 [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov A., Chernyakov I., Gu W., Hiley S. L., Hughes T. R., Grayhack E. J., Phizicky E. M. (2006) Mol. Cell 21, 87–96 [DOI] [PubMed] [Google Scholar]
- 21.Johansson M. J., Byström A. S. (2002) RNA 8, 324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chernyakov I., Whipple J. M., Kotelawala L., Grayhack E. J., Phizicky E. M. (2008) Genes Dev. 22, 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan T. H., Pach R., Crausaz A., Ivens A., Schneider A. (2002) Mol. Cell. Biol. 22, 3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. (2006) Mol. Cell 21, 97–108 [DOI] [PubMed] [Google Scholar]
- 25.Kambampati R., Lauhon C. T. (2003) Biochemistry 42, 1109–1117 [DOI] [PubMed] [Google Scholar]
- 26.Leidel S., Pedrioli P. G., Bucher T., Brost R., Costanzo M., Schmidt A., Aebersold R., Boone C., Hofmann K., Peter M. (2009) Nature 458, 228–232 [DOI] [PubMed] [Google Scholar]
- 27.Nakai Y., Umeda N., Suzuki T., Nakai M., Hayashi H., Watanabe K., Kagamiyama H. (2004) J. Biol. Chem. 279, 12363–12368 [DOI] [PubMed] [Google Scholar]
- 28.Nakai Y., Nakai M., Hayashi H., Kagamiyama H. (2001) J. Biol. Chem. 276, 8314–8320 [DOI] [PubMed] [Google Scholar]
- 29.Land T., Rouault T. A. (1998) Mol. Cell 2, 807–815 [DOI] [PubMed] [Google Scholar]
- 30.Johansson M. J., Esberg A., Huang B., Björk G. R., Byström A. S. (2008) Mol. Cell. Biol. 28, 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadaba S., Krueger A., Trice T., Krecic A. M., Hinnebusch A. G., Anderson J. (2004) Genes Dev. 18, 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider A. (2001) Int. J. Parasitol. 31, 1403–1415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.