Abstract
The bacterial protein-disulfide isomerase DsbC is a homodimeric V-shaped enzyme that consists of a dimerization domain, two α-helical linkers, and two opposing thioredoxin fold catalytic domains. The functional significance of the two catalytic domains of DsbC is not well understood yet. We have engineered heterodimer-like DsbC derivatives covalently linked via (Gly3-Ser) flexible linkers. We either inactivated one of the catalytic sites (CGYC), or entirely removed one of the catalytic domains while maintaining the putative binding area intact. Variants having a single active catalytic site display significant levels of isomerase activity. Furthermore, mDsbC[H45D]-dim[D53H], a DsbC variant lacking an entire catalytic domain but with an intact dimerization domain, also showed isomerase activity, albeit at lower levels. In addition, the absence of the catalytic domain allowed this protein to catalyze in vivo oxidation. Our results reveal that two catalytic domains in DsbC are not essential for disulfide bond isomerization and that a determining feature in isomerization is the availability of a substrate binding domain.
Disulfide bonds are critical for the proper folding and structural stability of many exocytoplasmic proteins. The Dsb family of thiol:disulfide oxidoreductase enzymes catalyzes oxidative protein folding in the periplasm of Escherichia coli by means of two independent pathways (1–3). In the DsbA-DsbB oxidation pathway, DsbA, a very strong oxidant, catalyzes the formation of disulfide bonds on newly translocated proteins (4). The DsbA disulfide is rapidly recycled by DsbB, a membrane protein that transfers electrons from DsbA onto quinones (5–7). In the DsbC-DsbD isomerization pathway, non-native disulfides are reduced or rearranged by DsbC. DsbC is maintained in a reduced, catalytically active state via the transfer of electrons from the inner membrane protein DsbD that in turn accepts electrons from thioredoxin 1 and ultimately from NADPH (via thioredoxin reductase) within the cytoplasm (8, 9). Large kinetic barriers keep the oxidation and isomerization pathways isolated, preventing the establishment of a futile cycle of electron transfer. Accordingly, reactions between enzymes of the two pathways, for example the oxidation of DsbC by DsbB or the reduction of DsbA by DsbD, are 103–107-fold slower than the physiologically relevant DsbA-DsbB and DsbC-DsbD reactions (10). Nonetheless, the kinetic barrier between DsbB and DsbC can be breached by introducing mutations that result in structural changes in DsbC (11, 12).
DsbC is a homodimer with each monomer comprising an N-terminal dimerization domain and a C-terminal thioredoxin-like catalytic domain fused by an α-helical linker. The crystal structure of DsbC reveals that the two monomers come together to form a V-shaped protein. The inner surface of the resulting cleft is patched with uncharged and hydrophobic residues suggesting an important role in the binding of substrate proteins. The active sites comprising the sequence Cys98-Gly99-Tyr100-Cys101 in each of the monomeric subunits are located in the arms of the “V” facing each other (13). Isomerization involves an attack onto a substrate disulfide by Cys98 resulting in the formation of a mixed disulfide, which then is resolved by either another cysteine from the substrate or by Cys101 from DsbC (14, 15). Besides its isomerase activity, DsbC is also known to display chaperone activity preventing protein aggregation during refolding (16). In E. coli, disulfide bond isomerization is the limiting step in the oxidative folding of many heterologous proteins that contain multiple cysteines. Overexpression of DsbC has been shown to enhance the yield of proteins such as human nerve growth factor, human tissue plasminogen activator (tPA)2 and immunoglobulins (17–19).
DsbC is topologically analogous to the eukaryotic protein-disulfide isomerase (PDI). The structural similarities between the two enzymes may have resulted from convergent evolution by thioredoxin-like domain replication in the case of PDI and domain recruitment in DsbC (20, 21). PDI comprises two thioredoxin-like catalytic domains (a and a′) separated by two non-catalytic domains (b and b′), in addition to a c domain (22). In PDI, the catalytic domains are different and functionally nonequivalent (23). Substrate binding is mediated primarily by the b′ domain; the two catalytic domains, a and a′, can catalyze oxidation of small model peptides indicating that they must also have low substrate binding affinity (24).
The DsbC monomer is essentially devoid of RNase A isomerase activity (25). Sun and Wang (44) reported that DsbC with one catalytic site impaired by carboxymethylation is also essentially inactive but, in separate studies, Zapun et al. (26) did not detect cooperativity between the two catalytic sites indicating that they function independently of each other (26). Moreover, unlike PDI, the significance of the putative peptide binding cleft of DsbC on disulfide isomerization has not been ascertained. However, while DsbA or TrxA with a PDI active site dipeptide (CGHC) display very little isomerase activity in vitro and in vivo (27–29), we recently showed that upon fusion to a dimerization region that provides a putative substrate binding surface (the E. coli peptidyl proline isomerase FkpA) they acquire the ability to assist the folding of periplasmically expressed multidisulfide heterologous proteins (30).
In the present work, we engineered heterodimer-like covalently linked DsbC derivatives in which one of the catalytic sites has been inactivated (Fig. 1A) or one of the catalytic domains has been entirely removed while maintaining the intact peptide binding cleft (which is normally formed by association of the N-terminal domains of the two monomers) (Fig. 3A). We show that DsbC forced monomers with one functional active site, or with one thioredoxin domain only, display significant isomerization activity. Interestingly, the latter variant is partially reduced in vivo indicating that the presence of both thioredoxin domains is important for the avoidance of protein oxidation by DsbB.
FIGURE 1.
A, protein structure of DsbC, and molecular models of mDsbC-mDsbC and the single active site covalently linked mutants. Dimerization domains are shown in gray, thioredoxin domains in black, and the active sites in white. B, gel filtration FPLC of DsbC and linked variants. Purified proteins were run on a SuperdexTM 200 column in PBS, 10% glycerol buffer.
FIGURE 3.
A, molecular model of mDsbC-dim. Dimerization domains are shown in gray, thioredoxin domain in black, and catalytic site in white. B, gel filtration FPLC of mDsbC-dim as compared with DsbC. Purified proteins were run on a SuperdexTM 200 column in PBS, 10% glycerol buffer. C, MALS measurement of the molar masses of the components of mDsbC-dim together with their hydrodynamic radii. The data show monomeric, dimeric, and tetrameric states. The relative concentrations were determined by the refractive index differences.
EXPERIMENTAL PROCEDURES
Strains and Plasmids
The bacterial strains and plasmids used in this study are listed in supplemental Table S1A. Genes encoding DsbC fusions containing a (GGGS)3GSA or (GGGS)7SA linker peptide were assembled by amplifying the individual monomer genes via PCR using the primers shown in supplemental Table S1B, digesting with XbaI-PvuII or PvuII-HindIII, and ligating into pBAD30. The complete fusion genes were then digested with XbaI and HindIII, and cloned either into pBAD33 (31), for in vivo testing, or into pET28(a) for protein purification purposes.
In Vivo Disulfide Bond Formation and Isomerization
E. coli PB351 (SF100 ΔdegP ΔdsbC) and PB403 (SF100 ΔdegP dsbA::kan) were co-transformed with pBAD33 plasmids encoding the DsbC or mDsbC-mDsbC variants, and with pTrcStIIvtPA, a derivative of pTrc99A encoding the tPA (Δ6–175) gene fused to the StII signal peptide (32). SF100 ΔdegP ΔdsbC cells were grown in LB medium and SF100 ΔdegP dsbA::kan cells in minimal medium (M9 salts, 0.1% casein amino acids, 2 mm MgSO4, 5 μg/ml thiamine, 0.4% glycerol with 50 μg/ml of kanamycin) supplemented with 25 μg/ml of chloramphenicol and 100 μg/ml of ampicillin at 37 °C. Induction of protein synthesis, cell harvesting, and assays for tPA activity were performed as described earlier (29). For the evaluation of in vivo oxidase activity of DsbC and its variants, E. coli LM106 (MC1000 dsbA::kan) and LM102 (MC1000 dsbB::kan) were transformed with the appropriate pBAD33 derivatives, grown in LB medium with 50 μg/ml of kanamycin and 25 μg/ml of chloramphenicol, and subcultured 1:50 in low phosphate minimal medium (MOPS salts, 0.2% glycerol, 0.2% casein amino acids, and 0.5 μg/ml thiamine, with 50 μg/ml of kanamycin and 25 μg/ml of chloramphenicol). When cultures reached an A600 of 0.4–0.5, arabinose was added to a final concentration of 0.2% (w/v). After 2 h of growth, 30 μl of cells were mixed with 20 μl of buffer (0.4 m iodoacetamide and lysis buffer (B-PERTM, Pierce) in a 1:2 ratio) in a 96-well plate and incubated at room temperature for 30 min. To measure alkaline phosphatase activity, 200 μl of 0.2 m Tris pH 8 and 0.25 mg/ml of p-nitrophenyl phosphate disodium hexahydrate (pNPP) (Sigma) were added, and the change in absorbance at A405 was recorded while incubating at room temperature for 2 h.
In Vivo Redox State
Overnight cultures of E. coli SF100 ΔdegP ΔdsbC harboring the appropriate pBAD33 derivatives were diluted 1:100 in LB medium containing 25 μg/ml of chloramphenicol and grown to A600 of ∼0.6–0.7, then expression was induced by adding arabinose to a final concentration of 0.0002%. 2 h after induction, a culture aliquot with an A600 equivalent to 1.0 was collected and mixed with an equal volume of 20% trichloroacetic acid to simultaneously quench disulfide exchange and precipitate the proteins in the sample (33). 15 mm 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt (AMS) was added and after a 45-min incubation at room temperature, proteins were pelleted and then solubilized in 75 μl of Laemmli 1× buffer without reducing agents (34). A reduced protein standard was prepared by first incubating 0.02 mg of purified protein with 100 mm DTT for 1 h followed by filtration through Sephadex G-25 for DTT removal and by trichloroacetic acid precipitation, AMS treatment, and solubilization, as above. Oxidized purified protein standards were generated by the same procedure except that incubation with DTT and AMS were omitted. The reduced and oxidized protein bands were resolved by SDS-PAGE electrophoresis on a 12% gel, and protein bands detected by Western blotting with an anti-histidine antibody (Sigma) at 1:20,000 dilution.
Expression, Purification, and Biochemical Assays
For the purification of the DsbC and mDsbC-mDsbC proteins, pET28(a) plasmids encoding the appropriate genes were transformed into E. coli BL21(DE3). Protein expression and purification was performed as previously described (29). All proteins used in this study were more than 95% pure as judged by Coomassie Blue-stained SDS-PAGE electrophoresis. The kinetics of insulin reduction and the renaturation of reduced, denatured RNase A were determined according to published procedures (35–37).
Light Scattering
Multi-angle light scattering (MALS) experiments were performed as previously described (38) with the following modifications: the buffer was 1× PBS (137 mm NaCl, 2.7 mm KCl, 12 mm Na2HPO4, 1.2 mm KH2PO4, pH 7.4); the protein concentration was ∼0.26 mm; the EOS photometer wavelength was 690 nm; a Wyatt model Optilab rEX differential refractometer followed the light scattering photometer in series; a TSK-GEL G3000PWXL size-exclusion column (TosoHaas, 300 × 7.8 mm) at a flow-rate of 0.4 ml/min was used; bovine serum albumin (Sigma A 1900) at 2.5 mg/ml was used for normalization of the light scattering detectors of the EOS. Dynamic light scattering: channel 13 of the EOS photometer together with a Wyatt QELS quasi-elastic light scattering instrument measured the diffusion coefficient from which the hydrodynamic radius of the protein was calculated. The data were analyzed with Astra software version 4.90.08 supplied by Wyatt.
RESULTS
Construction of a Linked Monomeric DsbC
A covalently linked DsbC was constructed by creating a gene fusion consisting of a dsbC gene in-frame with a sequence encoding a 15-amino acid Gly-Ser linker and a second dsbC gene. Gly-rich linkers have been used to join the two subunits of homodimeric proteins into a single polypeptide without disrupting activity (39–42). In the DsbC crystal structure, the distance between the C terminus of one subunit and the N terminus of the second subunit is ∼36 Å (13). To avoid introducing strain in the DsbC-DsbC fusion we employed a (Gly3-Ser)3-Gly-Ser-Ala linker estimated to span 57 Å in its extended conformation based on the theoretical 3.8 Å per residue planar length. The resulting fusion protein was designated mDsbC-mDsbC (Fig. 1A). The covalently linked DsbC was secreted into the periplasmic space via the DsbC signal peptide and purified by metal affinity chromatography. Gel filtration FPLC revealed that mDsbC-mDsbC elutes as a single peak with a retention time identical to that of DsbC (Fig. 1B). mDsbC-mDsbC exhibited slightly lower activity in the oxidative refolding of reduced RNase A and in the reduction of insulin (84 and 78%, respectively) relative to wt DsbC; however these differences in activity were not statistically significant. The in vivo disulfide isomerase activity of mDsbC-mDsbC in the E. coli periplasm was evaluated by determining the yield of correctly folded and active vtPA secreted via the StII signal peptide (29). vtPA is a truncated version of human tPA containing 9 disulfide bonds. Correct folding of tPA in the periplasmic space is entirely dependent upon disulfide bond isomerization and requires co-expression of DsbC (18). E. coli ΔdegP ΔdsbC cells containing plasmids encoding vtPA and the DsbC variant, transcribed from the pTrc and the arabinose promoters, respectively, were grown in LB medium, and protein synthesis was induced by isopropyl-1-thio-β-d-galactopyranoside and arabinose. Western blot analysis revealed that mDsbC-mDsbC and DsbC were expressed at the same level in E. coli (supplemental Fig. S1). The vtPA activity obtained by co-expressing mDsbC-mDsbC was essentially identical to that observed with wild type DsbC expressed from the same promoter indicating that the two proteins have identical activity in vivo (Fig. 2A).
FIGURE 2.
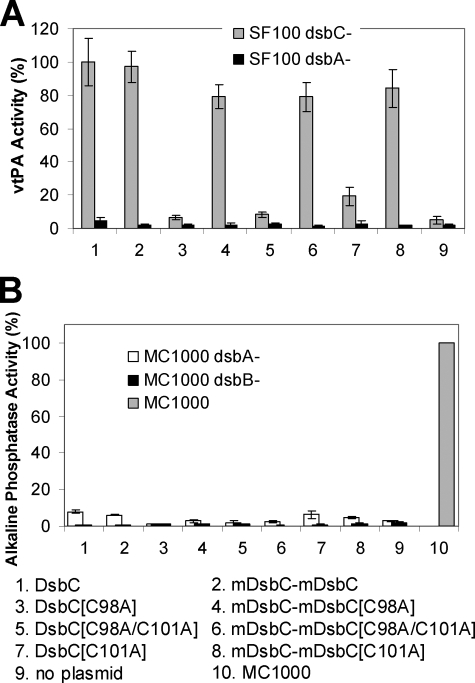
In vivo activity of single active site mutants. A, yield of active vtPA in dsbC (gray bars) or dsbA (black bars) cells. E. coli SF100 ΔdegP ΔdsbC and SF100 ΔdegP dsbA::kan (respectively) were transformed with pTrcStIIvtPA and pBAD33 encoding the respective DsbC derivatives. Protein synthesis was induced as described under “Experimental Procedures,” and the yield of active vtPA at 3 h after induction was determined. Relative activities were obtained by dividing the ΔA405 of each strain by the ΔA405 of the strain expressing wild type DsbC. B, PhoA activity in E. coli MC1000 dsbA::kan (white bars) and MC1000 dsbB::kan (black bars). The alkaline phosphatase activity in the parental isogenic strain MC1000 is shown by the gray bar.
Earlier we had shown that the orientation of the thioredoxin catalytic domains relative to each other in the DsbC homodimer is critical for avoiding misoxidation by DsbB. Single amino acid deletions in the α-helical linker that joins the catalytic and the dimerization domains render the protein susceptible to oxidation by DsbB. In turn, oxidized DsbC accumulates in the periplasm where it catalyzes the formation of disulfide bonds in substrate proteins and thus can partially complement dsbA mutants (12). We evaluated whether the covalent linkage of two DsbC monomers might have perturbed the tertiary structure of the molecule to make it susceptible to oxidation. Alkaline phosphatase (PhoA) contains two disulfide bonds that are essential for activity (43); in MC1000 dsbA cells grown in low phosphate medium, the PhoA level is about 3% of that observed in the wt cells (Fig. 2B). While overexpression of wt DsbC does not restore PhoA activity, the expression of DsbC variants with a perturbed tertiary structure results in PhoA levels >30% of those in wt cells (12). In contrast, expression of mDsbC-mDsbC conferred only background levels of PhoA activity. In addition, and unlike the DsbC variants that are susceptible to oxidation, mDsbC-mDsbC was unable to catalyze the in vivo folding of vtPA in a dsbA background.
We also determined the in vivo redox state of the covalently linked DsbC. Briefly, cells were grown in LB media, proteins were precipitated with trichloroacetic acid to prevent thiol:disulfide rearrangements, and the trichloroacetic acid pellets were treated with 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt (AMS). AMS reacts with free thiols increasing the molecular mass by 490 Da per thiol. When purified DsbC is pretreated with DTT, the thiols of the four disulfide-forming cysteines in each subunit react with AMS increasing the mass by ∼2 kDa per monomer. In vivo, however, the thiolates involved in the structural disulfide of DsbC are not accessible, and the protein is therefore found in a partially reduced state in which only the catalytic cysteine thiols have been modified by AMS increasing the mass by ∼1 kDa per monomer (9, 34). Comparison of the electrophoretic mobility of the in vitro generated standards with the in vivo samples of DsbC and mDsbC-mDsbC revealed that both proteins are found exclusively in the reduced state (supplemental Fig. S4). Collectively, these results demonstrate that the covalent linkage of two DsbC subunits via the 15-mer flexible linker has no effect on the structure of the enzyme and its ability to resist oxidation by DsbB (Fig. 2B).
Effect of Alanine Substitutions in One Catalytic Active Site
In earlier studies, Sun and Wang (44) reported that an in vitro prepared heterodimer of DsbC consisting of a wt subunit and a carboxymethylated subunit, is devoid of isomerase activity suggesting that both active sites in the DsbC homodimer are required for the catalysis of disulfide bond isomerization (1, 15, 44). We employed covalently linked DsbC fusions to examine this hypothesis. The Cys residues in one of the two active sites of mDsbC-mDsbC were substituted by Ala to give rise to mDsbC-mDsbC[C98A], mDsbC-mDsbC[C98A/C101A] and mDsbC-mDsbC[C101A] (Fig. 1A). All the mDsbC-mDsbC mutants eluted as single peaks with a retention time corresponding to an apparent molecular weight of 39 kDa that was identical to those of the wild type DsbC (Fig. 1B) and the catalytic site mutants.3 Substitution of one of the two nucleophilic active site Cys in the linked DsbC monomer (mDsbC-mDsbC[C98A]) resulted in only a modest reduction (∼19%) in the rate of refolding of reduced RNase A from 64 ± 8 × 10−3 to 52 ± 8 × 10−3 μm/min/μm enzyme. However, a more significant decrease (61%) was observed in the insulin reduction activity (Table 1). Mutation of both the Cys98 and Cys101 residues of one of the two active sites in mDsbC-mDsbC resulted in a slight further reduction in the isomerase activity relative to mDsbC-mDsbC[C98A] but had no additional effect on the disulfide reductase rate. For comparison, we also purified and characterized DsbC[C98A] and DsbC[C98A/C101A]. As expected, the substitution of Cys98 or both Cys in the DsbC homodimer completely abolished the isomerase and the insulin reductase activities (Ref. 45 and Table 1).
TABLE 1.
In vitro activities of purified enzymes
| Enzyme | RNAse refoldinga |
Insulin reduction |
||
|---|---|---|---|---|
| μm/min/μm Enzyme | % Activity | ×10−3 ΔA650/min2 | % Activity | |
| DsbC | 0.076 ± 0.007 | 100 | 11.1 ± 4.8 | 100 |
| mDsbC-mDsbC | 0.064 ± 0.008 | 84 | 8.63 ± 3.0 | 78 |
| DsbC[C98A] | 0.004 ± 0.001 | 5 | 0.04 ± 0.01 | 0 |
| mDsbC-mDsbC[C98A] | 0.052 ± 0.008 | 68 | 3.37 ± 1.0 | 30 |
| DsbC[C98A/C101A] | 0.001 ± 0.001 | 1 | 0.04 ± 0.01 | 0 |
| mDsbC-mDsbC[C98A/C101A] | 0.038 ± 0.005 | 50 | 3.19 ± 0.12 | 29 |
| DsbC[C101A] | 0.081 ± 0.009 | 107 | 12.88 ± 2.6 | 116 |
| mDsbC-mDsbC[C101A] | 0.079 ± 0.005 | 104 | 8.57 ± 2.0 | 77 |
| mDsbC-dim | 0.038 ± 0.005 | 50 | 1.95 ± 0.61 | 18 |
| mDsbC[H45D]-dim[D53H] | 0.024 ± 0.005 | 32 | 1.67 ± 0.13 | 15 |
| mDsbC[H45D]-dim[H45D] | 0.009 ± 0.002 | 12 | Ndb | Nd |
a The activities were determined from a plot of isomerization velocity against enzyme concentrations.
b Nd, not determined.
Consistent with the observation that a linked DsbC fusion having only one nucleophilic cysteine exhibits disulfide isomerase activity in vitro, the co-expression of mDsbC-mDsbC[C98A] or mDsbC-mDsbC[C98A/C101A] resulted in a vtPA yield that was 80% of the value obtained by expressing wt DsbC or mDsbC-mDsbC (Fig. 2A). In contrast, neither DsbC[C98A] nor DsbC[C98A/C101A] was able to catalyze the formation of active vtPA. Substitution of the resolving cysteine C101A alone decreased disulfide isomerization in vivo to 20% but had no effect on either isomerase or reductase activity in vitro (Fig. 2A and Table 1). None of the active site mutations in mDsbC-mDsbC compromised its ability to resist oxidation by DsbB, as evidenced by the finding that the proteins are present solely in the reduced state in vivo and also by their inability to catalyze the formation of active PhoA in a dsbA strain background (supplemental Fig. S4 and Fig. 2B).
A Linked DsbC with a Single Active Thioredoxin Domain
The finding that the mutational inactivation of one of the two catalytic sites in DsbC results only in a mild reduction in the disulfide isomerase activity, suggests that enzymes in which one of the two thioredoxin subdomains had been deleted in its entirety might be similarly active. To test this hypothesis, we fused a full-length DsbC monomer to a DsbC N-terminal dimerization domain (amino acids 1–64) using the (Gly3-Ser)3-Gly-Ser-Ala linker described above. The resulting molecule was designated mDsbC-dim and is shown schematically in Fig. 3A. mDsbC-dim expressed at a level comparable to DsbC (Fig. S1). Size exclusion FPLC on a SuperdexTM 200 column revealed that the purified protein elutes as several peaks (Fig. 3B). One peak with an apparent molecular mass of 22 kDa corresponded to the mass of a monomeric species and accounted for ∼60% of the A280. Additionally, a second peak that eluted as a 58-kDa protein was observed, as was a third peak corresponding to an even higher mass species. The 22- and 58-kDa species were isolated. Upon further incubation, the 58-kDa species gave rise to an elution profile similar to that of the starting material, whereas the 22-kDa species continued to elute as a single peak even after incubation at 4 °C for >1 week (supplemental Fig. S2). This suggests that the purified protein exists as a mixture of dimers and high molecular weight multimers that convert slowly to the monomeric form under the conditions tested. The oligomeric state of mDsbC-dim was further confirmed by multiangle light scattering experiments (Fig. 3C) in which three species with molar masses of ∼32.5, ∼75.4, and ∼127 kDa were identified, indicating the presence of monomers, dimers, and tetramers respectively. Dynamic light scattering showed a hydrodynamic radius (rH) increase from 2.8 nm for the monomer to 3.8 nm for the dimer, consistent with the radius increase of a sphere as the volume is doubled. The value of rH for the tetramer was 4.4 nm, which would be expected for a sphere of rH = 2.8 increasing 4-fold in volume, suggesting that the tetramer is compact and not a linear polymer of monomers. Monomeric molar masses were determined from the right half of the elution peak, thus avoiding the effect of larger complexes (46).
It has been established that in scFv antibodies, which comprise VH and VL immunoglobulin domains joined via a (Gly4-Ser)n repeat linker, the linker length plays an important role in dictating the monomer:multimer equilibrium. Linkers with more than 12 residues have been reported to strongly favor the monomeric state (47–49). To examine the role of the linker length on dimerization we constructed mDsbC-dim proteins having longer (Gly3-Ser) repeat linkers corresponding to 17, 19, or 30 amino acids. However, the respective proteins also eluted as a mixture of monomers and high molecular weight species, although the fusion with the 30-amino acid linker displayed a higher monomer to multimer ratio (supplemental Fig. S3).
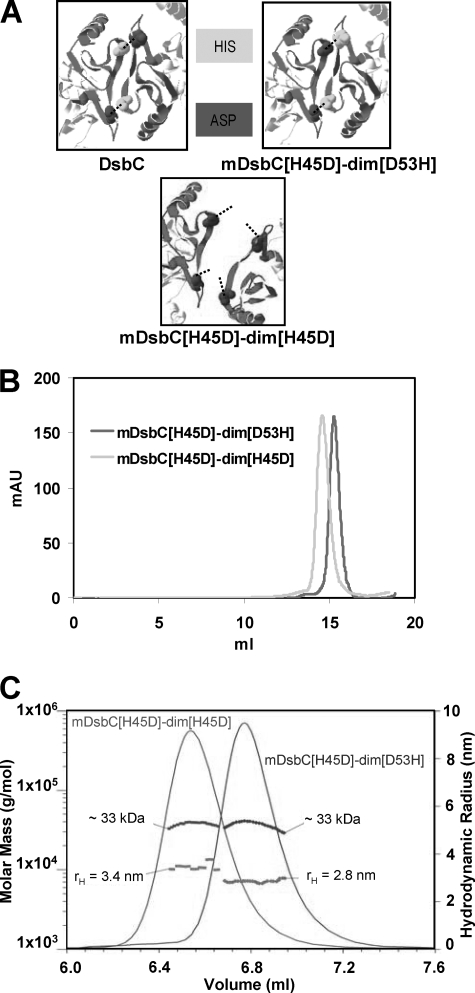
Earlier, Bader et al. (11) reported that a H45D substitution disrupts dimerization due to the interruption of a salt bridge that forms between His45 and Asp53. The monomeric DsbC[H45D] is susceptible to oxidation by DsbB and capable of complementing dsbA null mutants in vivo. We hypothesized that the His45Asp substitution in mDsbC-dim might prevent intermolecular dimerization. However, this could also disrupt the intramolecular association of the N-terminal regions and the formation of the peptide binding cleft. We reasoned that to prevent the oligomerization of mDsbC-dim it was necessary to weaken, but not completely disrupt, the interaction of the N-terminal regions; because the two N-terminal halves are covalently joined by the linker, their high effective concentration should overcome the loss of binding energy due to such mutations. To test this hypothesis we constructed and characterized a number of variants containing amino acid substitutions at the DsbC dimerization interface.
One of these variants, mDsbC[H45D]-dim[D53H], was constructed by fusing DsbC[H45D] to DsbC[Δ65–216, D53H] using (Gly3-Ser)7-Ser-Ala (Fig. 4). For comparison, we also constructed the mDsbC[H45D]-dim[H45D] variant, in which both histidines that normally form the His45-Asp53 salt bridges have been replaced with Asp; electrostatic repulsion of Asp45 and Asp53 is expected to disrupt the association of the two half-dimerization domains increasing its hydrodynamic radius (Fig. 4A). Consistent with this hypothesis we found that, even though the predicted molecular weights of mDsbC[H45D]-dim[D53H] and mDsbC[H45D]-dim[H45D] are very similar (32.89 and 32.85 kDa, respectively), the two proteins displayed distinctly different retention by gel filtration FPLC, eluting as single peaks (Fig. 4B). Further analysis by multiangle light scattering (Fig. 4C) revealed that the weight-average molar mass of mDsbC[H45D]-dim[D53H] was 33.0 (± 4%) kDa and that the protein appeared to be about 98% monomeric. mDsbC[H45D]-dim[H45D] was also found to be 99% monomeric with a weight-average molar mass of 33.5(± 5%) kDa. In agreement with the different size-exclusion elution patterns, dynamic light scattering determined that these two proteins have slightly different hydrodynamic radii, with rH = 2.8 nm for mDsbC[H45D]-dim[D53H] and 3.4 nm for mDsbC[H45D]-dim[H45D]. This discrepancy reflects a somewhat distinct shape consistent with the hypothesis that in mDsbC[H45D]-dim[H45D] the dimerization domains have failed to associate, while in mDsbC[H45D]-dim[D53H] the dim[53] domain packs against the N terminus of mDsbC[H45D] and thus gives rise to a molecule having the expected DsbC-like conformation. mDsbC[H45D]-dim[H45D] catalyzed RNase refolding only at a near background level. In contrast, the DsbC variant consisting of a thioredoxin domain and an intact putative binding domain (mDsbC[H45D]-dim[D53H]) displayed 32% of the activity of wild type DsbC. In comparison with mDsbC-mDsbC[C98A/C101A], which also has only one active catalytic site, mDsbC[H45D]-dim[D53H] displayed 63% of the activity of the former, indicating that the presence of the second thioredoxin domain makes a contribution to the catalytic activity, perhaps by mediating weak interactions with substrate proteins (Table 1). Expression of the single thioredoxin domain variant mDsbC[H45D]-dim[D53H] assisted the in vivo folding of active vtPA in SF100 degP dsbC cells to 75% of the level observed with DsbC (Fig. 5A). Given the absence of one thioredoxin domain, it was not surprising to find that unlike DsbC, mDsbC[H45D]-dim[D53H] was able to mediate protein oxidation and catalyzed the correct folding of vtPA in a dsbA background at a 40% level (Fig. 5A), suggesting that in vivo this enzyme exhibits dual oxidase and isomerase activity. Disulfide bond formation in vivo was further confirmed by the partial restoration of PhoA activity; this activity was dependent upon the presence of DsbB (Fig. 5B). Consistent with its dual oxidase and isomerase activities, mDsbC[H45D]-dim[D53H] was found to exist as a mixture of reduced and oxidized species at a ∼75:25 ratio in the periplasm of E. coli (supplemental Fig. S4).
FIGURE 4.
A, crystal structure of DsbC at the dimerization interface is shown. Residues His45 and Asp53 interact to establish salt bridges. In mDsbC[H45D]-dim[D53H], His45 is replaced by Asp in the first dimerization domain and Asp53 is replaced by His in the second dimerization domain to disrupt interdimerization but promote intradimerization. In mDsbC[H45D]-dim[H45D], residue His45 in each dimerization domain is replaced by Asp to fully disrupt dimerization. B, gel filtration FPLC of mDsbC[H45D]-dim[D53H] and mDsbC[H45D]-dim[H45D]. Purified proteins were run on a SuperdexTM 200 column in PBS-10% glycerol. C, MALS measurement of the molar masses of mDsbC[H45D]-dim[D53H] and mDsbC[H45D]-dim[H45D]. The relative concentrations are shown as determined by the refractive index differences. Both mutants have similar molar masses but elute at different volumes in the order predicted by their hydrodynamic radii.
FIGURE 5.
In vivo activity of single catalytic-domain mutants. A, yield of active vtPA in SF100 ΔdegP ΔdsbC (gray bars) or SF100 ΔdegP dsbA::kan (black bars) cells was determined as described under “Experimental Procedures.” B, PhoA activity in E. coli MC1000 dsbA::kan (white bars) and MC1000 dsbB::kan (black bars). The alkaline phosphatase activity in the parental isogenic strain MC1000 is shown by the gray bar.
DISCUSSION
Here we report the construction and characterization of covalently linked derivatives of DsbC joined via (Gly3-Ser) repeat flexible linkers. Linking the two monomers of DsbC via a sufficiently long linker resulted in an enzyme that displayed essentially identical catalytic and biochemical properties to the authentic dimeric DsbC. We showed that linked enzymes in which one or both of the active site Cys had been replaced with Ala exhibited significant isomerase activity. Similar to DsbC, the mDsbC-mDsbC[C98A], mDsbC-mDsbC[C98A/C101A] and mDsbC-mDsbC[C101A] variants were completely reduced in vivo and did not support the oxidation of PhoA in a dsbA background. In contrast, and consistent with earlier findings, substitution of the two N-terminal Cys98, or of both Cys98 and Cys101 in DsbC, completely eliminated isomerase activity; whereas replacing the resolving cysteine, Cys101, on both active sites affected this activity only partially (Refs. 9, 26, 45; Fig. 2 and Table 1). Earlier, Sun and Wang (44) sought to characterize the enzymatic properties of chimeric DsbC formed by mixing active and carboxymethylated monomers. Analysis of the in vitro reconstituted DsbC hybrid mixture led to speculation that both active sites are required for isomerization (1, 44). Our findings now reveal that the two catalytic sites of DsbC catalyze disulfide bond isomerization in RNase A independently of each other. Thus, mDsbC-mDsbC[C98A] and mDsbC-mDsbC[C98A/C101A] display half the activity of wt DsbC or of (mDsbC-mDsbC) simply because at the same molar concentration the latter enzyme contains two active sites. These results are consistent with the observation that substituting the N-terminal cysteine or both cysteines in either one of the two PDI catalytic sites leads to partial isomerase activity at varying degrees (21, 23, 50, 51).
The high level of in vitro isomerase activity displayed by mutants in which the resolving cysteine Cys101 had been replaced with Ala (DsbC[C101A] and mDsbC-mDsbC[C101A]) indicates that mixed disulfides with RNase A can be resolved efficiently via the attack of another cysteine in the substrate. Alternatively, isomerization may involve the formation of mixed disulfides with glutathione, which is present in the assay buffer. In contrast, the resolving cysteine, Cys101, has to participate in disulfide reduction, and therefore the finding that DsbC[C101A] and mDsbC-mDsbC[C101A] display high insulin reduction activity was unexpected. However these data are consistent with earlier findings that a DsbC variant with C101A and also C163A, the cysteine that normally forms the intramolecular bond of DsbC, exhibits 30% activity in this assay (45). It should be noted that the insulin reduction assay is performed in a buffer that contains excess DTT, which likely can substitute for Cys101 in the resolution of mixed disulfides between the enzyme and the substrate.
By engineering the dimer interface of the linked DsbC we constructed a variant, mDsbC[H45D]-dim[D53H] consisting of a single thioredoxin domain linked to an intact dimerization domain. This protein displayed isomerase activity with RNase A, and in the E. coli periplasm where it enhanced the folding of vtPA. The absence of a second catalytic domain also allowed this protein to catalyze disulfide bond formation, supporting the oxidation of PhoA and the formation of active vtPA in a dsbA strain background. Consistent with the in vivo activity of mDsbC[H45D]-dim[D53H], we showed that the protein is maintained in a predominantly reduced state.
The cleft forming upon interaction of the N-terminal dimerization domains of DsbC monomers, lined with uncharged and hydrophobic residues, is considered to be involved in the noncovalent binding of substrate proteins (13, 44). Previous studies reporting that the DsbC dimerization domain folds into its native conformation independent of the presence of the catalytic domain (52), together with our findings that disrupting the His-Asp interactions in mDsbC[H45D]-dim[H45D] results in an extended polypeptide, support the premise that mDsbC[H45D]-dim[D53H] in fact folds into a DsbC-like overall conformation, most likely preserving the substrate binding region. The level of in vitro isomerase activity observed with mDsbC[H45D]-dim[D53H] correlates well to that of the single-active-site mutant and is substantially higher relative to the background observed with monomeric DsbC, the active site mutant DsbC[C98A], or mDsbC[H45D]-dim[H45D], in which the N-terminal dimerization regions likely fail to interact (8, 5, and 12% respectively) ((25); Table 1).3 These data are in agreement with the published studies on PDI reporting that the minimal structure for simple disulfide isomerization includes one catalytic domain (a or a′) in combination with the non-catalytic b′ domain, the latter identified as the primary substrate binding site (22, 24).
The emergence of DsbB-dependent oxidase activity in mDsbC[H45D]-dim[D53H] which is structurally equivalent to DsbC lacking one thioredoxin domain, support the steric incompatibility model proposed by Inaba et al. (53) wherein interaction of DsbC with DsbB would result in its second thioredoxin domain clashing onto the membrane (53), and the experimental evidence showing that monomeric DsbC and α-helix deletion mutants of DsbC with re-oriented catalytic domains are able to catalyze DsbB-dependent oxidation (11, 12).
In summary, through the engineering of heterodimer-like mutants of DsbC we have demonstrated that only a single catalytic site or domain is essential for catalysis of isomerization in bacteria. In addition, we propose that the determining factor that converts the association of thioredoxin-fold-containing monomers from a mere pair of oxidoreductases in close proximity into an efficient isomerase is the emergence of a substrate binding region, only present when both N-terminal domains interact. We have also provided further experimental support to the notion that the kinetic barrier separating both pathways in bacteria derives from steric complications that dissipate with structural modifications. These findings contribute to understanding the role that dimerization plays in the catalysis of isomerization, highlight the indispensable features of DsbC, and expand the basis for the engineering of DsbC into a more PDI-like protein capable of catalyzing the oxidative folding of heterologous proteins in the bacterial periplasm in a highly efficient manner.
Supplementary Material
Acknowledgments
We thank Dr. Laura Segatori for helpful discussions related to this work and Claire K. Riggs for assistance in the light scattering experiments. The light scattering instruments were obtained with funding from National Science Foundation Grant MCB-0237651 (to A. F. R.).
This work was supported, in whole or in part, by National Institutes of Health Grant GM55090.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S4.
G. Georgiou, unpublished data.
- tPA
- tissue plasminogen activator
- PDI
- protein-disulfide isomerase
- RNAse A
- ribonuclease A
- TrxA
- thioredoxin
- vtPA
- truncated version of tPA
- AMS
- 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt
- DTT
- dithiothreitol
- MALS
- multi-angle laser light scattering
- PBS
- phosphate-buffered saline
- wt
- wild type
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Gleiter S., Bardwell J. C. (2008) Biochim. Biophys. Acta 1783, 530–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito K., Inaba K. (2008) Curr. Opin. Struct. Biol. 18, 450–458 [DOI] [PubMed] [Google Scholar]
- 3.Kadokura H., Katzen F., Beckwith J. (2003) Annu. Rev. Biochem. 72, 111–135 [DOI] [PubMed] [Google Scholar]
- 4.Zapun A., Bardwell J. C., Creighton T. E. (1993) Biochemistry 32, 5083–5092 [DOI] [PubMed] [Google Scholar]
- 5.Grauschopf U., Fritz A., Glockshuber R. (2003) EMBO J. 22, 3503–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilhot C., Jander G., Martin N. L., Beckwith J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 9895–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishigami S., Kanaya E., Kikuchi M., Ito K. (1995) J. Biol. Chem. 270, 17072–17074 [DOI] [PubMed] [Google Scholar]
- 8.Rietsch A., Belin D., Martin N., Beckwith J. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 13048–13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rietsch A., Bessette P., Georgiou G., Beckwith J. (1997) J. Bacteriol. 179, 6602–6608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozhkova A., Stirnimann C. U., Frei P., Grauschopf U., Brunisholz R., Grütter M. G., Capitani G., Glockshuber R. (2004) EMBO J. 23, 1709–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bader M. W., Hiniker A., Regeimbal J., Goldstone D., Haebel P. W., Riemer J., Metcalf P., Bardwell J. C. (2001) EMBO J. 20, 1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segatori L., Murphy L., Arredondo S., Kadokura H., Gilbert H., Beckwith J., Georgiou G. (2006) J. Biol. Chem. 281, 4911–4919 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy A. A., Haebel P. W., Törrönen A., Rybin V., Baker E. N., Metcalf P. (2000) Nat. Struct. Biol. 7, 196–199 [DOI] [PubMed] [Google Scholar]
- 14.Collet J. F., Bardwell J. C. (2002) Mol. Microbiol. 44, 1–8 [DOI] [PubMed] [Google Scholar]
- 15.Ritz D., Beckwith J. (2001) Annu. Rev. Microbiol. 55, 21–48 [DOI] [PubMed] [Google Scholar]
- 16.Chen J., Song J. L., Zhang S., Wang Y., Cui D. F., Wang C. C. (1999) J. Biol. Chem. 274, 19601–19605 [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa Y., Yanagi H., Yura T. (2001) J. Biol. Chem. 276, 14393–14399 [DOI] [PubMed] [Google Scholar]
- 18.Qiu J., Swartz J. R., Georgiou G. (1998) Appl. Environ. Microbiol. 64, 4891–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Li Z. H., Wang F., Fang M., Yin C. C., Zhou Z. Y., Lin Q., Huang H. L. (2002) Protein Expr. Purif. 26, 218–228 [DOI] [PubMed] [Google Scholar]
- 20.Gruber C. W., Cemazar M., Heras B., Martin J. L., Craik D. J. (2006) Trends Biochem. Sci. 31, 455–464 [DOI] [PubMed] [Google Scholar]
- 21.Tian G., Xiang S., Noiva R., Lennarz W. J., Schindelin H. (2006) Cell 124, 61–73 [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson B., Gilbert H. F. (2004) Biochim. Biophys. Acta 1699, 35–44 [DOI] [PubMed] [Google Scholar]
- 23.Lyles M. M., Gilbert H. F. (1994) J. Biol. Chem. 269, 30946–30952 [PubMed] [Google Scholar]
- 24.Hatahet F., Ruddock L. W. (2007) Febs. J. 274, 5223–5234 [DOI] [PubMed] [Google Scholar]
- 25.Ke H., Zhang S., Li J., Howlett G. J., Wang C. C. (2006) Biochemistry 45, 15100–15110 [DOI] [PubMed] [Google Scholar]
- 26.Zapun A., Missiakas D., Raina S., Creighton T. E. (1995) Biochemistry 34, 5075–5089 [DOI] [PubMed] [Google Scholar]
- 27.Jonda S., Huber-Wunderlich M., Glockshuber R., Mössner E. (1999) EMBO J. 18, 3271–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundström J., Krause G., Holmgren A. (1992) J. Biol. Chem. 267, 9047–9052 [PubMed] [Google Scholar]
- 29.Segatori L., Paukstelis P. J., Gilbert H. F., Georgiou G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10018–10023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arredondo S., Segatori L., Gilbert H. F., Georgiou G. (2008) J. Biol. Chem. 283, 31469–31476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessette P. H., Aslund F., Beckwith J., Georgiou G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 13703–13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishigami S., Akiyama Y., Ito K. (1995) FEBS Lett. 364, 55–58 [DOI] [PubMed] [Google Scholar]
- 34.Joly J. C., Swartz J. R. (1997) Biochemistry 36, 10067–10072 [DOI] [PubMed] [Google Scholar]
- 35.Holmgren A. (1979) J. Biol. Chem. 254, 9627–9632 [PubMed] [Google Scholar]
- 36.Lyles M. M., Gilbert H. F. (1991) Biochemistry 30, 613–619 [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Galisteo E., Padilla C. A., Garcia-Alfonso C., Lopez-Barea J., Barcena J. A. (1993) Biochimie (Paris) 75, 803–809 [DOI] [PubMed] [Google Scholar]
- 38.Callaway K. A., Rainey M. A., Riggs A. F., Abramczyk O., Dalby K. N. (2006) Biochemistry 45, 13719–13733 [DOI] [PubMed] [Google Scholar]
- 39.Bizub D., Weber I. T., Cameron C. E., Leis J. P., Skalka A. M. (1991) J. Biol. Chem. 266, 4951–4958 [PubMed] [Google Scholar]
- 40.Liang H., Sandberg W. S., Terwilliger T. C. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 7010–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Predki P. F., Regan L. (1995) Biochemistry 34, 9834–9839 [DOI] [PubMed] [Google Scholar]
- 42.Robinson C. R., Sauer R. T. (1996) Biochemistry 35, 109–116 [DOI] [PubMed] [Google Scholar]
- 43.Kamitani S., Akiyama Y., Ito K. (1992) EMBO J. 11, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X. X., Wang C. C. (2000) J. Biol. Chem. 275, 22743–22749 [DOI] [PubMed] [Google Scholar]
- 45.Liu X., Wang C. C. (2001) J. Biol. Chem. 276, 1146–1151 [DOI] [PubMed] [Google Scholar]
- 46.Zhu H., Ownby D. W., Riggs C. K., Nolasco N. J., Stoops J. K., Riggs A. F. (1996) J. Biol. Chem. 271, 30007–30021 [DOI] [PubMed] [Google Scholar]
- 47.Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 5879–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kortt A. A., Malby R. L., Caldwell J. B., Gruen L. C., Ivancic N., Lawrence M. C., Howlett G. J., Webster R. G., Hudson P. J., Colman P. M. (1994) Eur. J. Biochem. 221, 151–157 [DOI] [PubMed] [Google Scholar]
- 49.Todorovska A., Roovers R. C., Dolezal O., Kortt A. A., Hoogenboom H. R., Hudson P. J. (2001) J. Immunol. Methods 248, 47–66 [DOI] [PubMed] [Google Scholar]
- 50.Vuori K., Myllylä R., Pihlajaniemi T., Kivirikko K. I. (1992) J. Biol. Chem. 267, 7211–7214 [PubMed] [Google Scholar]
- 51.Walker K. W., Lyles M. M., Gilbert H. F. (1996) Biochemistry 35, 1972–1980 [DOI] [PubMed] [Google Scholar]
- 52.Yeh S. M., Koon N., Squire C., Metcalf P. (2007) Acta Crystallogr. D. Biol. Crystallogr. 63, 465–471 [DOI] [PubMed] [Google Scholar]
- 53.Inaba K., Murakami S., Suzuki M., Nakagawa A., Yamashita E., Okada K., Ito K. (2006) Cell 127, 789–801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.