Abstract
A cytochrome P450 (P450) enzyme in porcine liver that catalyzed the phenol-coupling reaction of the substrate (R)-reticuline to salutaridine was previously purified to homogeneity (Amann, T., Roos, P. H., Huh, H., and Zenk, M. H. (1995) Heterocycles 40, 425–440). This reaction was found to be catalyzed by human P450s 2D6 and 3A4 in the presence of (R)-reticuline and NADPH to yield not a single product, but rather (−)-isoboldine, (−)-corytuberine, (+)-pallidine, and salutaridine, the para-ortho coupled established precursor of morphine in the poppy plant and most likely also in mammals. (S)-Reticuline, a substrate of both P450 enzymes, yielded the phenol-coupled alkaloids (+)-isoboldine, (+)-corytuberine, (−)-pallidine, and sinoacutine; none of these serve as a morphine precursor. Catalytic efficiencies were similar for P450 2D6 and P450 3A4 in the presence of cytochrome b5 with (R)-reticuline as substrate. The mechanism of phenol coupling is not yet established; however, we favor a single cycle of iron oxidation to yield salutaridine and the three other alkaloids from (R)-reticuline. The total yield of salutaridine formed can supply the 10 nm concentration of morphine found in human neuroblastoma cell cultures and in brain tissues of mice.
Cytochrome P450 (P450)2 enzymes catalyze the most versatile chemical reactions in nature (1). There is, however, a discrepancy between the plant and the animal kingdoms with regard to the sheer number of these biocatalysts. Whereas in a single model plant, Arabidopsis thaliana, there are to date 273 P450 proteins, in the human genome, only 57 of these proteins are present. Whereas plants and animals share a multitude of highly regio- and stereospecific O-demethylation reactions, more complex reactions such as phenol coupling are much more abundant in plants than in animals, especially in the alkaloid field (2–11). The proposal of Barton and Cohen (12) correlated the structure of specific plant alkaloids in terms of this reaction mechanism and gave mechanistic proposals of how these phenol-coupled products may possibly be biosynthesized in nature. The oxidation of phenols by one-electron transfer affords radicals, which, by radical pairing, form new C-C or C-O bonds either by intra- or intermolecular coupling. The first two examples that unequivocally demonstrated the formation of C-C and C-O bonds in a stereo- and regioselective manner in plant metabolism are catalyzed by specific P450-linked microsomal-bound plant enzymes (13). One of these enzymes was salutaridine synthase from Papaver somniferum (opium poppy) (14). This synthase catalyzes the intramolecular formation of the critical C12-C13 carbon bridge and is a key enzyme in morphine biosynthesis.
The groups of Goldstein (15) and Spector (16) have published a number of reports over the past 25 years claiming that mammals are capable of synthesizing de novo traces of endogenous morphine. However, no convincing experimental data have been presented regarding the enzymes. Phenol-coupling reactions in mammals are extremely rare, and the only example described thus far is the formation of thyroxine, by radical pairing, in humans. If the key step of morphine synthesis, the formation of phenol-coupled salutaridine from (R)-reticuline, occurs in mammals then in analogy to plants, a P450 enzyme must be present (in mammals) to catalyze this reaction. In 1987, the first experiments were conducted in an attempt to discover the reaction by supplying uniformly labeled racemic [3H]reticuline in the presence of rat microsomes and NADPH to examine whether [3H]salutaridine can be formed under these conditions (15). A radioactive compound was formed in 1% yield and assumed to be the phenol-coupled product salutaridine. We later repeated this experiment using (R)-[N-14CH3]reticuline and NADPH-fortified microsomes from pig, rat, cow, and sheep. We observed the formation of [N-14CH3]salutaridine with the correct stereochemistry at carbon 9 (17). The enzyme from porcine liver was subsequently purified to homogeneity and the reaction product was characterized by mass spectrometry and physical parameters to be a product of a P450 enzyme, which we provisionally named “salutaridine synthase” (18). The aim of this report is the identification of the homogenous porcine P450 enzyme, its equivalent in humans, and the mechanism of the phenol-coupling reaction in the formation of precursors of morphine.
EXPERIMENTAL PROCEDURES
Chemicals
Alkaloids were from our departmental collection, and isoboldine, pallidine, and sinoacutine were gifts of Dr. André Cavé, University Paris-Sud, Dr. Shoei-Sheng Lee, National Taiwan University, and Dr. F. Bracher, Ludwig-Maximilians-University Munich. l-α-Dilauroyl-sn-glycero-3-phosphocholine, bovine liver catalase, and bovine erythrocytes superoxide dismutase were obtained from Sigma.
Enzymes
Human P450 2D6 and rat NADPH-P450 reductase were prepared as described (19). Human P450 2D6 and human P450 3A4 (with or without cytochrome b5) were purchased from BD Biosciences (Woburn, MA). Porcine salutaridine synthase was purified according to Ref. 18 and subjected to SDS-PAGE on a 10% (w/v) acrylamide gel. The protein band at ∼50 kDa (containing ∼10 μg of protein) was excised and eluted, and the N terminus was sequenced by Edman degradation using an Applied Biosystems Model 470 gas-phase sequencer at the Max Planck Institute for Biochemistry, Martinsried, Germany.
Enzyme Assays
Enzyme assays utilizing radioactive labeled substrate were conducted in a total volume of 140 μl containing 36 mm potassium phosphate buffer (pH 6.5), 20 pmol of P450 enzyme (BD Biosciences, microsomal preparation from baculovirus-infected insect cells coexpressing human NADPH-P450 reductase), 1.8 mm NADPH, 0.36 mm EDTA, and 0.2 μCi (R)- or (S)-[N-14CH3] reticuline (200,000–300,000 cpm, 6.75 nmol). The reaction mixture was incubated for 5 h at 37 °C. For identification of products, the reaction mixture was separated by two-dimensional TLC (solvent 1: toluene/acetone/ethanol/NH4OH, 45:45:7:3 (v/v/v/v); solvent 2: CHCl3/acetone/diethylamine, 5:4:1 (v/v/v)), and radioactive bands were detected by phosphorimaging with a Typhoon 9410 (Molecular Dynamics). For plant-feeding experiments, potential precursor solutions were obtained by separation of the reaction mixture by TLC using the solvent mixture chloroform/acetone/diethylamine, 5:4:1 (v/v/v) following the elution of corresponding bands with CH3OH and reconstitution in H2O. Standard reactions for kinetic data and pH profile analysis were conducted in a total volume of 250 μl containing 100 mm potassium phosphate buffer (pH 7.4), 15 μg of l-α-dilauroyl-sn-glycero-3-phosphocholine, 1 mm NADP+, 10 mm glucose 6-phosphate, 1 unit/ml glucose-6-phosphate dehydrogenase (yeast), 1000 units/ml catalase (bovine liver), 10 μg bovine erythrocyte superoxide dismutase, and 0.25 μm to 5 mm substrate. Reactions were started by adding 38 pmol of P450 2D6 (Escherichia coli-expressed protein) and 255 pmol of rat cytochrome P450 reductase (E. coli-expressed protein) or 9–18 pmol of P450 2D6 or P450 3A4 (BD Biosciences) and incubated for 10 min at 37 °C. Reaction mixtures were made alkaline with 400 μl of 1 m Na2CO3 buffer (pH 9.5) and extracted twice with 400 μl of CHCl3. The combined organic phases were dried, reconstituted with CH3OH, and subjected to LC-MS/MS. Kinetic parameters were estimated by non-linear regression with GraphPad Prism. For pH profile analysis enzymatic assays were conducted as described above in a reaction mixture containing 25 μmol of following buffer: sodium citrate (pH 4–6), potassium phosphate (pH 6–8), and Tris/HCl (pH 8–9).
Plant Feeding Experiments
Sterilized 5-day-old Papaver seedlings were incubated for 48 h with the potential precursor solution. The seedlings were extracted with 80% ethanol (v/v). The extracts were dried, redissolved in 20 μl 50% ethanol (v/v), and separated by TLC in solvent system toluene/ethyl acetate/diethylamine, 7:2:1 (v/v/v). Radioactive bands were detected by phosphorimaging.
LC-MS/MS Analysis
Enzyme activities were analyzed with a 4000 QTRAP LC-TIS-MS-MS system by monitoring the enhanced product ion (EPI) and multiple reaction monitoring (MRM) in the positive ionization mode. The system consisted of a CTC PAL autosampler (LEAP Technologies), a Shimadzu LC-20AD HPLC system, and a 4000 QTRAP mass spectrometer (Applied Biosystems). Separation of (10 μl) samples was achieved by using a Luna C18 octadecylsilane HPLC column (Phenomenex, 5 μm, 150 mm × 2 mm) combined with a C18 guard column (Phenomenex, 4 mm × 2 mm). The mobile phase total flow was set to 0.5 ml/min with binary gradient elution, using solvent A (5% CH3OH, 5% CH3CN, 10 mm NH4HCO3, 45 mm NH4OH) and B (90% CH3CN, 10 mm NH4HCO3, 15 mm NH4OH) (all v/v). The gradient started with 100% A for 2 min and was increased to 100% B over 10 min. Elution was continued for 2 min at 100% B followed by a 5-min equilibration with starting condition. The following TIS source parameters were used: CUR 30, CAD high, IS 5000, TEM 500, EP 10, CXP 17. Compound-dependent parameters for the compounds of interest (collision energy, declustering potential, quantifier MRM transition, qualifier MRM transition, dwell time) are listed in Table 1. Identification of an analyte was based on retention times, the quantifier-to-qualifier MRM transition ratio, and comparison with the expected values for standards. Quantitation was performed by constructing standard curves for each analyte and integrating the peak area of the quantifier MRM transition with Analyst 1.4.1 (Applied Biosystems, MDS SCIEX Instruments).
TABLE 1.
Compound-dependent parameters for the LC-MS-MS method
| Analyte | Collision energy | Declustering potential | Quantifier MRM transition | Qualifier MRM transition | Dwell time |
|---|---|---|---|---|---|
| V | V | ms | |||
| Reticuline | 35 | 45 | 330 → 192 | 330 → 137 | 50 |
| Corytuberine | 40 | 50 | 328 → 265 | 328 → 282 | 50 |
| Pallidine | 40 | 50 | 328 → 211 | 328 → 237 | 50 |
| Salutaridine | 40 | 50 | 328 → 211 | 328 → 237 | 50 |
| Isoboldine | 40 | 50 | 328 → 265 | 328 → 237 | 50 |
| Thebaine | 35 | 40 | 312 → 251 | 312 → 281 | 50 |
| Oripavine | 35 | 40 | 298 → 218 | 298 → 249 | 50 |
RESULTS
Sequencing of Porcine Salutaridine Synthase
The microsomal porcine liver enzyme catalyzing the formation of the phenol-coupling reaction from (R)-reticuline to salutaridine was purified to apparent electrophoretic homogeneity and subjected to Edman degradation. The N terminus yielded the amino acid sequence: Gly-Leu-Leu-Thr-Gly-Asp-Leu-Leu-Gly-Ile-Leu-Ala-Leu-Ala-Met-Val-Ile-Phe-Leu-Leu-Asn(Leu)-Val-Asp-Leu-Met-X-Arg.
Upon comparison of this peptide sequence to those present in the public databases, this is the exact N-terminal of pig P450 2D25, except for the missing N-terminal Met. Homology to human P450 2D6 was observed (56% identity). Consequently, human P450 2D6 was obtained from a commercial source (BD Biosciences) and found to indeed catalyze the phenol-coupling reaction from (R)-[N-14CH3]reticuline to salutaridine in the presence of NADPH. However, close examination of the reaction products of the porcine and the commercially available human P450 2D6 enzymes by two-dimensional TLC revealed four metabolites in addition to unconsumed 14C-labeled (R)-reticuline (vide infra).
Identification of P450 2D6-catalyzed Reaction Products
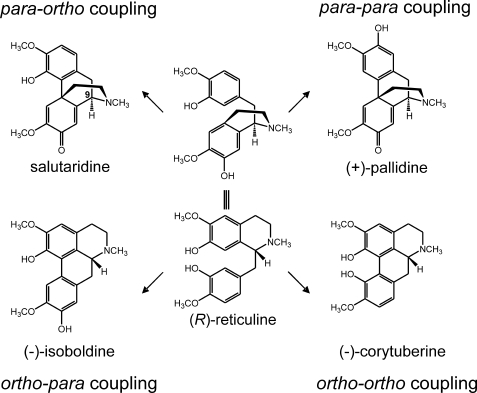
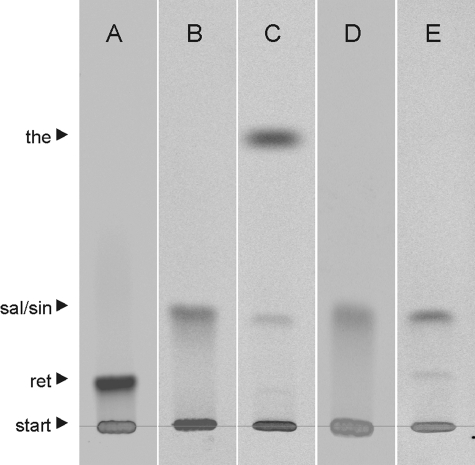
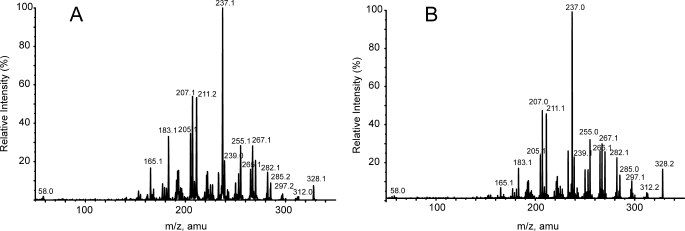
Incubation of (R)-reticuline in the presence of NADPH-P450 reductase and P450 2D6 resulted in the formation of four metabolites that were not found in controls with heat-inactivated enzymes. These metabolites were subjected to MS after separation by HPLC. Comparison of the four compounds with standards of alkaloids that were expected to be formed according to the phenol-coupling reactions allowed the identification of the unknown compounds as salutaridine (para-ortho coupling), (+)-pallidine (para-para coupling), (−)-isoboldine (ortho-para coupling), and (−)-corytuberine (ortho-ortho coupling) (Fig. 1). The identification of these compounds is consistent with the previous findings by the incubation of rat liver microsomes in the presence of racemic reticuline (20, 21). However, the important morphine precursor salutaridine was missed during that investigation. Salutaridine is formed unequivocally during the phenol-coupling reaction and was identified by MS and retention time. Application of the para-ortho coupled product to 5-day-old poppy seedlings verified salutaridine by its incorporation into thebaine (Fig. 2), while the other three phenol-coupled products of the (R)-series were not incorporated into thebaine. Analogous incubation of NADPH-P450 reductase in the presence of P450 2D6 and (S)-reticuline again gave four phenol-coupled products of the (S)-series, which were sinoacutine, (−)-pallidine, (+)-isoboldine, and (+)-corytuberine and did not serve as precursors to morphine. The collision-induced dissociation (CID) spectra of salutaridine [M+H]+ 328 by EPI yielded the characteristic fragment ions m/z 297, 285, 265, 239, and 237 (22) that were also found in CID spectra of the para-ortho coupled product. The CID spectra of salutaridine standard and para-ortho coupled enzymatic product are shown (Fig. 3).
FIGURE 1.
Oxidative phenol-coupling reaction of (R)-reticuline in mammals. The four phenol-coupled products salutaridine, (+)-pallidine, (−)-isoboldine, and (−)-corytuberine were formed from (R)-reticuline via an oxidative phenol-coupling reaction catalyzed by human P450 2D6 and P450 3A4.
FIGURE 2.
TLC radiogram of feeding of mammalian phenol-coupled alkaloids to P. somniferum seedlings. A, 14C-labeled reticuline standard. B, 14C-labeled salutaridine formed by enzymatic phenol-coupling of (R)-[N-14CH3]reticuline. C, 14C-labeled thebaine in extract of Papaver seedlings fed with 14C-labeled salutaridine shown in B. D, 14C-labeled sinoacutine formed by enzymatic phenol-coupling of (S)-[N-14CH3]reticuline; E, extract of Papaver seedlings fed with 14C-labeled sinoacutine shown in D. Sinoacutine was not incorporated into thebaine, an intermediate of plant morphine biosynthesis. the, thebaine; sal, salutaridine; sin, sinoacutine; ret, reticuline.
FIGURE 3.
CID spectra of salutaridine in EPI mode. A, MS of salutaridine standard (parent [M+H]+ 328, CE = 40V); B, MS of the enzymatic product salutaridine (parent [M+H]+ 328, CE = 40V).
Human P450 2D6 is known to catalyze a multitude of reactions both with natural compounds and xenobiotics and is involved in the oxidation of about 30% of drugs used by humans (19, 23). Interestingly, human P450 2D6 is known to catalyze several reactions within the putative pathway leading from l-tyrosine to morphine. These reactions include the hydroxylation of tyramine to dopamine (23, 24), the demethylation of thebaine to oripavine (25), and the demethylation of codeine to morphine (26); all of these reactions can be catalyzed by a single enzyme-P450 2D6. Tyramine hydroxylation to dopamine plays only a minor role, if any, in the production of dopamine in humans; however, three P450 2D6-catalyzed reactions may play a role in the biosynthesis of endogenous morphine.
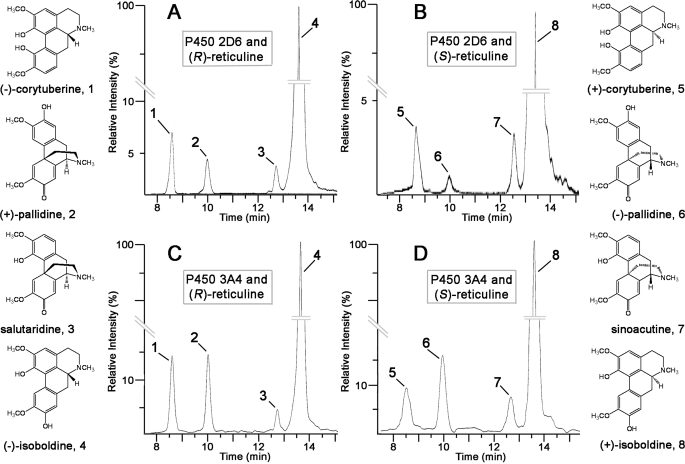
P450 3A4 has previously been shown to N-demethylate codeine to N-norcodeine (27). This similarity of P450 3A4 in accepting codeine as substrate prompted us to investigate whether P450 3A4 can catalyze the phenol coupling of (R)-reticuline. Indeed P450 3A4 showed the product pattern known for 2D6, i.e. the formation of salutaridine, (+)-pallidine, (−)-isoboldine, and (−)-corytuberine from the precursor (R)-reticuline as determined by MS (Fig. 4). The morphine biosynthetic precursor salutaridine is indeed formed by P450 3A4 as well. P450 3A4 was also tested to determine if it can catalyze the phenol-coupling reaction with the (S)-configuration of reticuline as substrate. Incubation of P450 3A4 with (S)-reticuline again yielded four products, sinoacutine, (−)-pallidine, (+)-isoboldine, and (+)-corytuberine, as determined by MS.
FIGURE 4.
LCMS analysis of products formed by human P450 2D6 and P450 3A4. The MRM transition from m/z 328 to m/z 237 shows product formation after 2 h of incubation of: A, human P450 2D6 and (R)-reticuline; B, human P450 2D6 and (S)-reticuline; C, human P450 3A4 and (R)-reticuline; and D, human P450 3A4 and (S)-reticuline.
The four phenol-coupled alkaloids formed in the presence of human P450 2D6 and 10 μm (R)-reticuline were individually quantitated after an incubation time of 10 min, in which time product formation is in the linear range: (−)-corytuberine (4 nm), (−)-isoboldine (54 nm), (+)-pallidine (24 nm), and salutaridine (6 nm). With the (S)-configuration of reticuline as substrate under the same incubation condition the yields of phenol-coupled products were: (+)-corytuberine (1 nm), (+)-isoboldine (61 nm), (−)-pallidine (15 nm), and sinoacutine (3 nm). With P450 3A4 as catalyst, the same set of phenol-coupled alkaloids yielded the following with (R)-reticuline as substrate: (−)-corytuberine (7 nm), (−)-isoboldine (30 nm), (+)-pallidine (18 nm), and salutaridine (28 nm). With (S)-reticuline as substrate, under the same conditions as above and P450 3A4 as catalyst, the yields were: (+)-corytuberine (5 nm) (+)-isoboldine (15 nm), (−)-pallidine (7 nm), and sinoacutine (15 nm). Similar yields were obtained for the (R)- and (S)-series of phenol-coupled products suggesting that the two different P450s oxidize reticuline via radical formation without stereoselectivity. A pH profile for both P450 enzymes catalyzing the phenol coupling of (R)-reticuline to salutaridine is shown in supplemental Fig. S1. The relative distribution of the four phenol-coupled products was the same after 10 min and 120 min of incubation as well as at any pH between 5 and 9.
Kinetic Parameters of the Phenol Coupling Catalyzed by P450 2D6 and 3A4
Both human P450 enzymes described herein, P450 2D6 and P450 3A4, catalyze the same reaction by converting (R)-reticuline to the four phenol-coupled products but with distinct kinetics that can be derived from their Michaelis-Menten parameters (Table 2 and supplemental Figs. S2-S4). P450 2D6 had a lower apparent Km of 2–3 μm for the substrate (R)-reticuline as compared with P450 3A4 (Km = 500–1000 μm) whereas a higher maximum rate (kcat) for the conversion of (R)-reticuline is observed for P450 3A4. Including cytochrome b5 in the P450 3A4 system increased the rate of product formation by about 6 to 7 times whereas the Km was only slightly changed. These findings support other studies showing that the activity of P450 3A4 can be enhanced by cytochrome b5 whereas P450 2D6 is not affected (28). Catalytic efficiencies for both enzymes, P450 2D6 and P450 3A4-utilizing (R)-reticuline as a substrate, show that P450 2D6 seems to be more efficient; however, if cytochrome b5 is added to the P450 3A4 system, catalytic efficiencies of both enzymes become comparable. Additionally, human P450 2D6 shows a higher Km value for two other substrates, thebaine and codeine, i.e. 190–250 and 48 μm, respectively (Table 2 and supplemental Fig. S5), as compared with (R)-reticuline. However, increased catalytic rates for the substrates thebaine and codeine lead to catalytic efficiencies of same order of magnitude for all three substrates of P450 2D6 that described herein.
TABLE 2.
Catalytic parameters for human P450 2D6 and P450 3A4
| Enzyme | Substrate | Product | Km | kcat | kcat//Km |
|---|---|---|---|---|---|
| μm | pmol/min/pmol P450 | mm−1s−1 | |||
| P450 2D6 | (R)-Reticuline | Corytuberine | 2.7 ± 0.37 | 0.015 ± 0.001 | 0.09 |
| Pallidine | 1.8 ± 0.26 | 0.045 ± 0.002 | 0.42 | ||
| Salutaridine | 2.5 ± 0.21 | 0.011 ± 0.001 | 0.08 | ||
| Isoboldine | 1.9 ± 0.36 | 0.390 ± 0.026 | 3.42 | ||
| Thebaine | Oripavine | 48 ± 9.6 | 4.55 ± 0.54 | 1.60 | |
| Codeine | Morphine | 250a | 14a | 0.93 | |
| 190b | 6.4b | 0.56 | |||
| P450 3A4 with cytochrome b5 | (R)-Reticuline | Corytuberine | 384 ± 40 | 1.3 ± 0.1 | 0.06 |
| Pallidine | 474 ± 30 | 14.7 ± 0.3 | 0.52 | ||
| Salutaridine | 1961 ± 181 | 12.3 ± 0.5 | 0.12 | ||
| Isoboldine | 4860 ± 860 | 158 ± 17 | 0.54 | ||
| P450 3A4 | (R)-Reticuline | Corytuberine | 534 ± 66 | 0.16 ± 0.14 | 0.005 |
| Pallidine | 490 ± 38 | 2.97 ± 0.14 | 0.101 | ||
| Salutaridine | 993 ± 220 | 2.20 ± 0.36 | 0.037 | ||
| Isoboldine | 577 ± 130 | 9.37 ± 1.34 | 0.271 |
DISCUSSION
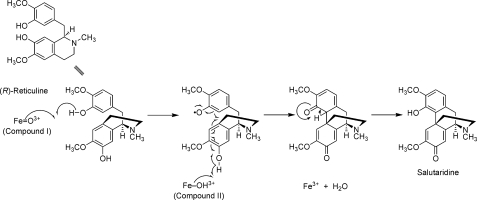
It has been shown that human P450 2D6 and P450 3A4 catalyze what appear to be radical-induced phenol-coupling reactions with both (R)-and (S)-reticuline, yielding four (R)-configured and four (S)-configured phenol-coupled products. As shown in Fig. 5, a mechanism can be postulated that starts with the fully activated P450 (“compound I” form), which abstracts a hydrogen atom to create a phenoxy radical. The substrate rotates in juxtaposition to the P450 iron, which is now in the “compound II” form and can still abstract a second phenolic hydrogen, which then leads to immediate coupling within a single cycle of P450 action, yielding a water molecule. A simple enolization of the cyclohexanedienal ring yields the final product salutaridine as one of the (R)-coupled alkaloids and the missing link in human morphine biosynthesis. The intramolecular C-C phenol-coupling reaction of (S)-reticuline to corytuberine catalyzed by CYP80G2 in the plant Coptis japonica is postulated to occur by a diradical mechanism (7). This postulate was based on a previously proposed mechanism in the first report of a plant cytochrome P450 that catalyzes a phenol-coupling reaction, berbamunine synthase from Berberis stolonifera (2). The concept of a diradical mechanism in phenol-coupling reactions finds origin in the proposal of Barton and Cohen (12) in which radical pairing furnishes diphenyl ether or aryl-aryl bonds. A new C-C phenol-coupling reaction catalyzed by CYP121 of Mycobacterium tuberculosis has recently been reported (29). It is proposed therein that the intramolecular C-C coupling of the two tyrosyl side chains of cYY to cyclodipeptide proceeds via activation of the tyrosyl residues through a radical mechanism. A diradical mechanism can also be proposed herein; however, the mechanism proposed in Fig. 5 avoids the energetic instability of a formal diradical species.
FIGURE 5.
Proposed mechanism of the oxidative phenol-coupling reaction in mammals. The formation of salutaridine from (R)-reticuline is catalyzed by P450 2D6 and P450 3A4 and passes through a single cycle of iron oxidation.
Salutaridine has previously escaped detection using rat liver microsomes (20, 21), being a minor phenol-coupled alkaloid. Salutaridine is the para-ortho phenol-coupled product, which is the biosynthetic precursor of morphine both in the poppy plant and in humans and other mammals. This finding adds considerable credibility to the controversial discussion about whether endogenous morphine occurs in mammals. It should be pointed out that the occurrence of P450 2D6, not only in liver tissue but also in human brain, has been unequivocally proven (30). P450 2D6 occurs mainly in the neocortex, telencephalon, hippocampus, diencephalon, mesencephalon, cerebellum, and myelencephalon. The second P450 that catalyzes the phenol-coupling reactions with (R)-reticuline as substrate forming again four phenol-coupled products, including salutaridine-3A4- is found to occur in brain, mainly in pons and cervical cord (31) and in liver and small intestine (32).
The presence of endogenous morphine in brain of humans and mammals has been suggested by various researchers (16, 33) and in sterile human cell cultures, e.g. neuroblastoma cells SH-SY5Y (34–36). The possible occurrence of morphine in human and mammalian cells has been a matter of controversy for more than 30 years. A resolution of this problem can come only by the application of state-of-the-art analytical chemistry, biochemistry, and molecular biology, with the application of critical controls to exclude introduction of morphine from food sources to the animals and exclusion of laboratory contamination with morphine.
It has been suggested that human P450 2D6 might catalyze the transformation of (R)-reticuline to salutaridine (37). In a recent report, Hawkins and Smolke (38) tested whether P450 2D6 might catalyze the formation of salutaridine from (R)-reticuline in recombinant yeast. (R,S)-Norlaudanosoline was fed to the recombinant yeast strain which produced racemic (R,S)-reticuline. When P450 2D6 was introduced into the (R,S)-reticuline-producing yeast strain, a product was formed that had the MS fragment ions of m/z 297 and m/z 265 and was designated salutaridine (38). The yield of the morphine precursor was reported to be between 6 and 8%. The yield could not be further increased (38) by available techniques.
P450 2D6 (and P450 3A4) catalyze reactions that can be interpreted as a diradical mechanism or alternatively involving a single cycle of iron oxidation, vide supra (Fig. 5). This mechanism will lead to four final alkaloid products, each alkaloid in a different but consistent concentration. Only one of these products is the desired morphine precursor salutaridine, in 0.06% concentration. Use of racemic (R,S)-reticuline (via (R,S)-norlaudanosoline in the recombinant yeast system) leads to another four phenol-coupled products of the (S)-series, namely the alkaloids (+)-corytuberine, (+)-isoboldine, sinoacutine, (−)-pallidine, all known natural products. This addition of the (S)-configured compounds would produce seven alkaloids in the recombinant system; only one product (salutaridine) is the morphine precursor. All eight phenol-coupled products derived from (R,S)-reticuline yield the fragment ions m/z 297 and 265 upon mass fragmentation (supplemental Figs. S6 and S7). Eight different structures in different concentrations will result in the recombinant yeast strain; the yield of salutaridine will be ∼0.2%. Although the low and inherent concentration of salutaridine generated by P450 2D6 and P450 3A4 is low for a biotechnological production of morphine, it is expected to be sufficient to generate the ∼10 nm concentration of morphine (via salutaridine) in human cells (35) and mouse cerebellum (36).
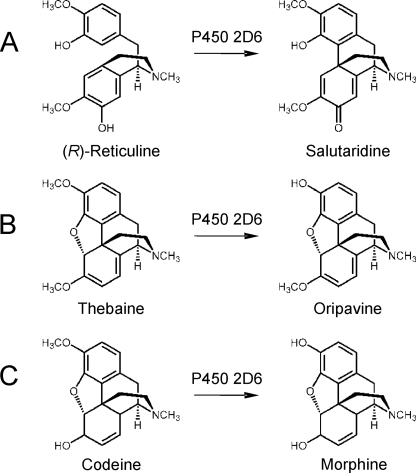
The phenol-coupling reaction of (R)-reticuline to salutaridine is the key reaction in the biosynthesis of morphine in plants and animals (Fig. 5). The enzyme in endogenous morphine biosynthesis in mammals (P450 2D6) is unique (Fig. 6) in that it catalyzes the 3-O-demethylation of codeine to morphine (26), the 3-O-demethylation of thebaine to oripavine (25), and the phenol coupling of (R)-reticuline to salutaridine (this work). A minor reaction, the oxidation of tyramine to dopamine that is also catalyzed by P450 2D6 (23, 24), does not seem to play a role in endogenous morphine biosynthesis (39). These different reaction types, which are catalyzed by one enzyme in morphine biosynthesis, are rather unique in nature.
FIGURE 6.
Human P450 2D6 is involved in three reactions in the biosynthesis of morphine. A, phenol coupling of (R)-reticuline to salutaridine; B, 3-O-demethylation of thebaine to oripavine; C, 3-O-demethylation of codeine to morphine.
Supplementary Material
Acknowledgments
We thank Dr. F. Lottspeich, Max Planck Institute for Biochemistry, Martinsried, Germany, for sequencing the porcine P450 (done on November 20, 1992) and recognizing the homology to human P450 2D6. We also thank Dr. André Cavé, University Paris-Sud, Dr. Shoei-Sheng Lee, National Taiwan University, and Dr. F. Bracher, Ludwig-Maximilians-University Munich, for their generous gift of the alkaloids isoboldine, pallidine, and sinoacutine. Finally we thank Dr. Leslie Hicks and Dr. Sophie Alvarez, Donald Danforth Plant Science Center, for sequencing the commercially available P450 2D6. Funding for the QTRAP LC-MS was provided by NSF-MRI Grant DBI-0521250.
This work was supported, in whole or in part, by National Institutes of Health Grants R21 DA0224418 and R37 CA090426 and the Deutsche Forschungsgemeinschaft.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- P450
- cytochrome P450
- EPI
- enhanced product ion
- LC
- liquid chromatography
- MRM
- multiple reaction monitoring
- MS
- mass spectrometry
- TLC
- thin layer chromatography.
REFERENCES
- 1.Coon M. J. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 1–25 [DOI] [PubMed] [Google Scholar]
- 2.Stadler R., Zenk M. H. (1993) J. Biol. Chem. 268, 823–831 [PubMed] [Google Scholar]
- 3.Kraus P. F., Kutchan T. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2071–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasreen A., Rueffer M., Zenk M. H. (1996) Tetrahedron Lett. 37, 8161–8164 [Google Scholar]
- 5.Meier U. H., Zenk M. H. (1997) Tetrahedron Lett. 38, 7357–7360 [Google Scholar]
- 6.Eichhorn J., Takada T., Kita Y., Zenk M. H. (1998) Phytochemistry 49, 1037–1047 [Google Scholar]
- 7.Ikezawa N., Iwasa K., Sato F. (2008) J. Biol. Chem. 283, 8810–8821 [DOI] [PubMed] [Google Scholar]
- 8.Zenk M. H. (1994) Pure Appl. Chem. 66, 2023–2028 [Google Scholar]
- 9.Rueffer M., Zenk M. H. (1987) Tetrahedron Lett. 28, 5307–5310 [Google Scholar]
- 10.Rueffer M., Zenk M. H. (1998) FEBS Lett. 438, 111–113 [DOI] [PubMed] [Google Scholar]
- 11.Sagner S., Shen Z.-W., Deus-Neumann B., Zenk M. H. (1998) Phytochemistry 47, 375–387 [DOI] [PubMed] [Google Scholar]
- 12.Barton D. H., Cohen T. (1957) Some Biogenetic Aspects of Phenol Oxidation. “Festschrift Prof. Dr. Arthur Stoll,”Birkhäuser Verlag, Basel [Google Scholar]
- 13.Zenk M. H., Gerardy R., Stadler R. (1989) J. Chem. Soc. Chem. Commun. 22, 1725–1727 [Google Scholar]
- 14.Gerardy R., Zenk M. H. (1993) Phytochemistry 32, 79–86 [Google Scholar]
- 15.Weitz C. J., Faull K. F., Goldstein A. (1987) Nature 330, 674–677 [DOI] [PubMed] [Google Scholar]
- 16.Donnerer J., Oka K., Brossi A., Rice K. C., Spector S. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4566–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amann T., Zenk M. H. (1991) Tetrahedron Lett. 32, 3675–3678 [Google Scholar]
- 18.Amann T., Roos P. H., Huh H., Zenk M. H. (1995) Heterocycles 40, 425–440 [Google Scholar]
- 19.Hanna I. H., Krauser J. A., Cai H., Kim M. S., Guengerich F. P. (2001) J. Biol. Chem. 276, 39553–39561 [DOI] [PubMed] [Google Scholar]
- 20.Kametani T., Ohta Y., Takemura M., Ihara M., Fukumoto K. (1977) Bioorg. Chem. 6, 249–256 [Google Scholar]
- 21.Kametani T., Kanaya N., Ohta Y., Ihara M. (1980) Heterocycles 14, 963–970 [Google Scholar]
- 22.Raith K., Neubert R., Poeaknapo C., Boettcher C., Zenk M. H., Schmidt J. (2003) J. Am. Soc. Mass Spectrom. 14, 1262–1269 [DOI] [PubMed] [Google Scholar]
- 23.Guengerich F. P., Miller G. P., Hanna I. H., Sato H., Martin M. V. (2002) J. Biol. Chem. 277, 33711–33719 [DOI] [PubMed] [Google Scholar]
- 24.Hiroi T., Imaoka S., Funae Y. (1998) Biochem. Biophys. Res. Commun. 249, 838–843 [DOI] [PubMed] [Google Scholar]
- 25.Mikus G., Somogyi A. A., Bochner F., Eichelbaum M. (1991) Xenobiotica 21, 1501–1509 [DOI] [PubMed] [Google Scholar]
- 26.Dayer P., Desmeules J., Leemann T., Striberni R. (1988) Biochem. Biophys. Res. Commun. 152, 411–416 [DOI] [PubMed] [Google Scholar]
- 27.Yue Q. Y., Säwe J. (1997) Eur. J. Clin. Pharmacol. 52, 41–47 [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki H., Nakamura M., Komatsu T., Ohyama K., Hatanaka N., Asahi S., Shimada N., Guengerich F. P., Shimada T., Nakajima M., Yokoi T. (2002) Protein Expr. Purif. 24, 329–337 [DOI] [PubMed] [Google Scholar]
- 29.Belin P., Le, Du M. H., Fielding A., Lequin O., Jacquet M., Charbonnier J. B., Lecoq A., Thai R., Courǫn M., Masson C., Dugave C., Genet R., Pernodet J. L., Gondry M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7426–7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegle I., Fritz P., Eckhardt K., Zanger U. M., Eichelbaum M. (2001) Pharmacogenetics 11, 237–245 [DOI] [PubMed] [Google Scholar]
- 31.Agarwal V., Kommaddi R. P., Valli K., Ryder D., Hyde T. M., Kleinman J. E., Strobel H. W., Ravindranath V. (2008) PLoS One 3, e2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guengerich F. P. (2005) in Cytochrome P450: Structure, Mechanism, and Biochemistry, (Ortiz de Montellano P. R. ed) 3rd Ed., pp. 377–530, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 33.Kodaira H., Spector S. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 1267–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boettcher C., Fellermeier M., Boettcher C., Dräger B., Zenk M. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8495–9500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poeaknapo C., Schmidt J., Brandsch M., Dräger B., Zenk M. H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14091–14096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller A., Glattard E., Taleb O., Kemmel V., Laux A., Miehe M., Delalande F., Roussell G., Van Dorsselaer A., Metz-Boutigue M. H., Aunis D., Goumon Y. (2008) PLoS One 3, e1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu W., Cadet P., Baggerman G., Mantione K. J., Stefano G. B. (2005) J. Immunol. 175, 7357–7362 [DOI] [PubMed] [Google Scholar]
- 38.Hawkins K. M., Smolke C. D. (2008) Nat. Chem. Biol. 4, 564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boettcher C., Fischer W., Zenk M. H. (2006) J. Immunol. 176, 5703–5704 [DOI] [PubMed] [Google Scholar]
- 40.Oscarson M., Hidestrand M., Johansson I., Ingelman-Sundberg M. (1997) Mol. Pharmacol. 52, 1034–1040 [DOI] [PubMed] [Google Scholar]
- 41.Yu A., Kneller B. M., Rettie A. E., Haining R. L. (2002) J. Pharmacol. Exp. Ther. 303, 1291–1300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.