Abstract
Lysophosphatidic acid (LPA), a bioactive phospholipid, induces a wide range of cellular effects, including gene expression, cytoskeletal rearrangement, and cell survival. We have previously shown that LPA stimulates secretion of pro- and anti-inflammatory cytokines in bronchial epithelial cells. This study provides evidence that LPA enhances pulmonary epithelial barrier integrity through protein kinase C (PKC) δ- and ζ-mediated E-cadherin accumulation at cell-cell junctions. Treatment of human bronchial epithelial cells (HBEpCs) with LPA increased transepithelial electrical resistance (TER) by ∼2.0-fold and enhanced accumulation of E-cadherin to the cell-cell junctions through Gαi-coupled LPA receptors. Knockdown of E-cadherin with E-cadherin small interfering RNA or pretreatment with EGTA (0.1 mm) prior to LPA (1 μm) treatment attenuated LPA-induced increases in TER in HBEpCs. Furthermore, LPA induced tyrosine phosphorylation of focal adhesion kinase (FAK) and overexpression of the FAK inhibitor, and FAK-related non-kinase-attenuated LPA induced increases in TER and E-cadherin accumulation at cell-cell junctions. Overexpression of dominant negative protein kinase δ and ζ attenuated LPA-induced phosphorylation of FAK, accumulation of E-cadherin at cell-cell junctions, and an increase in TER. Additionally, lipopolysaccharide decreased TER and induced E-cadherin relocalization from cell-cell junctions to cytoplasm in a dose-dependent fashion, which was restored by LPA post-treatment in HBEpCs. Intratracheal post-treatment with LPA (5 μm) reduced LPS-induced neutrophil influx, protein leak, and E-cadherin shedding in bronchoalveolar lavage fluids in a murine model of acute lung injury. These data suggest a protective role of LPA in airway inflammation and remodeling.
The airway epithelium is the site of first contact for inhaled environmental stimuli, functions as a physical barrier to environmental insult, and is an essential part of innate immunity. Epithelial barrier disruption is caused by inhaled allergens, dust, and irritants, resulting in inflammation, bronchoconstriction, and edema as seen in asthma and other respiratory diseases (1–4). Furthermore, increased epithelial permeability also results in para-cellular leakage of large proteins, such as albumin, immunoglobulin G, and polymeric immunoglobulin A, into the airway lumen (5, 6). The epithelial cell-cell junctional complex is composed of tight junctions, adherens junctions, and desmosomes. These adherens junctions play a pivotal role in regulating the activity of the entire junctional complex because the formation of adherens junctions subsequently leads to the formation of other cell-cell junctions (7–9). The major adhesion molecules in the adherens junctions are the cadherins. E-cadherin is a member of the cadherin family that mediates calcium-dependent cell-cell adhesion. The N-terminal ectodomain of E-cadherin contains homophilic interaction specificity, and the cytoplasmic domain binds to catenins, which interact with actin (10–13). Plasma membrane localization of E-cadherin is critical for the maintenance of epithelial cell-cell junctions and airway epithelium integrity (7, 10, 14). A decrease of adhesive properties of E-cadherin is related to the loss of differentiation and the subsequent acquisition of a higher motility and invasiveness of epithelial cells (10, 14, 15). Dislocation or shedding of E-cadherin in the airway epithelium induces epithelial shedding and increases airway permeability in lung airway diseases (10, 14, 16). In an ovalbumin-challenged guinea pig model of asthma, it has been demonstrated that E-cadherin is dislocated from the lateral margins of epithelial cells (10). Histamine increases airway para-cellular permeability and results in an increased susceptibility of airway epithelial cells to adenovirus infection by interrupting E-cadherin adhesion (14). Serine phosphorylation of E-cadherin by casein kinase II, GSK-3β, and PKD1/PKC2 μ enhanced E-cadherin-mediated cell-cell adhesion in NIH3T3 fibroblasts and LNCaP prostate cancer cells (11, 17). However, the regulation and mechanism by which E-cadherin is localized within the pulmonary epithelium is not fully known, particularly during airway remodeling.
LPA, a naturally occurring bioactive lipid, is present in body fluids, such as plasma, saliva, follicular fluid, malignant effusions, and bronchoalveolar lavage (BAL) fluids (18–20). Six distinct high affinity cell-surface LPA receptors, LPA-R1–6, have been cloned and described in mammals (21–26). Extracellular activities of LPA include cell proliferation, motility, and cell survival (27–30). LPA exhibits a wide range of effects on differing cell types, including pulmonary epithelial, smooth muscle, fibroblasts, and T cells (31–35). LPA augments migration and cytokine synthesis in lymphocytes and induces chemotaxis of Jurkat T cells through Matrigel membranes (34). LPA induces airway smooth muscle cell contractility, proliferation, and airway repair and remodeling (35, 36). LPA also potently stimulates IL-8 (31, 37–39), IL-13 receptor α2 (IL-13Rα2) (40), and COX-2 gene expression and prostaglandin E2 release (41) in HBEpCs. Prostaglandin E2 and IL-13Rα2 have anti-inflammatory properties in pulmonary inflammation (42, 43). These results suggest that LPA may play a protective role in lung disease by stimulating an innate immune response while simultaneously attenuating the adaptive immune response. Furthermore, intravenous injection with LPA attenuated bacterial endotoxin-induced plasma tumor necrosis factor-α production and myeloperoxidase activity in the lungs of mice (44), suggesting an anti-inflammatory role for LPA in a murine model of sepsis.
We have reported that LPA induces E-cadherin/c-Met accumulation in cell-cell contacts and increases TER in HBEpCs (45). Here, for the first time, we report that LPA-induced increases in TER are dependent on PKCδ, PKCζ, and FAK-mediated E-cadherin accumulation at cell-cell junctions. Furthermore, we demonstrate that post-treatment of LPA rescues LPS-induced airway epithelial disruption in vitro and reduces E-cadherin shedding in a murine model of ALI. This study identifies the molecular mechanisms linking the LPA and LPA receptors to maintaining normal pulmonary epithelium barrier function, which is critical in developing novel therapies directed at ameliorating pulmonary diseases.
EXPERIMENTAL PROCEDURES
Materials
1-Oleoyl (18:1)LPA, Ki16425, LPS, and antibody to HA tag were purchased from Sigma. Antibodies for PKCζ and PKCδ were procured from Cell Signaling Technology (Beverly, MA). c-Met antibody was from Upstate Biotechnology, Inc. (Lake Placid, NY). Myr-PKCζ peptide inhibitor was from Biomol (Plymouth Meeting, PA). Scrambled siRNA and LPA1–3 siRNA were from Dharmacon (Lafayette, CO). E-cadherin siRNA, TLR4 siRNA, TLR4 antibody, and E-cadherin antibody (k-20) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DECMA-1 antibody was from Abcam Inc. (Cambridge, MA). FAK antibody was from BD Biosciences. Phospho-FAK antibody was from Millipore Inc. (Billerica, MA). Transmembrane transfection reagent was from Qiagen (Chatsworth, CA). Horseradish peroxidase-conjugated rabbit anti-goat was purchased from Molecular Probes (Eugene, OR). ECL kit for detection of proteins by Western blotting was obtained from Amersham Biosciences. All other reagents were of analytical grade.
Epithelial Cell Culture
Primary small airway epithelial cells (HSAEpCs) were obtained from Lonza (Walkersville, MD). Primary bronchial epithelial cells (HBEpCs) were isolated from organ donors at the University of Chicago Medical Center. Briefly, trachea was opened longitudinally to expose the airway lumen and washed to remove mucus. Fine forceps, ophthalmic scissors, and a binocular dissecting microscope were used to carefully remove the epithelial layer. The epithelial tissue was digested with 1% Protease type IX in serum-free media at 37 °C for 2 h with mild shaking. The epithelial tissue was disrupted by trituration using a pipette. The cell suspension was collected and pelleted to remove the dissociation media. The cells were incubated in serum-free basal essential growth medium (supplied by Lonza, Walkersville, MD) supplemented with growth factors, at 37 °C in 5% CO2 and 95% air to ∼80% confluence, and subsequently propagated in 6-well collagen-coated dishes. All experiments were carried out between passages 1 and 3. MLE12 cells were purchased from ATCC, and cells were incubated in Dulbecco's modified Eagle's medium/F-12 medium with 10% fetal bovine serum and growth factors.
Phospholipid Treatments
LPA was stored as a solution in ethanol (5 mm) and was dried under N2 prior to resuspension in BEBM media containing 0.1% endotoxin-free bovine serum albumin. Uniform dispersion was ensured by vortexing.
Immunofluorescence Microscopy
HBEpCs grown on chamber slides to ∼90% confluence were treated with LPA, LPS, or agonist. Chamber slides were permeabilized for 2 min in PBS containing 0.1% Triton X-100 and 3.7% formaldehyde and fixed in 3.7% formaldehyde for 20 min. Changes in E-cadherin localization were examined with E-cadherin antibody, and images were examined with a Nikon ECLIPSE TE 300 inverted microscope. Pixel intensities in cell margins were measured with MetaVue software.
Measurement of TER by Electrical Cell-Substrate Impedance Sensing System (ECIS)
HBEpCs were grown to confluence on gold microelectrodes. TER was measured in an ECIS (Applied BioPhysics, Foster City, CA). The total electrical resistance measured dynamically across the epithelial monolayer was determined as the combined resistance between the basal surface of the cell and the electrode, reflecting alterations in cell-cell adhesion.
Transfections of Small Interfering RNA (siRNA)
HBEpCs (P1 or P2) were cultured onto 6-well plates. At 50–60% confluence, transient transfection of siRNA was carried out using Transmessenger transfection reagent (Qiagen, Chatsworth, CA). Briefly, siRNA (50–100 nm) was condensed with Enhancer R and formulated with Transmessenger reagent, according to the manufacturer's instruction. The transfection complex was diluted into 900 μl of BEBM medium and added directly to the cells. The medium was replaced with complete basal essential growth medium after 3 h. Cells were treated at 72 h after transfection.
Adenoviral Infection
Infection of HBEpCs (∼60% confluence) with adenoviral empty vector and adenoviral dominant negative PKCδ and -ζ was carried out in 6-well plates. After infection with 20 m.o.i. in 1 ml of basal essential growth medium for 24 h, experiments were performed.
Plasmid Transfection
HBEpCs grown on gold microelectrodes or 6-well plates (∼60–70% confluence) were transfected with HA-tagged FRNK plasmid (1 μg/ml) using FuGENE HD transfection reagent according to the manufacturer's protocol. Cells were analyzed after 48 h.
Western Blotting
HBEpCs were lysed with buffer containing 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 2 mm EGTA, 5 mm β-glycerophosphate, 1 mm MgCl2, 1% Triton X-100, 1 mm sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. Cell lysates were sonicated on ice for 10 s and centrifuged at 500 × g for 5 min at 4 °C. Equal amounts of protein (20 μg) or concentrated media (20 μl) were subjected to 10% SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, blocked with 5% (w/v) bovine serum albumin in TBST (25 mm Tris-HCl (pH 7.4), 137 mm NaCl, and 0.1% Tween 20) for 1 h, and incubated with primary antibodies in 5% (w/v) bovine serum albumin in TBST for 2 h at room temperature. The membranes were washed at least three times with TBST at 15-min intervals and then incubated with either mouse or rabbit or goat horseradish peroxidase-conjugated secondary antibody (1:3,000) for 1 h. The membranes were developed with enhanced chemiluminescence detection system according to the manufacturer's instructions.
Statistical Analyses
All results were subjected to statistical analysis using one-way analysis of variance and, whenever appropriate, analyzed by Student-Newman-Keuls test. Data were expressed as means ± S.D. of triplicate samples from at least three independent experiments, and the level of significance was taken to p < 0.05.
RESULTS
LPA Induces Increases in TER
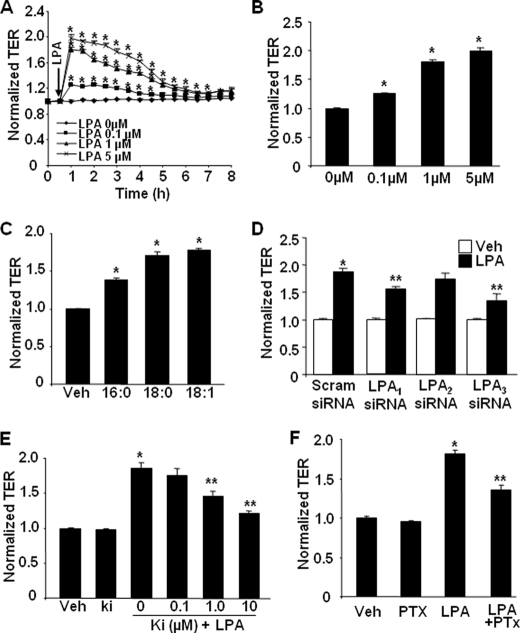
As pulmonary epithelium provides a physical barrier between underlying tissue and respiratory gases (1–4), we utilized ECIS to determine the effect of LPA on pulmonary epithelial barrier function. Exposure of HBEpCs to LPA (0.1–5.0 μm) rapidly induced increases in TER by ∼2.0-fold over the control value (Fig. 1, A and B), suggesting LPA-mediated enhancement of epithelial barrier integrity. LPA (1 μm) also induced the increases in TER in HSAEpCs and MLE12 cells (data not shown). These results show that LPA-mediated increases in TER are very similar, if not identical, in all three cell types, and further experiments with LPA were carried out with HBEpCs. To determine the effect of LPA with varying acyl chain lengths on epithelial barrier function, HBEpCs were challenged with 1 μm of 16:0, 18:0, and 18:1 LPA. All three LPAs induced increases in TER; the magnitude of effect on increasing TER was 18:1LPA ≥ 18:0LPA > 16:0LPA (Fig. 1C).
FIGURE 1.
LPA induces increases in TER in HBEpCs. A, HBEpCs grown on ECIS gold electrodes were challenged with LPA at different concentrations (0.1–5 μm), and changes in TER were measured with the ECIS. B shows changes in TER at 1 h of LPA treatment. *, p < 0.01, compared with vehicle control. C, HBEpCs grown on ECIS gold electrodes were challenged with 1 μg/ml 16:0LPA, 18:0LPA, and 18:1LPA, and changes in TER at 1 h were measured with the ECIS. *, p < 0.01, compared with vehicle (Veh) control. D, HBEpCs grown on ECIS gold electrodes were transfected with 100 nm scrambled (Scram) siRNA, LPA1 siRNA, LPA2 siRNA, or LPA3 siRNA for 72 h, and then cells were challenged with LPA (1 μm). The changes in TER at 1 h were measured with ECIS. *, p < 0.01, compared with scrambled siRNA; **, p < 0.01, compared with scrambled siRNA + LPA. E, HBEpCs grown on ECIS gold electrodes were pretreated with Ki16425 (Ki) (0.1–10 μm) for 1 h, and then cells were challenged with LPA (1 μm). The changes of TER at 1 h were measured with ECIS. *, p < 0.01, compared with LPA-treated cells; **, p < 0.01, compared with LPA treatment. F, HBEpCs grown on ECIS gold electrodes were pretreated with pertussis toxin (PTx) (100 ng/ml, 4 h) prior to LPA (1 μm) challenge, and changes in TER at 1 h were measured with ECIS. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPA treatment.
The expression of LPA receptors (LPA1–5) in HBEpCs was examined by real time RT-PCR. As shown in Table 1, the relative abundance of mRNA detected was LPA1 > LPA3 > LPA2 >LPA4, whereas LPA5 was not detectable in HBEpCs. We have shown that transfection of HBEpCs with LPA1–3 siRNA down-regulated expression of LPA1–3 and attenuated LPA-induced IL-8 secretion (37). Using a similar approach, HBEpCs were transfected with scrambled siRNA, LPA1-, LPA2-, or LPA3-specific siRNA and then challenged with LPA (1 μm). Fig. 1D shows that down-regulation of LPA1 and LPA3 by transfection of LPA1 and LPA3 siRNA-attenuated LPA (100 nm, 72 h) induced the increases in TER around 53 and 64%, respectively; however, down-regulation of LPA2 expression had no effect on LPA-induced increases in TER. Pretreatment with LPA1,3-specific antagonist, Ki16425 (0.1, 1.0, and 10 μm), attenuated LPA (1 μm) induced the increases in TER in a dose-dependent fashion (Fig. 1E). Furthermore, pretreatment with a specific Gαi inhibitor, pertussis toxin (100 ng/ml, 4 h) attenuated LPA, induced the increases in TER (Fig. 1F). These results suggest that LPA induces pulmonary epithelial barrier enhancement, in part, through LPA-R1,3 coupled to Gαi.
TABLE 1.
Expression of LPA receptors in HBEpCs
Total RNA was isolated from HBEpCs, and LPA receptor mRNAs were examined by real time RT-PCR. The data were normalized to 18 S.
| Human LPA receptors | LPA1 | LPA2 | LPA3 | LPA4 | LPA5 |
| Normalized to 18 S | 145.1 ± 47.7 | 14.3 ± 1.7 | 51.0 ± 14.0 | 1.2 ± 0.1 | 0.1 ± 0.7 |
LPA Induces E-cadherin Accumulation at Cell-Cell Junctions
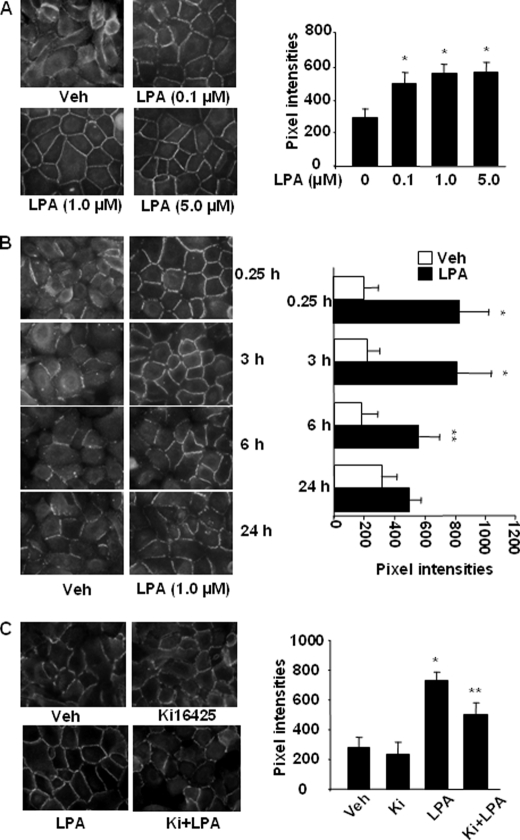
We previously demonstrated that LPA induced E-cadherin accumulation at cell-cell junctions in 1 h (45). To further determine the effect of LPA on E-cadherin redistribution, HBEpCs were challenged with LPA (0.1, 1.0, and 5.0 μm) for 15 min, and E-cadherin localization was determined with an antibody specificity to the extracellular domain of E-cadherin. As shown in Fig. 2A, LPA-induced E-cadherin accumulation at cell-cell junctions was dose-dependent. However, the effect of LPA was maintained up to 6 h and reduced at 24 h (Fig. 2B). Furthermore, LPA (1 μm, 15 min) induced E-cadherin accumulation at cell-cell junctions in both HSAEpCs and MLE12 (data not shown). Next we determined which LPA receptor isotype(s) was involved in LPA-induced E-cadherin accumulation at cell-cell junctions. HBEpCs were incubated with Ki16425 (5 μm) for 1 h and then challenged with LPA (1 μm, 15 min). As shown in Fig. 2C, Ki16425 partly attenuated LPA-induced E-cadherin accumulation at cell-cell junctions. These results suggest that LPA1 and LPA3 are involved in LPA-induced E-cadherin accumulation at cell-cell junctions in pulmonary epithelial cells.
FIGURE 2.
LPA induces E-cadherin accumulation at cell-cell junctions. A, HBEpCs grown on glass chamber slides were treated with LPA (0.1, 1.0, and 5.0 μm) for 15 min, and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody (k-20). The intensities of E-cadherin at cell-cell junctions were measured by MetaVue software. *, p < 0.01, compared with vehicle (Veh) control. B, HBEpCs grown on glass chamber slides were treated with LPA (1 μm) for 15 min and 3, 6, and 24 h, and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01; **, p < 0.05, compared with vehicle control. C, HBEpCs grown on glass chamber slides were treated with Ki16425 (5 μm) for 1 h prior to LPA challenge (1 μm, 15 min), and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPA treatment.
Role of E-cadherin in LPA-induced Increases in TER
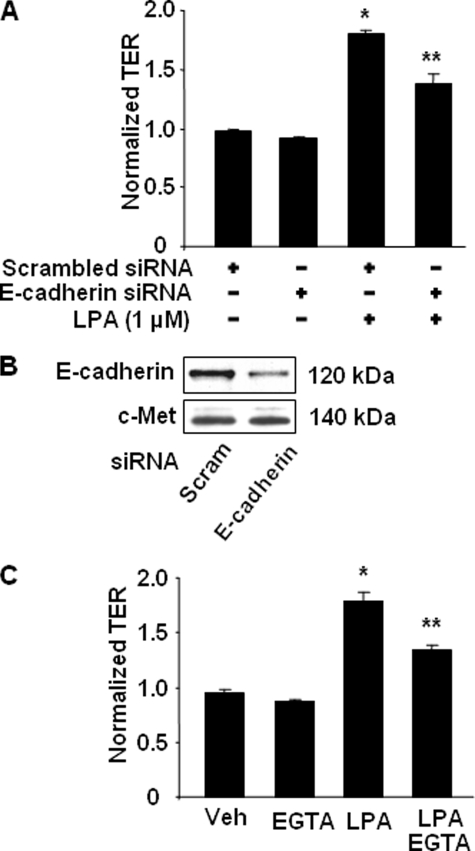
The mechanism(s) of LPA-mediated enhanced epithelial barrier integrity has yet to be fully defined. To determine whether LPA-induced enhancement of TER is dependent on E-cadherin accumulation to cell-cell junctions, HBEpCs were transfected with E-cadherin siRNA (100 nm, 72 h) prior to LPA treatment. As shown in Fig. 3A, E-cadherin siRNA attenuated LPA-induced increases in TER. The specificity of E-cadherin siRNA was confirmed by Western blotting with antibody to E-cadherin. E-cadherin siRNA transfection decreased E-cadherin expression, without altering c-Met expression (Fig. 3B).
FIGURE 3.
LPA induces the increases in TER via E-cadherin. A, HBEpCs grown on ECIS gold electrodes were transfected with scrambled (Scram) siRNA (100 nm) or E-cadherin siRNA (100 nm) for 72 h and then treated with LPA (1 μm). The changes in TER at 1 h were measured with ECIS. *, p < 0.01, compared with scrambled siRNA; **, p < 0.01, compared with scrambled siRNA + LPA. B, Western blotting confirmed E-cadherin expression after E-cadherin siRNA transfection with antibodies to E-cadherin and c-Met. C, HBEpCs grown on ECIS gold electrodes were pretreated with EGTA (0.1 mm) for 0.5 h prior to LPA (1 μm) challenge. Changes in TER at 1 h were measured with ECIS. *, p < 0.01, compared with vehicle (Veh) control; **, p < 0.01, compared with LPA treatment.
To further understand the mechanism of regulation of TER by LPA via E-cadherin, we adopted a well established technique to study E-cadherin-mediated cell-cell junctions by disruption of Ca2+-dependent E-cadherin-mediated adhesion with EGTA (46). HBEpCs were pretreated with EGTA (0.1 mm) for 30 min and then challenged with LPA (1 μm), and TER was measured by the ECIS system. As shown in Fig. 3C, LPA increased TER, and pretreatment with EGTA attenuated LPA-induced TER (from ∼1.7- to ∼1.3-fold). These data suggest that the LPA-induced increase in TER is via Ca2+-dependent E-cadherin-mediated cell-cell adhesion.
Role of PKC Isoforms in LPA-induced Airway Epithelial Barrier Function
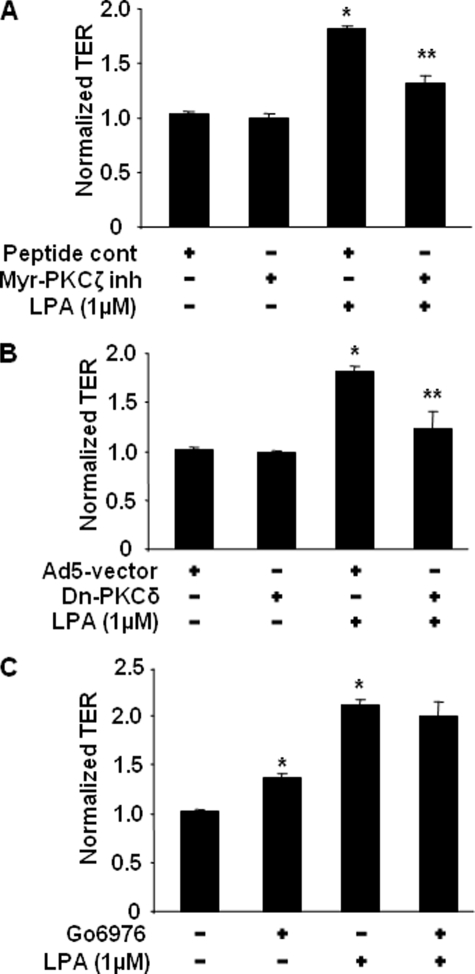
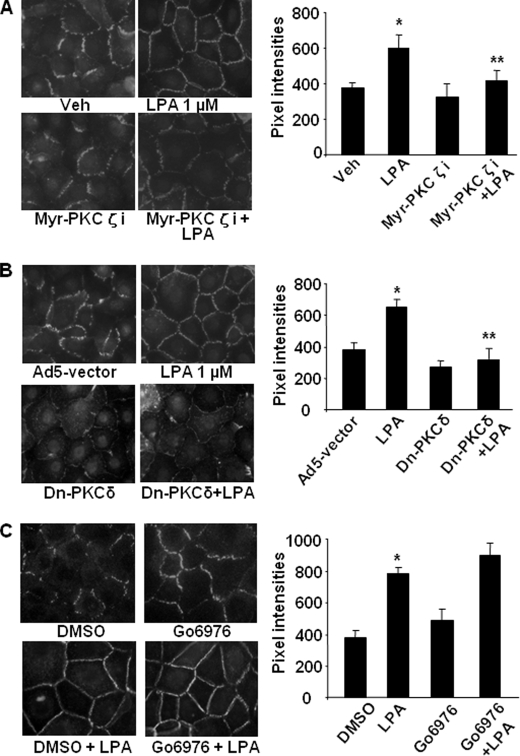
Human bronchial epithelial cells express PKC isoforms PKCα, PKCδ, PKCζ, and PKCλ (31). We have shown earlier that PKCδ regulated LPA-induced c-Met intracellular trafficking from cytoplasm to plasma membrane in HBEpCs (45). Here we examined the role of specific PKC isoforms in LPA-induced increases in TER and E-cadherin accumulation at cell-cell junctions in HBEpCs. Pretreatment with myristoylated (myr)-PKCζ peptide inhibitor (10 μm, 1 h) or overexpression of dn-PKCδ (20 m.o.i., 24 h) attenuated the LPA-induced increases in TER (Fig. 4, A and B) and E-cadherin accumulation at cell-cell junctions (Fig. 5, A and B). Western blotting with a specific antibody to PKCδ confirmed the overexpression of dn-PKCδ (data not shown). Pretreatment with the PKCα inhibitor Go6976 (5 μm, 1 h) increased TER and E-cadherin accumulation at cell-cell junctions, although it had no effect on LPA-induced increases in TER (Fig. 4C) and E-cadherin accumulation at cell-cell junctions (Fig. 5C). These results suggest that LPA-induced increases in TER and E-cadherin accumulation at cell-cell junctions are dependent on activation of PKCδ and PKCζ in HBEpCs.
FIGURE 4.
PKCδ and PKCζ regulate LPA-induced increases in TER. HBEpCs grown on ECIS gold electrodes were treated with myr-PKCζ peptide inhibitor (10 μm, 24 h) (A), or infected with adenovirus empty vector or adenoviral dn-PKCδ (20 m.o.i., 24 h) (B), or were treated with Go6976 (5 μm, 1 h) (C) prior to LPA (1 μm) challenge. The changes of TER at 1 h were measured with ECIS. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPA treatment.
FIGURE 5.
PKCδ and PKCζ regulate LPA-induced E-cadherin accumulation at cell-cell junctions. HBEpCs grown on glass chamber slides were treated with myr-PKCζ peptide inhibitor (10 μm, 1 h) (A), or infected with adenovirus empty vector or adenoviral dn-PKCδ (20 m.o.i., 24 h) (B), or treated with Go6976 (5 μm, 1 h) (C) prior to LPA (1 μm) challenge for 15 min. E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vehicle (Veh) control; **, p < 0.01, compared with LPA treatment.
Role of FAK in LPA-induced Barrier Function
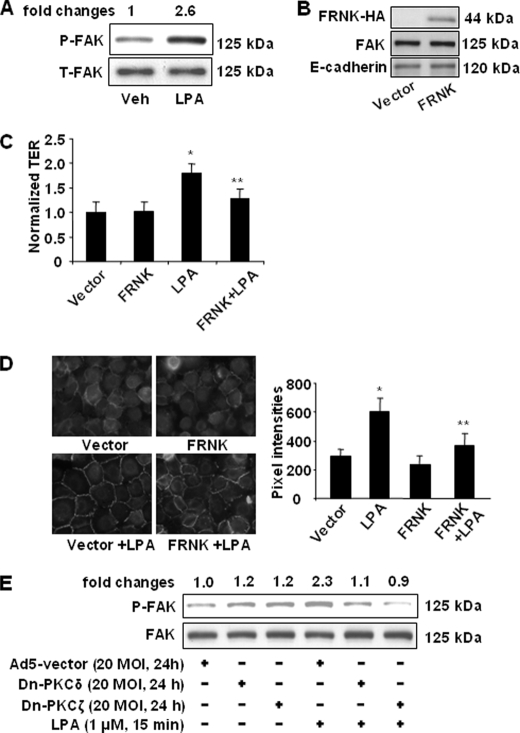
FAK has been implicated as an important regulator of cell integrity (47). Treatment of HBEpCs with LPA (1 μm) for 15 min induced phosphorylation of FAK (Fig. 6A). Overexpression of HA-tagged FAK inhibitor, termed FAK-related non-kinase (FRNK) (1 μg/ml, 48 h), attenuated LPA-induced increases in TER (Fig. 6C) and E-cadherin accumulation at cell-cell junctions (Fig. 6D). The overexpression of HA-tagged FRNK was confirmed by Western blotting with a specific antibody to HA tag, and overexpression of FRNK had on effect on expression of FAK and E-cadherin (Fig. 6B). To further determine the involvement of PKC isoforms in LPA-induced phosphorylation of FAK, HBEpCs were infected with adenovirus empty vector, dn-PKCδ, or dn-PKCζ (20 m.o.i., 24 h) and then challenged with LPA (1 μm) for 15 min. As shown in Fig. 6E, overexpression of dn-PKCδ or dn-PKCζ slightly increased phosphorylation of FAK and attenuated LPA-induced phosphorylation of FAK. These results suggest a role for FAK in LPA-induced epithelial barrier function and that activation of FAK is regulated by PKCδ and PKCζ.
FIGURE 6.
FAK regulates LPA-induced pulmonary epithelial barrier function. A, HBEpCs grown on 6-well plates were challenged with LPA (1 μm) for 15 min. Cell lysates were analyzed by Western blotting with antibodies to phospho(Tyr-397)-FAK (P-FAK) and FAK. Changes in FAK phosphorylation are expressed as fold changes and normalized to total FAK (T-FAK). Shown are representative blots from three independent experiments. Veh, vehicle. B, HBEpCs were transfected with empty vector or HA-tagged FRNK plasmid (1 μg/ml) for 48 h. Cell lysates were analyzed by Western blotting with antibodies to HA tag, FAK, or E-cadherin. Shown are representative blots for three independent experiments. C, HBEpCs were transfected with empty vector or HA-tagged FRNK plasmid for 48 h and then challenged with LPA (1 μm). Changes in TER at 1 h were measured with ECIS. *, p < 0.01, compared with vector control; **, p < 0.01, compared with LPA treatment. D, HBEpCs were transfected with empty vector and HA-tagged FRNK plasmid for 48 h and then challenged with LPA (1 μm) for 15 min, and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vector control; **, p < 0.01, compared with LPA treatment. E, HBEpCs were infected with dn-PKCδ and dn-PKCζ (20 m.o.i., 24 h) and then challenged with LPA (1 μm) for 15 min. Cell lysates were analyzed by Western blotting with antibodies to phospho(Tyr-397)-FAK and FAK. Changes in FAK phosphorylation are expressed as fold changes and normalized for total FAK (T-FAK). Shown are representative blots from three independent experiments.
LPS Induces Epithelial Barrier Disruption via TLR4/Myd88
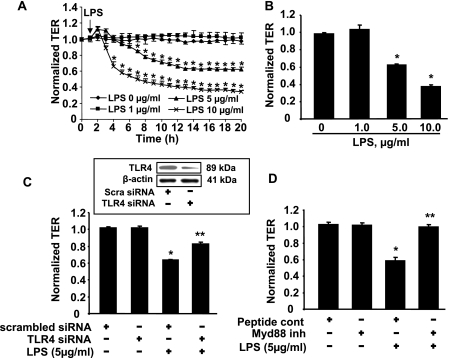
Intratracheally instilled LPS induces epithelial injury, inflammation, and barrier dysfunction in both of the bronchial and alveolar epithelium in a murine model of ALI (48–50). To investigate the effect of LPS in pulmonary epithelial barrier function, HBEpCs were challenged with LPS for 18 h. As shown in Fig. 7A, LPS (1 μg/ml) had no effect on TER, whereas LPS (5 and 10 μg/ml) induced decreases in TER in HBEpCs (Fig. 7, A and B). Furthermore, treatment of HSAEpCs and MLE12 with LPS (5–10 μg/ml) decreased TER (data not shown). These results suggest that LPS challenge causes pulmonary epithelial barrier disruption. To further determine whether LPS-mediated epithelial barrier disruption is mediated via the LPS receptor, Toll-like receptor 4 (TLR4), HBEpCs were transfected with TLR4 siRNA (50 nm, 48 h) prior to LPS challenge (5 μg/ml). As shown in Fig. 7C, TLR4 siRNA reversed LPS-induced decreases in TER. Western blotting with an antibody specific to TLR4 showed that TLR4 siRNA transfection down-regulated TLR4 protein levels by 65%. Furthermore, pretreatment with a peptide inhibitor of Myd88 (100 μm, 24 h), which is a downstream signaling protein of TLR4, completely blocked LPS-induced decreases in TER (Fig. 7C), suggesting that the effects of LPS on barrier disruption are dependent on TLR4 and Myd88 in pulmonary epithelial cells.
FIGURE 7.
LPS decreases TER in pulmonary epithelial cells. A, HBEpCs grown on ECIS gold electrodes were challenged with LPS (1, 5, and 10 μg/ml), and changes in TER were measured with ECIS. *, p < 0.01, compared with untreated cells. B shows changes in TER at 18 h of LPS treatment. *, p < 0.01, compared with vehicle control. C, HBEpCs grown on ECIS gold electrodes were transfected with scrambled siRNA (50 nm) or TLR4 siRNA (50 nm) for 48 h, and then cells were challenged with LPS (10 μg/ml). Changes in TER at 18 h were measured by ECIS. *, p < 0.01, compared with scrambled siRNA; **, p < 0.01, compared with scrambled siRNA + LPS. Inset shows TLR4 expression by Western blotting with an antibody specific to TLR4. D, HBEpCs grown on ECIS gold electrodes were treated with Myd88 peptide inhibitor (100 μm, 24 h) prior to LPS (5 μg/ml) challenge. The changes in TER at 18 h were measured with ECIS. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPS treatment.
LPS Reduces E-cadherin Distribution at Cell-Cell Junctions
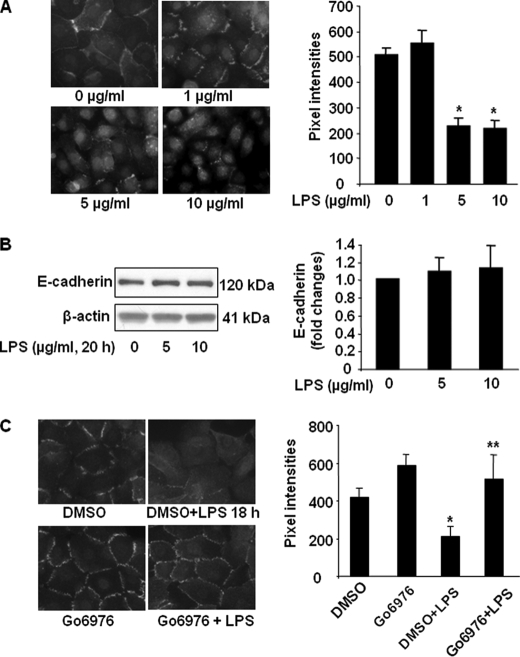
As loss of E-cadherin-mediated adherence junctions is involved in epithelial barrier dysfunction (10, 14), we next investigated the effect of LPS on pulmonary epithelial barrier function. HBEpCs were challenged with LPS (1, 5, and 10 μg/ml) for 18 h, and E-cadherin localization was determined by immunocytochemistry with antibody specificity to the extracellular domain of E-cadherin. In vehicle and 1 μg/ml LPS-treated cells, E-cadherin was primarily localized in plasma membrane; however, 5 and 10 μg/ml LPS treatment reduced the E-cadherin immunostaining in cell-cell junctions and increased E-cadherin immunostaining in the cytoplasm (Fig. 8A). LPS treatment (5 and 10 μm) for 18 h did not change the total E-cadherin protein levels (Fig. 8B). These results suggest that LPS induces E-cadherin intracellular trafficking from plasma membrane to cytoplasm without altering E-cadherin protein expression. Similar to HBEpCs, challenge of MLE12 with LPS (5 μg/ml, 18 h) induced less E-cadherin localization in cell-cell junctions (data not shown), which is consistent with LPS-induced pulmonary epithelial barrier disruption. Furthermore, pretreatment with the PKCα inhibitor Go6976 (5 μm, 1 h) partly prevented LPS-mediated reduction of E-cadherin staining at cell-cell junctions (Fig. 8C). These results suggest that LPS induces epithelial barrier dysfunction through PKCα-mediated E-cadherin redistribution from cell-cell junctions to cytoplasm.
FIGURE 8.
LPS decreases E-cadherin localization at cell-cell contacts. A, HBEpCs grown on glass chamber slides were challenged with LPS (1, 5, and 10 μg/ml) for 18 h, and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vehicle control. B, HBEpCs were challenged with LPS (5 and 10 μg/ml) for 18 h, and cell lysates were analyzed by Western blotting with specific antibodies to E-cadherin and β-actin. Shown are representative blots from three independent experiments. C, HBEpCs grown on glass chamber slides were treated with Go6976 (5 μm, 1 h) prior to LPS (5 μg/ml, 18 h) challenge. E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPS treatment.
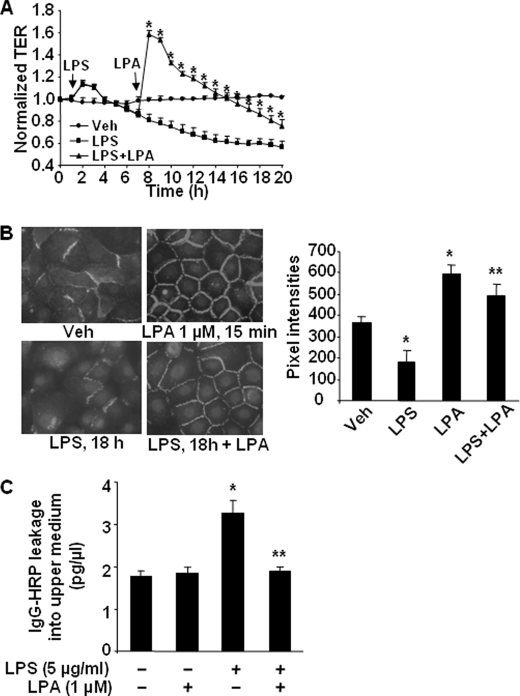
LPA Post-treatment Restores LPS-induced Epithelial Barrier Disruption
To investigate whether LPS-induced barrier disruption will be rescued by LPA, HBEpCs were challenged with LPS for 6 h and then treated with LPA (1 μm), and TER was measured by the ECIS system. As shown in Fig. 9A, LPS (5 μg/ml) decreased TER in a time-dependent fashion, whereas LPA post-treatment reversed the effect of LPS for a period of ∼5 h, and eventually TER returned back to the pre-LPA-treated state. Next, the effect of LPA on LPS-mediated E-cadherin localization was examined. LPS (5 μg/ml, 18 h) treatment reduced E-cadherin immunostaining at cell-cell junctions, whereas LPA post-treatment restored E-cadherin redistribution to cell-cell junctions (Fig. 9B). In addition to the measurement of TER and E-cadherin localization to reflect the epithelial barrier function, we performed an in vitro permeability assay using permeable polycarbonate inserts containing 0.4-μm pores. As shown in Fig. 9C, LPS treatment alone significantly increased IgG-HRP levels in the upper compartment, whereas LPA post-treatment attenuated the LPS-induced IgG-HRP leakage from the lower compartment to the upper compartment. These results further support the data obtained from the ECIS experiments suggesting a protective role of LPA in LPS-induced epithelial barrier dysfunction.
FIGURE 9.
LPA post-treatment restores LPS-induced epithelial barrier disruption. A, HBEpCs grown on ECIS gold electrodes were challenged with LPS (5 μg/ml) for 6 h; cells were treated with LPA (1 μm), and changes in TER were measured with ECIS. *, p < 0.01, compared with LPS treatment. Veh, vehicle. B, HBEpCs grown on glass chamber slides were challenged with LPS (5 μg/ml) for 18 h, then cells were treated with LPA (1 μm) for 15 min, and E-cadherin localization was detected by immunofluorescence staining with an E-cadherin antibody. The intensities of E-cadherin at cell-cell contacts were measured by MetaVue software. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPS treatment. C, HBEpCs were grown on permeable inserts containing 0.4-μm pores. IgG-HRP was added to the lower compartment, and LPS (10 μg/ml) was added to the upper compartment for 1 h, followed by LPA (2 μm) addition for 8 h. The medium from upper compartment was collected, and the IgG-HRP levels were measured by enzyme-linked immunosorbent assay kit. *, p < 0.01, compared with vehicle control; **, p < 0.01, compared with LPS treatment.
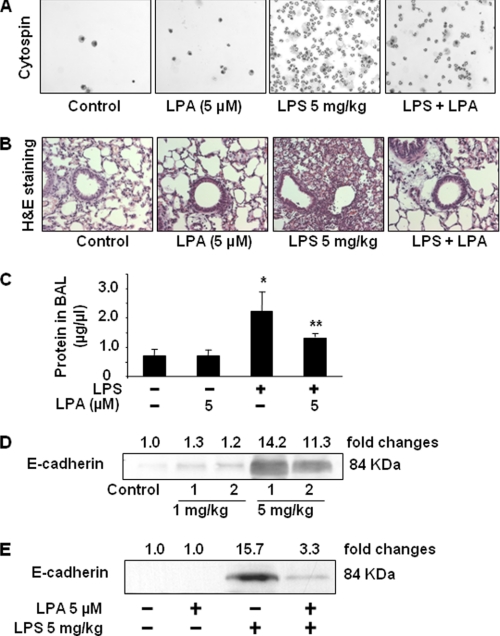
LPA Post-treatment Reduces Neutrophil Influx and E-cadherin Shedding in BAL Fluids of LPS-induced Murine Model of ALI
The LPS-mediated murine model of ALI has been well established (48, 49). Next, we investigated the effect of LPA post-treatment in LPS-induced ALI by examining infiltration of neutrophils, hematoxylin and eosin staining, and E-cadherin shedding in BAL fluids. As shown in Fig. 10, A and B, post-administration of LPA after 1 h of LPS (5 mg/kg body weight) challenge attenuated the LPS-induced infiltration of neutrophils as assessed by cytospin and histological examination. Protein concentration in BAL fluids was assessed by protein assay. Administration of LPA significantly attenuated LPS-induced protein (∼50%) in BAL fluids (Fig. 10C). Furthermore, LPS challenge (1 and 5 mg/kg body weight, 24 h) resulted in an increase of the extracellular domain of E-cadherin (84 kDa) in BAL fluids (Fig. 10D) and was consistent with an earlier report (16). Intratracheal administration of LPA (5 μm in 50 μl saline) delivered 1 h after LPS challenge (5 mg/kg body weight) attenuated LPS-induced increase of soluble E-cadherin in BAL fluids (Fig. 10E). These results suggest that LPA post-treatment exhibits multiple protective effects, including enhancement of airway barrier function and the reduction of inflammatory cell infiltration in the lungs of LPS-challenged mice.
FIGURE 10.
LPA post-treatment reduces LPS-induced neutrophil influx and E-cadherin shedding in a murine model of ALI. 1 h following intratracheal LPS challenge (5 mg/kg body weight), mice (n = 5–6) were intratracheally instilled with LPA (5 μm in 25 μl of saline). After 24 h, differential cell counts in BAL fluids were performed by cytospin (A). B, lung tissues were examined by hematoxylin and eosin (H&E) staining. C, protein concentrations in BAL fluids were detected. *, p < 0.01, compared with control; **, p < 0.05, compared with LPS challenge. D, mice were intratracheally instilled with LPS (1 and 5 mg/kg body weight); BAL fluids were collected at 24 h, and then E-cadherin expression was detected in BAL fluids by Western blotting with antibody to mouse E-cadherin extracellular domain (DECMA-1). Shown are representative blots from 5 to 6 challenged mice. E, mice were intratracheally instilled with LPS (5 mg/kg body weight), and after 1 h LPA (5 μm in 25 μl saline) was administered intratracheally. BAL fluids were collected after 24 h, and E-cadherin shedding was detected by Western blotting with antibody to mouse E-cadherin extracellular domain (DECMA-1). Shown are representative blots from each group (n = 5–6) mice.
DISCUSSION
We have previously demonstrated that LPA regulates cytokine (37–39), prostaglandin E2 (41), and IL-13 receptor decoy receptor (IL-13Rα2) (40) release in HBEpCs. Here, we provide evidence that LPA enhances pulmonary epithelial barrier integrity through PKCδ- and PKCζ-mediated E-cadherin accumulation at cell-cell junctions. Furthermore, post-treatment with LPA reversed endotoxin-induced pulmonary epithelial barrier disruption. This study, for the first time, addresses the underlying mechanism(s) by which LPA mediates a protective role in LPS-induced pulmonary barrier disruption.
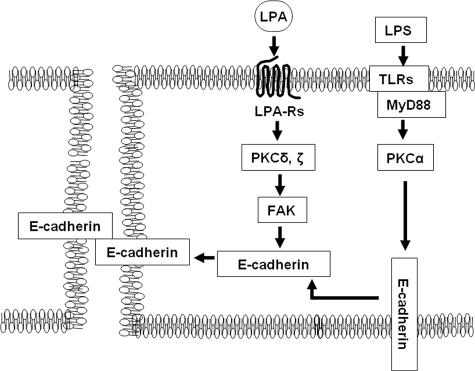
E-cadherin intracellular trafficking between plasma membrane and cytoplasm is critical for the maintenance of epithelial cell-cell junctions and functional integrity of the paracellular barrier in the epithelium (7, 10, 11). Here we show that LPA treatment of pulmonary epithelial cells, including main bronchial, small airway, and alveolar epithelial cells, enhances epithelial barrier function as reflected by increases in TER and E-cadherin accumulation at cell-cell junctions. In addition, the contribution of E-cadherin redistribution in LPA-induced epithelial barrier function was determined by knockdown of E-cadherin with siRNA transfection or EGTA pretreatment. The regulation of cadherin-mediated cell-cell junctions by LPA has been reported in various cell types (51, 52). In Schwann cells, LPA induces actin rearrangements and increases N-cadherin-mediated cell-cell junctions, resulting in cell clustering (51). The treatment of a rat neuronal cell line with LPA leads to cadherin-dependent cell adhesion following cell aggregation (52). Our previous study has shown that LPA treatment enhances airway epithelial cell clustering (45), whereas the current study suggests that LPA enhances pulmonary epithelial barrier function by inducing E-cadherin accumulation at cell-cell junctions in pulmonary epithelial cells. Furthermore, post-treatment of LPA reverses the LPS-induced epithelial barrier disruption, suggesting a potential role of LPA in repairing and remodeling injured pulmonary epithelial cells after endotoxin-induced acute lung injury (Fig. 11).
FIGURE 11.
LPA protects LPS-induced airway epithelial barrier disruption through regulation of E-cadherin intracellular trafficking. Ligation of LPA to LPA receptor(s) activates PKCδ and PKCζ, which regulate E-cadherin accumulation at cell-cell junctions and airway epithelial barrier integrity. However, LPS activates PKCα, resulting in cytoplasm mislocalization of E-cadherin and disruption of airway epithelial barrier. LPA post-treatment reverses LPS-induced airway epithelial barrier disruption.
The mechanisms of regulation of E-cadherin trafficking between plasma membrane and cytoplasm are still unclear. The PKCδ inhibitor rottlerin induces cell dissociation in urinary bladder carcinoma cells (53). In mouse blastocysts, upon activator treatment, PKCδ and PKCζ co-relocate to cell junctions (54), suggesting that PKCδ and PKCζ may play a central role in regulating cell junction membrane assembly. Our previous studies have shown that PKCδ or PKCζ regulates LPA-induced signaling and biological function, including NF-κB activation (31, 41), epidermal growth factor receptor transactivation (38), IL-8 secretion (31), COX-2 expression (41), and c-Met accumulation at cell-cell contacts (45) in HBEpCs. Furthermore, this study demonstrates that both PKCδ and PKCζ regulate the LPA-mediated E-cadherin accumulation at cell-cell junctions and increases in TER in bronchial epithelial cells. PKC may directly phosphorylate E-cadherin at serine residue(s) or E-cadherin-associated proteins, such as catenins and FAK. E-cadherin serine phosphorylation is involved in cell motility; however, the mechanism(s) of regulation of E-cadherin serine phosphorylation and redistribution has yet to be defined. Furthermore, we characterized that PKCδ and PKCζ regulate FAK (Tyr-397) phosphorylation in pulmonary epithelial cells. FAK Tyr-397 is an autophosphorylation site that, when phosphorylated, recruits Src, Grb7, Shc, phospholipase γ, and the p85 subunit of phosphoinositide 3-kinase (55). We have previously shown that LPA treatment induces accumulation of c-Met at cell-cell contacts and interacts with E-cadherin in HBEpCs and that PKCδ, but not PKCα, regulated LPA-induced serine phosphorylation of c-Met and c-Met intracellular trafficking (45). Down-regulation of c-Met by c-Met siRNA-attenuated LPA induced the increases in TER and E-cadherin accumulation at cell-cell contacts,3 suggesting a potential role of PKC isoforms in regulating E-cadherin intracellular trafficking via phosphorylation of c-Met and FAK in pulmonary epithelial cells.
LPA is present in human BAL fluids as determined by tandem mass spectrometry, and significantly increases following segmental allergen challenge (56). Recent studies by Tager et al. (57) showed increases in LPA levels in BAL fluids from a murine model of bleomycin-induced pulmonary fibrosis and from idiopathic pulmonary fibrosis patients, compared with normal controls. Furthermore, a recent study has shown that LPA reduces barrier function in human umbilical vein cells (58). However, this study shows that LPA post-treatment partly mitigated LPS-induced epithelial barrier disruption. Thus, the observable difference of LPA action in bronchial epithelial versus umbilical cells suggest variable regulation in different cell types. Other related studies also support the effect of LPA on barrier enhancement. The LPA acyltransferase inhibitor lysofylline attenuated LPA degradation and protected lung leak in vivo (59), and LPA enhanced barrier function in corneal endothelial cells (60). Furthermore, a recent study from Fan et al. (44) showed that the administration of LPA has an anti-inflammatory effect in murine models of pulmonary inflammatory disease. Intravenous injection of LPA attenuated bacterial endotoxin-induced plasma tumor necrosis factor-α production and myeloperoxidase activity in the lung of mice suggesting that exogenously administered LPA plays a protective role in pulmonary inflammatory diseases (44). This study supports the potential role LPA and LPA analogs as novel therapeutic agents against endotoxin-induced pulmonary epithelial barrier disruption.
Acknowledgments
We greatly appreciate Drs. Nigel Pyne and Michael A. O'Reilly for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL091916 (to Y. Z.) and RO1 HL079396 (to V. N.). This work was also supported by American Cancer Society-Institutional Research Grant 58-044-47 (to Y. Z.).
D. He and Y. Zhao, unpublished data.
- PKC
- protein kinase C
- LPA
- lysophosphatidic acid
- HBEpC
- human bronchial epithelial cell
- HSAEpC
- human small airway epithelial cell
- TER
- transepithelial electrical resistance
- FAK
- focal adhesion kinase
- FRNK
- FAK-related non-kinase; lipopolysaccharide
- BAL
- bronchoalveolar lavage
- ALI
- acute lung injury
- siRNA
- small interfering RNA
- IL
- interleukin
- PKC
- protein kinase C
- HBEpC
- human bronchial epithelial cell
- ECIS
- electrical cell-substrate impedance sensing system
- HA
- hemagglutinin
- dnPKC
- dominant negative PKC
- HRP
- horseradish peroxidase.
REFERENCES
- 1.Budinger G. R., Sznajder J. I. (2006) Clin. Chest Med. 27, 655–669; abstract ix [DOI] [PubMed] [Google Scholar]
- 2.Knight D. A., Holgate S. T. (2003) Respirology 8, 432–446 [DOI] [PubMed] [Google Scholar]
- 3.Matthay M. A., Robriquet L., Fang X. (2005) Proc. Am. Thoracic Soc. 2, 206–213 [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., Usatyuk P. V., Gorshkova I. A., He D., Wang T., Moreno-Vinasco L., Geyh A. S., Breysse P. N., Samet J. M., Spannhake E. W., Garcia J. G., Natarajan V. (2009) Am. J. Respir. Cell Mol. Biol. 40, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gourgoulianis K. I., Domali A., Molyvdas P. A. (1999) Mediat. Inflamm. 8, 261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulsmann A. R., Raatgeep H. R., den Hollander J. C., Stijnen T., Saxena P. R., Kerrebijn K. F., de Jongste J. C. (1994) Am. J. Respir. Crit. Care Med. 149, 519–525 [DOI] [PubMed] [Google Scholar]
- 7.Bush K. T., Tsukamoto T., Nigam S. K. (2000) Am. J. Physiol. Renal Physiol. 278, F847–F852 [DOI] [PubMed] [Google Scholar]
- 8.Chen X., Gumbiner B. M. (2006) Curr. Opin. Cell Biol. 18, 572–578 [DOI] [PubMed] [Google Scholar]
- 9.Huntley G. W., Gil O., Bozdagi O. (2002) Neuroscientist 8, 221–233 [DOI] [PubMed] [Google Scholar]
- 10.Goto Y., Uchida Y., Nomura A., Sakamoto T., Ishii Y., Morishima Y., Masuyama K., Sekizawa K. (2000) Am. J. Respir. Cell Mol. Biol. 23, 712–718 [DOI] [PubMed] [Google Scholar]
- 11.Jaggi M., Rao P. S., Smith D. J., Wheelock M. J., Johnson K. R., Hemstreet G. P., Balaji K. C. (2005) Cancer Res. 65, 483–492 [PubMed] [Google Scholar]
- 12.Thiery J. P. (2003) Curr. Opin. Genet. Dev. 13, 365–371 [DOI] [PubMed] [Google Scholar]
- 13.Wheelock M. J., Johnson K. R. (2003) Annu. Rev. Cell Dev. Biol. 19, 207–235 [DOI] [PubMed] [Google Scholar]
- 14.Zabner J., Winter M., Excoffon K. J., Stoltz D., Ries D., Shasby S., Shasby M. (2003) J. Appl. Physiol. 95, 394–401 [DOI] [PubMed] [Google Scholar]
- 15.Otto T., Rembrink K., Goepel M., Meyer-Schwickerath M., Rübben H. (1993) Urol. Res. 21, 359–362 [DOI] [PubMed] [Google Scholar]
- 16.Evans S. M., Blyth D. I., Wong T., Sanjar S., West M. R. (2002) Am. J. Respir. Cell Mol. Biol. 27, 446–454 [DOI] [PubMed] [Google Scholar]
- 17.Lickert H., Bauer A., Kemler R., Stappert J. (2000) J. Biol. Chem. 275, 5090–5095 [DOI] [PubMed] [Google Scholar]
- 18.Sugiura T., Nakane S., Kishimoto S., Waku K., Yoshioka Y., Tokumura A. (2002) J. Lipid Res. 43, 2049–2055 [DOI] [PubMed] [Google Scholar]
- 19.Tokumura A. (2002) Biochim. Biophys. Acta 1582, 18–25 [DOI] [PubMed] [Google Scholar]
- 20.Westermann A. M., Havik E., Postma F. R., Beijnen J. H., Dalesio O., Moolenaar W. H., Rodenhuis S. (1998) Ann. Oncol. 9, 437–442 [DOI] [PubMed] [Google Scholar]
- 21.An S., Bleu T., Hallmark O. G., Goetzl E. J. (1998) J. Biol. Chem. 273, 7906–7910 [DOI] [PubMed] [Google Scholar]
- 22.An S., Dickens M. A., Bleu T., Hallmark O. G., Goetzl E. J. (1997) Biochem. Biophys. Res. Commun. 231, 619–622 [DOI] [PubMed] [Google Scholar]
- 23.Im D. S., Heise C. E., Harding M. A., George S. R., O'Dowd B. F., Theodorescu D., Lynch K. R. (2000) Mol. Pharmacol. 57, 753–759 [PubMed] [Google Scholar]
- 24.Lee C. W., Rivera R., Gardell S., Dubin A. E., Chun J. (2006) J. Biol. Chem. 281, 23589–23597 [DOI] [PubMed] [Google Scholar]
- 25.Noguchi K., Ishii S., Shimizu T. (2003) J. Biol. Chem. 278, 25600–25606 [DOI] [PubMed] [Google Scholar]
- 26.Tabata K., Baba K., Shiraishi A., Ito M., Fujita N. (2007) Biochem. Biophys. Res. Commun. 363, 861–866 [DOI] [PubMed] [Google Scholar]
- 27.Kranenburg O., Poland M., van Horck F. P., Drechsel D., Hall A., Moolenaar W. H. (1999) Mol. Biol. Cell 10, 1851–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postma F. R., Jalink K., Hengeveld T., Offermanns S., Moolenaar W. H. (2001) Curr. Biol. 11, 121–124 [DOI] [PubMed] [Google Scholar]
- 29.Takuwa Y., Takuwa N., Sugimoto N. (2002) J. Biochem. 131, 767–771 [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Cummings R., Zhao Y., Kazlauskas A., Sham J. K., Morris A., Georas S., Brindley D. N., Natarajan V. (2003) J. Biol. Chem. 278, 39931–39940 [DOI] [PubMed] [Google Scholar]
- 31.Cummings R., Zhao Y., Jacoby D., Spannhake E. W., Ohba M., Garcia J. G., Watkins T., He D., Saatian B., Natarajan V. (2004) J. Biol. Chem. 279, 41085–41094 [DOI] [PubMed] [Google Scholar]
- 32.Ediger T. L., Danforth B. L., Toews M. L. (2002) Am. J. Physiol. Lung Cell Mol. Physiol. 282, L91–L98 [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto T., Yamashita M., Ohata H., Momose K. (2003) J. Pharmacol. Sci. 91, 8–14 [DOI] [PubMed] [Google Scholar]
- 34.Rubenfeld J., Guo J., Sookrung N., Chen R., Chaicumpa W., Casolaro V., Zhao Y., Natarajan V., Georas S. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 290, L66–L74 [DOI] [PubMed] [Google Scholar]
- 35.Toews M. L., Ustinova E. E., Schultz H. D. (1997) J. Appl. Physiol. 83, 1216–1222 [DOI] [PubMed] [Google Scholar]
- 36.Ediger T. L., Toews M. L. (2000) J. Pharmacol. Exp. Ther. 294, 1076–1082 [PubMed] [Google Scholar]
- 37.Saatian B., Zhao Y., He D., Georas S. N., Watkins T., Spannhake E. W., Natarajan V. (2006) Biochem. J. 393, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., He D., Saatian B., Watkins T., Spannhake E. W., Pyne N. J., Natarajan V. (2006) J. Biol. Chem. 281, 19501–19511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y., Usatyuk P. V., Cummings R., Saatian B., He D., Watkins T., Morris A., Spannhake E. W., Brindley D. N., Natarajan V. (2005) Biochem. J. 385, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y., He D., Zhao J., Wang L., Leff A. R., Spannhake E. W., Georas S., Natarajan V. (2007) J. Biol. Chem. 282, 10172–10179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He D., Natarajan V., Stern R., Gorshkova I. A., Solway J., Spannhake E. W., Zhao Y. (2008) Biochem. J. 412, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vancheri C., Mastruzzo C., Sortino M. A., Crimi N. (2004) Trends Immunol. 25, 40–46 [DOI] [PubMed] [Google Scholar]
- 43.Wood N., Whitters M. J., Jacobson B. A., Witek J., Sypek J. P., Kasaian M., Eppihimer M. J., Unger M., Tanaka T., Goldman S. J., Collins M., Donaldson D. D., Grusby M. J. (2003) J. Exp. Med. 197, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan H., Zingarelli B., Harris V., Tempel G. E., Halushka P. V., Cook J. A. (2008) Mol. Med. 14, 422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., He D., Stern R., Usatyuk P. V., Spannhake E. W., Salgia R., Natarajan V. (2007) Cell. Signal. 19, 2329–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collares-Buzato C. B., McEwan G. T., Jepson M. A., Simmons N. L., Hirst B. H. (1994) Biochim. Biophys. Acta 1222, 147–158 [DOI] [PubMed] [Google Scholar]
- 47.Giehl K., Menke A. (2008) Front. Biosci. 13, 3975–3985 [DOI] [PubMed] [Google Scholar]
- 48.Eutamene H., Theodorou V., Schmidlin F., Tondereau V., Garcia-Villar R., Salvador-Cartier C., Chovet M., Bertrand C., Bueno L. (2005) Eur. Respir. J. 25, 789–796 [DOI] [PubMed] [Google Scholar]
- 49.Poynter M. E., Irvin C. G., Janssen-Heininger Y. M. (2003) J. Immunol. 170, 6257–6265 [DOI] [PubMed] [Google Scholar]
- 50.Vernooy J. H., Dentener M. A., van Suylen R. J., Buurman W. A., Wouters E. F. (2001) Am. J. Respir. Cell Mol. Biol. 24, 569–576 [DOI] [PubMed] [Google Scholar]
- 51.Weiner J. A., Fukushima N., Contos J. J., Scherer S. S., Chun J. (2001) J. Neurosci. 21, 7069–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanagida K., Ishii S., Hamano F., Noguchi K., Shimizu T. (2007) J. Biol. Chem. 282, 5814–5824 [DOI] [PubMed] [Google Scholar]
- 53.Koivunen J., Aaltonen V., Koskela S., Lehenkari P., Laato M., Peltonen J. (2004) Cancer Res. 64, 5693–5701 [DOI] [PubMed] [Google Scholar]
- 54.Eckert J. J., McCallum A., Mears A., Rumsby M. G., Cameron I. T., Fleming T. P. (2005) Dev. Biol. 288, 234–247 [DOI] [PubMed] [Google Scholar]
- 55.Hanks S. K., Ryzhova L., Shin N. Y., Brábek J. (2003) Front. Biosci. 8, d982–996 [DOI] [PubMed] [Google Scholar]
- 56.Georas S. N., Berdyshev E., Hubbard W., Gorshkova I. A., Usatyuk P. V., Saatian B., Myers A. C., Williams M. A., Xiao H. Q., Liu M., Natarajan V. (2007) Clin. Exp. Allergy 37, 311–322 [DOI] [PubMed] [Google Scholar]
- 57.Tager A. M., LaCamera P., Shea B. S., Campanella G. S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B. A., Kim N. D., Hart W. K., Pardo A., Blackwell T. S., Xu Y., Chun J., Luster A. D. (2008) Nat. Med. 14, 45–54 [DOI] [PubMed] [Google Scholar]
- 58.van Nieuw Amerongen G. P., Vermeer M. A., van Hinsbergh V. W. (2000) Arterioscler. Thromb. Vasc. Biol. 20, E127–133 [DOI] [PubMed] [Google Scholar]
- 59.Hybertson B. M., Bursten S. L., Leff J. A., Lee Y. M., Jepson E. K., Dewitt C. R., Zagorski J., Cho H. G., Repine J. E. (1997) J. Appl. Physiol. 82, 226–232 [DOI] [PubMed] [Google Scholar]
- 60.Yin F., Watsky M. A. (2005) Invest. Ophthalmol. Vis. Sci. 46, 1927–1933 [DOI] [PubMed] [Google Scholar]