Abstract
Terminally differentiated neurons are unable to reenter the cell cycle. Aberrant cell cycle activation provokes neuronal cell death, whereas cell cycle inhibition elevates neuronal survival. However, the molecular mechanism regulating the cell cycle and cell death in mature neurons remains elusive. Here we show that SRPK2, a protein kinase specific for the serine/arginine (SR) family of splicing factors, triggers cell cycle progression in neurons and induces apoptosis through regulation of nuclear cyclin D1. Akt phosphorylates SRPK2 on Thr-492 and promotes its nuclear translocation leading to cyclin D1 up-regulation, cell cycle reentry, and neuronal apoptosis. In addition, SRPK2 phosphorylates SC35 and, thus, inactivates p53, resulting in cyclin D1 up-regulation. 14-3-3 binding to SRPK2, regulated by Akt phosphorylation, inhibits these events. We find that SRPK2 is phosphorylated in ischemia-attacked brain, correlating with the observed increase in cyclin D1 levels. Hence, phosphatidylinositol 3-kinase/Akt mediates the cell cycle and cell death machinery in the nervous system through phosphorylation of SRPK2.
In the central nervous system the nascent neuroblasts leave ventricular zone or subventricular zone and migrate to the destination where they differentiate and become permanently post-mitotic cells (1). It is well established that terminally differentiated neurons are unable to reenter the cell cycle, but accumulating evidence has demonstrated the up-regulation of cell cycle regulatory proteins in degenerating neurons of Alzheimer disease (AD)2 brain (2). Elevated levels of Cdc2, Cdk4, p16, Ki-67, cyclin B1, and cyclin D have been found in pathologically affected or vulnerable neurons in AD (3–7). Moreover, several of these regulators have been observed in vulnerable neurons before lesion formation (8). Together these findings suggest that the activity of certain cell cycle regulators plays a critical upstream role in the AD neurodegenerative process. Greene and co-workers (9–12) have shown that drugs that block cell cycle advances are efficient in preventing the death of PC12 cells as well as sympathetic neurons. Dominant negative forms of the Cdk4 and Cdk6 preventing the cell death induced by camptothecin (a topoisomerase inhibitor) are effectively blocked by the G1/S blockers, such as deferoxamine and mimosine, as well as by the cyclin-dependent kinases (Cdk) inhibitors flavopiridol and olomoucine. In addition, they show that neurons treated with DNA-damaging agents such as UV irradiation or camptothecin also require cyclin D and Cdk4/6 activity to induce neuronal death (13). Thus, this evidence supports that mature neurons might retain certain elements of the cell cycle and have the capability of reactivating additional aspects of the replication mechanism when under stresses. Any events that force a mature neuron back into the cell cycle are lethal rather than mitogenic for the neuron.
SRPK, a family of cell cycle-regulated protein kinases, phosphorylate serine/arginine (SR) domain-containing proteins in nuclear speckles and mediate the pre-mRNA splicing. SRPK1 and SRPK2 are highly specific kinases for the SR family of splicing factors. SRPK1 is predominantly expressed in pancreas, whereas SRPK2 is highly expressed in brain, although both are coexpressed in other human tissues and in many experimental cell lines (14). SRPK1 was originally identified as a kinase of SC35, a key pre-mRNA splicing factor in the nuclear speckles, in the extracts from HeLa cells (15, 16). The addition of purified SRPK1 to permeabilized cells or overexpression of SRPK1 in transfected cells results in an apparent disassembly of the nuclear speckles (15). These results suggest that phosphorylation or hyperphosphorylation causes release of these factors from the speckles or perhaps that the integrity of these structures is compromised. SRPK1 and -2 are 92% identical. SRPK2 distinguishes from SRPK1 in the middle portion of the protein, which has an acidic domain insertion (17). Both SRPK1 and SRPK2 are primarily localized in the cytoplasm despite having putative nuclear localization signals (17). Most recently we show that SRPK2 binds and phosphorylates acinus, an SR protein essential for RNA splicing, and redistributes it from the nuclear speckles to the nucleoplasm, resulting in cyclin A1 but not A2 up-regulation (18). Ablation of acinus or SRPK2 abrogates cyclin A1 expression in leukemia cells and arrests cells at the G1 phase. Acinus or SRPK2 overexpression increases leukemia cell proliferation. Furthermore, both SRPK2 and acinus are highly expressed in some of human AML patients and correlate with elevated cyclin A1 expression levels, fitting with the oncogenic activity of cyclin A1 in leukemia. Thus, our findings establish a molecular mechanism by which SR splicing machinery regulates cell cycle and contributes to leukemia tumorigenesis.
The 14-3-3 proteins are a family of phosphoserine/phosphothreonine binding molecules that control the function of a wide array of cellular proteins and promote cell survival. 14-3-3 binds the client proteins through an amphipathic binding cleft that preferentially recognizes the phosphorylated motifs RSXpSXP or RXXXpSXP (19), which share a common region in the consensus Akt phosphorylation elements preserved in numerous Akt substrates. Therefore, 14-3-3 proteins frequently interact with a variety of Akt substrates and regulate diverse biological processes including signal transduction, cell cycle control, and apoptosis (20). For instance, Akt phosphorylates the proapoptotic Bcl-2 family member BAD and provokes its binding to 14-3-3, thereby inhibiting BAD pro-apoptotic functions (21, 22). Akt also controls a major class of transcription factors (the Forkhead box transcription factor by phosphorylating FOXOs (Forkhead box, group O)) and inhibiting their ability to induce the expression of death genes (23–25). In the absence of survival factors, when Akt is inactive, FOXOs are localized in the nucleus and activate gene transcription. In the presence of survival factors, Akt becomes activated and phosphorylates FOXOs at several regulatory sites, leading to the FOXO association with 14-3-3 proteins, cytoplasmic retention, and down-regulation of apoptotic gene transcription. In this report we show that Akt phosphorylates SRPK2 and elicits its binding to 14-3-3. Akt phosphorylation substantially enhances SRPK2 stimulatory activity in triggering cyclin D1 expression and promoting apoptosis in neurons, which can be blocked by 14-3-3. We show that SRPK2 enhances cyclin D1 expression through phosphorylating SC35 and suppressing p53. Interestingly, phosphorylated SRPK2 tightly couples to cyclin D1 expression in vulnerable neurons.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HEK293, HeLa, mouse embryonic fibroblast cells were maintained in Dulbecco's modified Eagle's medium including 10% fetal bovine serum and 100 units of penicillin-streptomycin. All cells were maintained at 37 °C with 5% CO2 atmosphere in a humidified incubator. EGF was from Roche Applied Science. Myc antibody, Cdk2, and Cdk4 inhibitors were from Calbiochem. Wortmannin, LY294002, GF109203X, and GST-horseradish peroxidase were from Sigma. Anti-phospho-Akt Ser-473, anti-p27, anti-p53, phosphor-p53, and phospho-Rb antibodies were from Cell Signaling. Akt (sc5298), Cdk4, cyclin D1, and green fluorescent antibodies were from Santa Cruz. Anti-p21 antibody was form BD Pharmingen. BrdUrd antibody was from Abcam. Active Akt protein was from Upstate Biotechnology, Inc. Akt knock-out mouse embryonic fibroblast cells were gifts from Dr. Morris Birnbaum at University of Pennsylvania. Cdk2 inhibitor II (compound 3) and Cdk4 inhibitor were from Calbiochem. All the chemicals not included above were from Sigma.
In Vitro Kinase Assay
Purified GST fusion proteins were incubated with various His-SRPK2 purified protein in 20 μl of kinase reaction buffer (20 mm Tris, pH 7.5, with 10 mm MgCl2) containing 25 μm ATP and 2.5 μCi of [γ-32P]ATP for 20 min at 30 °C. Reactions were terminated by adding 7 μl of Laemmli sample buffer and boiling for 5 min. A portion of the sample (15 μl) was separated on a SDS-polyacrylamide gel and autoradiographed or analyzed by phosphorimaging analysis.
Coimmunoprecipitation and in Vitro Binding Assay
A 10-cm plate of transfected HEK293 cells was washed once in PBS and lysed in 1 ml of lysis buffer (50 mm Tris, pH 7.4, 40 mm NaCl, 1 mm EDTA, 0.5% Triton X-100, 1.5 mm Na3VO4, 50 mm NaF, 10 mm sodium pyrophosphate, 10 mm sodium β-glycerophosphate, protease inhibitor mixture) and centrifuged for 10 min at 16,000 × g at 4 °C. The supernatant were transferred to a fresh tube and mixed with a variety of antibody. After SDS-PAGE, the samples were transferred to a nitrocellulose membrane. Western blotting analysis was performed with a variety of antibodies.
Fluorescence Microscope Analysis
Cells were split onto coverslips in a 12-well plate. After overnight culture, cells were transfected and incubated for 36 h. Cells were fixed by 3.7% paraformaldehyde in PBS for 15 min at room temperature. The cells were then permeabilized and blocked by 0.2% Triton X-100 and 2% fetal bovine serum in PBS at room temperature for 5 min, washed with PBS three times, and treated with several antibodies. Fluorescent images were taken by Zeiss Axioplan 2 microscope equipped with a confocal laser scanning unit (Carl Zeiss LSM510 META).
Genomic DNA Fragmentation
Oligonucleosomal fragmentation of genomic DNA was determined as described below. In brief, after transfection or infection, the cells were incubated with 50 μm VP16 or 1 μm staurosporine for 16 h. At the end of incubation, cells were pelleted, washed twice with ice-cold PBS, and lysed at 37 °C for 2 h in 500 μl of lysis buffer (100 mm Tris/HCl, pH 8.5, 5 mm EDTA, 200 mm NaCl, 0.2% SDS, and 0.2 mg/ml proteinase K). Samples were treated with phenol/chloroform and then centrifuged, and the supernatants were treated with isopropyl alcohol precipitation. This pellet was dissolved in Tris-EDTA buffer containing RNase A (10 mg/ml) at 37 °C for 2 h, then the same amount DNA (10 μg) was electrophoresed at 100 V for 1 h through a 2% agarose gel containing ethidium bromide in TAE buffer (0.04 m Tris acetate, 0.001 M EDTA, pH 8.0). DNA bands were visualized under UV light.
In Vivo Labeling with [32P]Orthophosphate
293 cells were cultured and transfected as described above. Two days after transfection, the cells were washed with phosphate-free Dulbecco's modified Eagle's medium and preincubated for 90 min. The cells were incubated for 4 h with the same medium containing [32P]orthophosphate (PerkinElmer Life Sciences). Labeling was stopped by washing the cells several times with ice-cold PBS. The cells were lysed in lysis buffer. Mammalian GST-SC35 was purified from cell extracts by glutathione-Sepharose 4B. Mammalian GST-SC35 beads complex were separated on a SDS-polyacrylamide gel and autoradiographed.
Primary Rat Cortical Neuron Culture and Apoptotic Assay
Primary cultured rat cortical neurons were prepared as follows. E17 rat pups were decapitated, and the cortices were extirpated, cross-chopped, and suspended by pipetting for separation in 5% fetal calf serum, 5% horse serum, and Dulbecco's modified Eagle's medium gently. The cell suspension was then centrifuged at 250 × g for 5 min. This operation was repeated again. Cells were seeded into polyethyleneimine-coated 10-cm dishes and 12-well plates including coated coverslips and incubated at 37 °C in 5% CO2, 95% air. After 3 h culture medium was changed to Neurobasal containing B-27 supplement (Invitrogen) and incubated for 4 days. For maintenance, a half-medium was changed to fresh Neurobasal/B27 every 4 days. After 1 week the dished-cultured neurons are employed in various experiments. The neuronal apoptosis was analyzed as described (26).
BrdUrd Incorporation Assay
Cortical neurons were seeded into 6-well plates at 1 × 104 cells/well, cultured for 7 days (7 days in vitro), and infected with various lentivirus expressing wild-type and mutated SRPK2. Infected neurons were grown in 20 μm BrdUrd including medium for 2 h and fixed by 3% formaldehyde in PBS. After washing with PBS, neurons were treated with prewarmed 2 m HCl for 15 min at 37 °C and neutralized by 0.1 m boric acid (pH 8.4) for 15 min at 37 °C twice. Then neurons were blocked by 2% fetal bovine serum/PBS and stained by anti-BrdUrd (1:50) and anti-MAP2 (1:200) antibody. Finally, neurons were counterstained with 4,6-diamidino-2-phenylindole to visualize the nuclei. Fluorescent images were taken by Zeiss Axioplan 2 microscope equipped with a confocal laser scanning unit (Carl Zeiss LSM510 META).
Stroke Experiment
The middle cerebral artery occlusion-induced stroke in 2–3-month-old male mice was performed as previously reported (27). Six wild-type mice were anesthetized with 4% chloral hydrate. The rectal and masseter muscle temperatures were controlled at 37 °C with a homeothermic blanket. Cerebral perfusion (CP) in the distribution of the middle cerebral artery was monitored throughout the surgical procedure with a laser Doppler (Perimed Inc.), and only animals with a >80% decrease in CP were included in this study.
Statistical Analysis
Continuous variables are expressed as the means ± S.D. Means of multiple treatment groups were compared with controls by using of analysis of variance with a post hoc test. A p value of p < 0.05 (*) and p < 0.001 (**) was considered statistically significant.
RESULTS
Akt Phosphorylates SRPK2 on the Thr-492 Residue
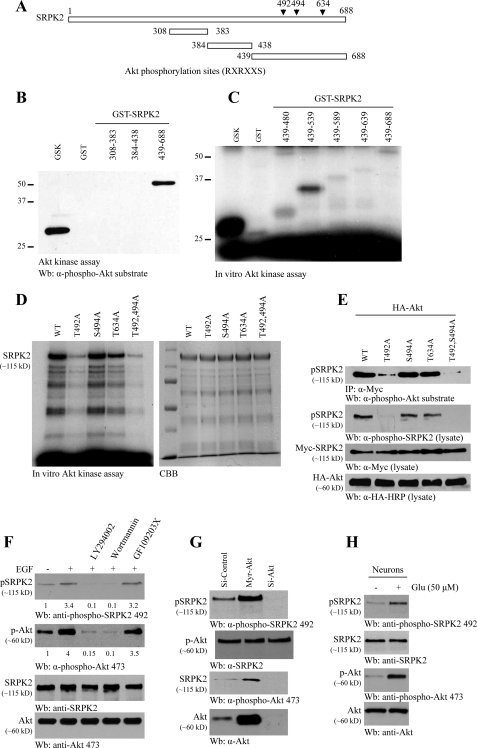
In exploring the sequence of SRPK2, we noticed that amino acid sequences in the C terminus correspond to a motif that is identified as a consensus Akt phosphorylation element (RXRXX(S/T)) present in numerous Akt substrates. To test whether Akt can phosphorylate SRPK2, we performed an in vitro kinase assay with purified SRPK2 fragments. The C-terminal fragment (amino acids 439–688) and positive control glycogen synthase kinase were robustly phosphorylated, whereas other fragments were not, suggesting that SRPK2 might be a substrate of Akt (Fig. 1, A and B). To search for the specific phosphorylation residue on SRPK2, we conducted a mapping assay with a variety of C-terminal truncates. Kinase assay revealed that fragment 439–539 was potently phosphorylated, indicating that this region might contain the major phosphorylation site (Fig. 1C). The putative Akt phosphorylation site (amino acids 487–492 HDRSRT) locates within this phosphorylation region. Mutation of Thr-492 into Ala substantially abrogated SRPK2 phosphorylation by Akt, whereas mutation of Ser-494 or Thr-634 into Ala failed to do so (Fig. 1D). The remnant SRPK2 phosphorylation by Akt in T492A mutants indicates that it might contain other minor Akt phosphorylation sites. To explore whether SRPK2 can be phosphorylated by Akt in intact cells, we cotransfected HA-Akt into HEK293 cells with Myc-tagged wild-type and mutated SRPK2. The immunoprecipitated SRPK2 was monitored with anti-phospho-Akt substrate antibody. In alignment with in vitro Akt phosphorylation, we observed that T492A mutation evidently abolished SRPK2 phosphorylation (Fig. 1E, top panel). To determine whether SRPK2 is the physiological substrate of Akt, we generated phospho-Thr-492-specific antibody. SRPK2 phosphorylation tightly correlated with anti-phospho-Akt substrate antibody results (Fig. 1E, second panel). These results suggest that Thr-492 might be the major phosphorylation residue. Thr-492 locates in the variable spacer domain between the N- and C-terminal kinase domains and is not conserved in SRPK1. Therefore, SRPK phosphorylation by Akt is specific for SRPK2.
FIGURE 1.
Akt phosphorylates SRPK2 on Thr-492 in vitro and in vivo. A, diagram of SRPK2. SRPK2 has three putative Akt phosphorylation motifs (RXRXX(S/T)) as indicated (▾). B, C, and D, in vitro Akt kinase assay. Purified recombinant GST fusion proteins were incubated with active Akt and monitored by immunoblotting with anti-phospho-Akt substrate antibody. Fragment 439–688 was robustly phosphorylated, whereas other fragments were not. B, Akt kinase assay. In vitro kinase assay with various SRPK2 fragments and active Akt in the presence of [γ-32P]ATP. The radiolabeled SRPK2 fragments were resolved on SDS-PAGE. Fragment 439–539 displayed the strongest phosphorylation activity. C and D, Thr-492 residue in SRPK2 is phosphorylated by Akt. Wild type (WT), T494A, and T634A but not T492A mutant were strongly phosphorylated (left panel). An equal amount of His proteins was employed (right panel). E, phospho-Thr-492 antibody selectively recognizes phosphorylated SRPK2. Although Thr-492 site was markedly phosphorylated in wild type, S494A, and T634A, no Thr-492 phosphorylation was detected in T492A or T492AS494A mutant (second panel). Expression of Myc-SRPK2 WT, mutants, and Akt was verified (third and fourth panels). Immunoblotting with phospho-Akt substrate antibody verified phospho-Thr-492 antibody results (top panel). HRP, horseradish peroxidase; IP, immunoprecipitate. F, PI 3-kinase inhibitors block SRPK2 phosphorylation on Thr-492. HEK293 cells were pretreated with 20 nm wortmannin, 10 μm LY294002, or 2 μm GF109203X for 30 min. The cells were then treated with EGF for 10 min. EGF stimulated SRPK2 phosphorylation, which was diminished by PI 3-kinase but not protein kinase C inhibitor pretreatment (top panel). Akt phosphorylation was verified (second panels). The relative signal strength of the blots was labeled underneath the blots. G, Akt is required for SRPK2 phosphorylation on Thr-492. HEK293 cells were infected with adenovirus expressing active myristoylated Akt or short hairpin RNA of Akt1. Myristoylated Akt provoked strong phosphorylation in endogenous SRPK2, and depletion of Akt abolished SRPK2 phosphorylation (top panel). Verification of Akt and SRPK2 expression (second and fourth panels). Akt phosphorylation was verified (third panel). H, glutamate triggers both Akt and SRPK2 phosphorylation in cortical neurons.
To explore whether Akt mediates SRPK2 phosphorylation in intact cells, we pretreated HEK293 cells with various pharmacological inhibitors followed by EGF stimulation. Inhibition of Akt by PI 3-kinase inhibitors Wortmannin and LY293002 blocked SRPK2 phosphorylation on Thr-492, whereas protein kinase C inhibitor GF109203X failed to suppress SRPK2 phosphorylation (Fig. 1F, top panel), suggesting that Akt dictates SRPK2 phosphorylation in intact cells. Overexpression of Akt increased SRPK2 phosphorylation, whereas depletion of Akt abolished endogenous SRPK2 Thr-492 phosphorylation (Fig. 1G). In cortical neurons glutamate treatment elicited significant Akt activation. Concomitantly, SRPK2 was also markedly phosphorylated (Fig. 1H). Taken together, these results demonstrate that SRPK2 is a physiological substrate of Akt which phosphorylates SRPK2 on the Thr-492 residue.
14-3-3 Interacts with Akt-phosphorylated SRPK2 and Blocks Its Nuclear Translocation
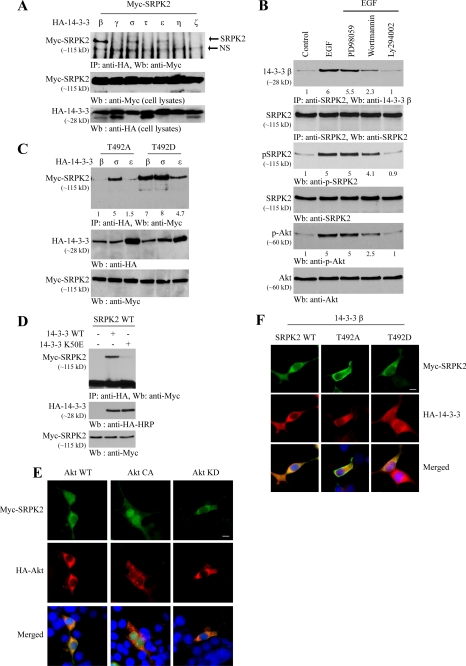
In addition to phosphorylating SRPK2, Akt also tightly interacts with SRPK2 in a phosphorylation-dependent manner, for which the N-terminal PH domain and C-terminal regulatory domain in Akt are implicated in the association (supplemental Fig. 1). Akt substrates often form a complex with 14-3-3. To examine whether SRPK2 also binds 14-3-3, we conducted a coimmunoprecipitation assay with Myc-SRPK2 and various 14-3-3 isoforms. SRPK2 selectively interacted with β, σ, and ϵ isoforms but not any other isoforms. Noticeably, the binding affinity by SRPK2 to the β isoform was the strongest (Fig. 2A). To investigate whether Akt phosphorylation plays any role in the association between 14-3-3 and SRPK2, we pretreated cortical neurons with PI 3-kinase inhibitors or MEK1 inhibitor (PD98059) followed by EGF treatment. EGF strongly promoted the binding by endogenous SRPK2 to 14-3-3β, which was substantially attenuated by PI 3-kinase inhibitors but not by MEK1 inhibitor (Fig. 2B, top panel). Akt activation status tightly coupled to SRPK2 phosphorylation activity, in alignment with the binding activities by SRPK2 to14-3-3β (Fig. 2B, third and fifth panels), indicated that Akt phosphorylation of SRPK2 is critical for its binding to 14-3-3. To further test this notion, we cotransfected SRPK2 T492D, a phosphorylation mimetic mutant, and T492A, an unphosphorylatable mutant, into HEK293 cells with various 14-3-3 isoforms. A coimmunoprecipitation study demonstrated that T492D strongly interacted with all three isoforms, whereas β and ϵ isoforms lost the affinity to T492A, indicating that Akt phosphorylation selectively regulates the binding by SRPK2 to both β and ϵ isoforms (Fig. 2C). Interestingly, σ isoform robustly interacted with both T492D and T492A SRPK2 mutants, suggesting that this action might be independent of Akt phosphorylation. K50E mutation disrupts the interaction by 14-3-3 to various binding targets (20). We also found that the association by SRPK2 to14-3-3β was abolished by this mutation (Fig. 2D), supporting that the interaction between SRPK2 and 14-3-3 is specific.
FIGURE 2.
14-3-3 interacts with Akt-phosphorylated SRPK2 and inhibits its nuclear translocation. A, SRPK2 binds to 14-3-3. HEK293 cells were transfected with Myc-SRPK2 and a variety of HA-14-3-3 isoforms. 14-3-3 was immunoprecipitated using HA antibody and was detected with Myc antibody (top panel). Expression of Myc-SRPK2 and HA-14-3-3s was verified (middle and bottom panels). IP, immunoprecipitate; NS, nonspecific. B, PI 3-kinase signaling regulates the interaction between endogenous SRPK2 and 14-3-3β. HEK293 cells were pretreated with PD98059, wortmannin, LY294002 for 30 min before EGF was introduced. Endogenous SRPK2 was immunoprecipitated and analyzed using anti-14-3-3β-specific antibody. The relative signal strength is labeled underneath of each blot. Equal amounts of SRPK2 were immunoprecipitated (second panel). Confirmation of Akt and SRPK2 phosphorylation status (third and fifth panels). C, SRPK2 binding to 14-3-3 is Akt phosphorylation-dependent. Myc-SRPK2 T492A and T492D were cotransfected into 293 cells with HA-14-3-3β, -σ, and -ϵ isoforms. 14-3-3 was immunoprecipitated using HA antibody and detected with Myc antibody. 14-3-3σ bound to both SRPK2 T492A and T492D proteins.14-3-3β and -ϵ isoforms selectively bound to SRPK2/T492D but not T492A (top panel). The expression of Myc-SRPK2 and HA-14-3-3s were examined (middle and bottom panels). D, 14-3-3 K50E mutant fails to interact with SRPK2. SRPK2 construct alone or together with HA-14-3-3 wild type or K50E mutant was transfected into HEK293 cells. 14-3-3 was immunoprecipitated using HA antibody and detected using Myc antibody (top panel). The expression of Myc-SRPK2, HA-14-3-3 wild type and mutant was examined (middle and bottom panels). E, Akt phosphorylation regulates SRPK2 subcellular location. Various HA-Akt constructs (WT, CA (constitutively active), KD) were cotransfected with SRPK2 wild type into HEK293 cells and monitored by immunofluorescent staining. F, effects of 14-3-3 on intracellular localization of SRPK2. Myc-SRPK2 wild type, T492A, and T492D were cotransfected with HA-14-3-3β into 293 cells, and indirect immunofluorescent staining was performed. Cotransfection with HA-14-3-3 inhibited SRPK2 T492D from translocating into the nucleus. The experiments described in E and F have been done three times. In total, 100 cells in E and 150 cells in F, were counted.
SRPK2 predominantly localizes in the cytoplasm, but it is also detectable in the nucleus as well (14). To explore whether Akt phosphorylation regulates its subcellular localization, we conducted immunofluorescent staining with Akt and SRPK2-cotransfected cells. SRPK2 occurred in the nucleus of some wild-type Akt-cotransfected cells, whereas it exclusively resided in the cytoplasm in the kinase-dead Akt-KD-cotransfected cells. Interestingly, SRPK2 completely distributed in the nucleus in constitutively active Akt-CA-cotransfected cells (Fig. 2E). These results suggest that Akt phosphorylation promotes SRPK2 nuclear translocation and SRPK2 phosphorylation is required for its nuclear residency. We made a similar observation with endogenous SRPK2 as well (supplemental Fig. 2). To further assess the effect of Akt phosphorylation on this event, we employed T492D and T492A mutants. Immunostaining showed that wild-type SRPK2 localized in both the cytoplasm and the nucleus, whereas kinase-dead SRPK2 (K110A) exclusively distributed in the cytoplasm. As expected, T492D evidently resided in the nucleus; in contrast, T492A mainly resided in the cytoplasm (Fig. 2F). To determine whether 14-3-3 plays any role in this event, we cotransfected 14-3-3β with various SRPK2 constructs into HEK293 cells. Both wild-type SRPK2 and T492A mutant predominantly localized in the cytoplasm of the cotransfected cells. Strikingly, cotransfection of 14-3-3 inhibited SRPK2 T492D from translocating into the nucleus (Fig. 2F), indicating that overexpressed 14-3-3 might sequestrate Akt-phosphorylated SRPK2 in the cytoplasm. Together, these data demonstrate that 14-3-3 binds SRPK2, which is regulated by Akt phosphorylation. Akt phosphorylation also provokes SRPK2 nuclear translocation that is blocked by 14-3-3.
14-3-3 Inhibits SRPK2-provoked Cell Cycle Progression in Neurons
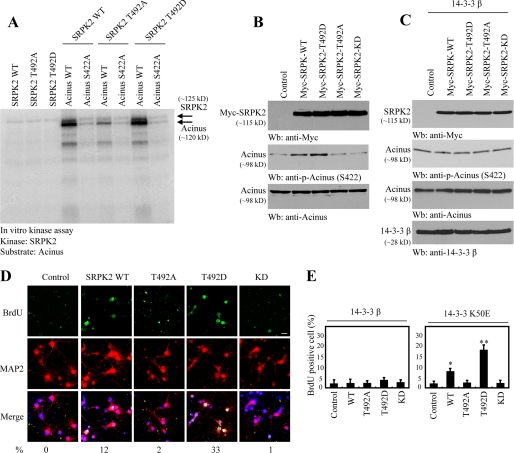
Our recent study demonstrates that acinus is a physiological substrate of SRPK2, which phosphorylates acinus on Ser-422 (18). To assess the effect of Akt phosphorylation on SRPK2 kinase activity toward acinus, we conducted an in vitro kinase assay using purified acinus protein as a substrate. Wild-type SRPK2 strongly phosphorylated wild-type acinus, which was substantially diminished in acinus S422A mutant. Interestingly, T492D, Akt phosphorylation mimetic mutant, exhibited a stronger kinase activity than wild-type SRPK2. By contrast, the kinase activity was significantly abrogated in SRPK2 T492A (Fig. 3A). SRPK2 KD failed to phosphorylate acinus (data not shown). Immunoblotting analysis showed that endogenous acinus was robustly phosphorylated in SRPK2 wild-type-infected neurons. The strongest effect occurred to T492D. In contrast, acinus phosphorylation was negligible in T492A, SRPK2-KD, and control cells (Fig. 3B), supporting that Akt phosphorylation is required for SRPK2 to actively phosphorylate acinus in intact cells. Surprisingly, coexpression of 14-3-3β completely abolished acinus Ser-422 phosphorylation by SRPK2 (Fig. 3C). Presumably, 14-3-3β blocks SRPK2 nuclear translocation, leading to inhibition of acinus phosphorylation by SRPK2.
FIGURE 3.
14-3-3 inhibits the biological activities of SRPK2. A, in vitro SRPK2 kinase assay. Purified His-SRPK2 recombinant proteins were incubated with acinus wild type and acinus S422A in the presence of [γ-32P]ATP. Wild-type SRPK2 and T492D strongly phosphorylated wild-type acinus and weakly phosphorylated S422A. By contrast, the kinase activity was significantly decreased in SRPK2 T492A. B, Akt-phosphorylated SRPK2 strongly phosphorylates acinus in cells. Lysates prepared from cortical neurons were infected with various lentiviruses encoding various SRPK2 proteins and analyzed by immunoblotting with various antibodies. Wild type and T492D SRPK2 provoked strong phosphorylation in endogenous acinus, but T492A and KD mutants failed to phosphorylate acinus (middle panel). Verification of SRPK2 and acinus expression (top and bottom panels). C, 14-3-3β inhibits SRPK2 kinase activity. Cortical neurons were co-infected with14-3-3β, and various SRPK2 proteins and acinus phosphorylation was monitored by immunoblotting. D, SRPK2 increases BrdUrd incorporation in neurons. Cortical neurons were infected with various SRPK2 proteins expressing lentiviruses. The infection efficiency was about 80∼90%. The cortical neurons were labeled with BrdUrd and stained 4 days after infection. The numbers under the figures are BrdUrd-positive percentage. E, 14-3-3 but not K50E blocks BrdUrd incorporation in neurons by SRPK2. Quantitative analysis of BrdUrd incorporation in cortical neurons by SRPK2 in the presence of wild-type 14-3-3β and K50E. Results are expressed as the mean ± S.D. from three independent experiments. p < 0.05 () and p < 0.001 (**), multiple comparison versus control group by analysis of variance with post hoc test.
To assess whether SRPK2 impinges on the cell cycle progression in mature neurons, we infected primary cortical neuronal cultures with various SRPK2 constructs. BrdUrd incorporation assay demonstrated that T492D markedly provoked cortical neuron proliferation. A decreased but significant activity was also observed with wild-type SRPK2. In contrast, no demonstrable cell proliferative activity was detected with SRPK2-KD or T492A (Fig. 3D). Hence, SRPK2 kinase activity and Akt phosphorylation on SRPK2 are indispensable for the stimulatory effect on neuronal cell proliferation by SRPK2. Coexpression of wild-type 14-3-3β in cortical neurons strongly inhibited the BrdUrd incorporation activity by T492D and wild-type SRPK2. Remarkably, 14-3-3β K50E mutant failed to block the stimulatory activity by wild-type SRPK2 and T492D (Fig. 3E), underscoring that association is required for 14-3-3 to exert its inhibitory activity on neuronal cell proliferation activity by SRPK2.
14-3-3 Suppresses the SRPK2 Effect on Cyclin D1 Expression and Apoptosis
SRPK2 robustly provoked cyclin A1 expression in leukemia cells, and it also weakly enhanced cyclin D1 expression in other cells in addition to leukemia (18). To explore whether Akt phosphorylation and SRPK2 kinase activity play any role in provoking cyclin D1 expression, we conducted a luciferase assay with cyclin D1 promoter construct. Compared with control, SRPK2 wild type evidently elevated cyclin D1 transcription, and the catalytic activity was lost in SRPK2 KD, suggesting that kinase activity of SRPK2 is required for this effect. Noticeably, T492D exhibited the strongest activity, which was markedly impaired in T492A, indicating that Akt phosphorylation is essential for SRPK2 to trigger cyclin D1 transcription (Fig. 4A, upper panel). Coexpression of wild-type 14-3-3β dramatically abrogated the stimulatory activity by SRPK2; by contrast,14-3-3β K50E lost its inhibitory effect (Fig. 4A, lower panels), confirming that the interaction between 14-3-3 and SRPK2 is critical for 14-3-3 to block the cyclin D1 transcription activity by SRPK2.
FIGURE 4.
SRPK2 regulates cyclin D1 expression and its localization. A, SRPK2 mediates cyclin D1 promoter activity, which is regulated by Thr-492 phosphorylation. HEK293 cells were transfected various SRPK2 constructs and/or14-3-3β and 14-3-3 K50E mutant. Empty vector was used to normalize the same total amount of DNA in all experiments. Values are the means (±S.D.) of three independent experiments. SRPK2 T492A and KD mutants lost the activity, whereas T492D strongly elevated cyclin D1 promoter activity (top panel). Coexpression of14-3-3β with SRPK2 abolished SRPK2 activity (bottom left panel). The 14-3-3 K50E mutant displayed a negligible effect on SRPK2 activity (bottom right panel). B, SRPK2 mediates cyclin D1 nuclear location in cortical neurons. Cortical neurons were infected with various SRPK2 constructs. After 4 days, neurons were fixed with 3.7% paraformaldehyde and stained with anti-cyclin D1 and anti-MAP2 antibodies. SRPK2 wild type and T492D mutant strongly elevated cyclin D1 nuclear location (data represent the mean ± S.D. of three independent experiments; *, p < 0.05, multiple comparison versus control group by analysis of variance with post hoc test). C, SRPK2 controls cyclin D1 expression in cortical neurons. SRPK2 wild type, T492A, T492D, KD, and control lentivirus were co-infected with control or 14-3-3 adenovirus into cortical neurons. Reverse transcription-PCR was conducted. SRPK2 wild type and T492D mutant increased cyclin D1 expression (top left panel), whereas coexpressed 14-3-3 blocked it (top right panel). D, quantitative apoptotic assay. A variety of SRPK2 constructs were infected into cortical neurons and incubated for 4 days. The infection efficiency was about ∼80–90%. The numbers under the figures are MR(DEVD)2-positive percentage. The apoptotic neurons were analyzed with MR(DEVD)2, a fluorescent dye turning to red upon caspase-3 cleavage. E, 14-3-3 prevents SRPK2-provoked apoptosis. A variety of SRPK2 constructs were co-infected with 14-3-3 wild type and mutant into cortical neurons and incubated for 4 days. The apoptotic neurons were analyzed with MR(DEVD)2. Coexpression of14-3-3β with SRPK2 abolished its pro-apoptotic activity (left panel). The 14-3-3 mutant almost had no effect (right panel). p < 0.05 (*) and p < 0.001 (**) multiple comparison versus control group by analysis of variance with post hoc test.
Cyclin D1 becomes predominantly cytoplasmic as primary cortical progenitor cells undergo cell cycle withdrawal and terminal differentiation (28). Accumulating evidence suggests that nuclear localization of ectopic cyclin D1 induced apoptosis. For instance, DNA-damaging compound camptothecin causes nuclear accumulation of endogenous cyclin D1 accompanied by Rb phosphorylation (28). Nuclear-localized cyclin D1 is up-regulated during kainic acid-evoked death of hippocampal neurons, and the Cdk inhibitor flavopiridol blocks the delayed death of cultured neurons (13). To explore whether SRPK2 regulates cyclin D1 expression in primary neuronal cultures, we conducted an immunofluorescent co-staining with cyclin D1 and MAP2, a neuronal marker. SRPK2 wild type and T492D robustly augmented cyclin D1 expression in the infected cortical neurons. Nevertheless, SRPK2 KD and T492A displayed the similar effect on cyclin D1 expression as control. Quantitative analysis revealed that 57% of SRPK2 T492D-infected neurons contained nuclear cyclin D1, and 33% occurred to wild-type SRPK2. Approximately 10% lentivirus-infected neurons were nuclear cyclin D1-positive for control, SRPK2-KD, and T492A (Fig. 4B). The representative pictures are shown in supplemental Fig. 3. Reverse transcription-PCR analysis verified that cyclin D1 was evidently augmented in SRPK2 wild-type and T492D-infected neurons. The stimulatory effect was completely suppressed by coexpression of wild-type 14-3-3β (Fig. 4C), fitting with the results from the luciferase activity assay. To investigate whether nuclear cyclin D1 provokes apoptosis in the infected neurons, we conducted an apoptotic assay with MR(DEVD)2, a caspase-3-cleavable fluorescent dye that turns red upon cleavage. Quantitative analysis showed that 68% of cells were in apoptosis in T492D-infected neurons, and 42% occurred to wild-type SRPK2. 10.1, 5.2, and 3% apoptotic cells were detected in T492A, SRPK2-KD, and control cells, respectively (Fig. 4D). Coexpression of 14-3-3β wild-type but not K50E mutant cells substantially repressed the apoptotic activity by T492D and wild-type SRPK2 (Fig. 4E). We made the similar observation with terminal dUTP nick-end labeling staining (data not shown). Therefore, 14-3-3 antagonizes the SRPK2 stimulatory effect on cyclin D1 expression, resulting in blockage of neuronal apoptosis elicited by SRPK2.
SRPK2 Promotes Neuronal Apoptosis through Up-regulating Cyclin D1
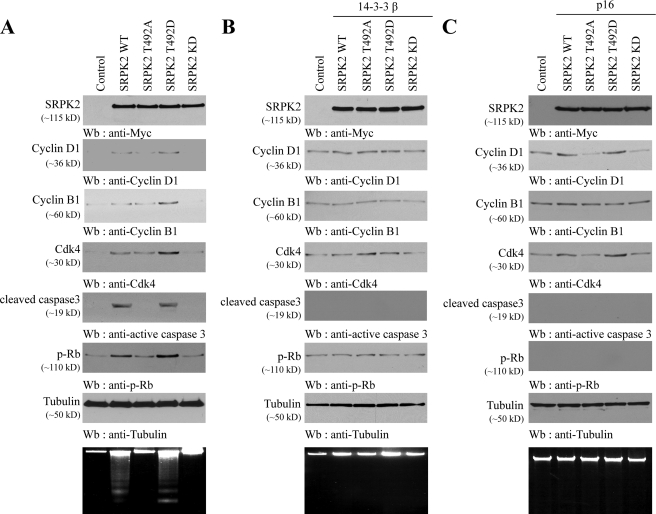
Cyclin D1 promotes G1 phase progression through forming a complex with Cdk4 and Cdk6. Previous studies demonstrate that the cyclin D1-dependent kinases are apoptotic. For example, chemical inhibitors of Cdks and dominant-negative forms of Cdk4 and -6 promote the survival of nerve growth factor-deprived sympathetic neurons (11). Cdk4/6 inhibitors prevent apoptosis of post-mitotic motoneurons (29). To determine whether SRPK2-provoked cyclin D1 is responsible for triggering apoptosis in neurons, we monitored cell cycle protein expression and caspase-3 activation in infected cortical neurons. Overexpression of T492D and wild-type SRPK2 evidently provoked cyclin D1, cyclin B1, and Cdk4 expression compared with control lentivirus, whereas SRPK2-KD failed. The elevated cyclin D1 and Cdk4 expression was accompanied by an increased Rb phosphorylation. Concomitantly, robust activation of caspase-3 and DNA fragmentation were demonstrated in these neurons. Although T492A slightly augmented cell cycle protein expression, it was unable to trigger apoptosis in infected neurons (Fig. 5A). Coinfection of 14-3-3β substantially diminished SRPK2 wild type and T492D effect on cyclin D1 and Cdk4 expression, leading to suppression of Rb phosphorylation. Consequently, neuronal apoptosis including caspase-3 activation and DNA fragmentation was markedly blocked (Fig. 5B), fitting with the pro-survival function of 14-3-3. To assess whether the SRPK2 apoptotic effect is mediated through cyclin D1-Cdk4 complex, we infected cortical neurons with INKp16, an inhibitor for cyclin D1-Cdk4 complex. As expected, SRPK2 wild type and T492D increased cyclin D1 and Cdk4 expression in cortical neurons even in the presence of p16. Nevertheless, Rb phosphorylation was completely blocked by INKp16. Consequently, cyclin B1 expression was not elevated in wild-type or T492D SRPK2-infected neurons. Accordingly, neuronal apoptosis was completely suppressed (Fig. 5C), suggesting that SRPK2-provoked cyclin D1-Cdk4 complex is responsible for the neuronal apoptotic effect. To further test this notion, we employed pharmacological agents; that is, cell-permeable Cdk2 inhibitor (compound 3, IC50 = 60 nm) and Cdk4 inhibitor (2-bromo-12,13-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7(6H)-dione, IC50 = 76 nm). These inhibitors block cyclin E- and cyclin D-dependent Cdks, respectively. Immunoblotting revealed that Cdk4 inhibitor did not affect cyclin D1 and Cdk4 up-regulation by SRPK2 wild type and T492D, but it blocked cyclin B1 induction; because of lacking cyclin D1-Cdk4 kinase activity, cells cannot proceed to S or G2 phase when cyclin B1 is expressed. Accordingly, Rb phosphorylation was completely abolished; so was neuronal apoptosis. By contrast, Cdk2 inhibitor failed to block Rb phosphorylation by cyclin D1-Cdk4 complex; therefore, it was unable to inhibit neuronal apoptosis, although cyclin B1 induction by SRPK2 was repressed due to cell cycle interruption by Cdk2 inhibitor (supplemental Fig. 4). Taken together, these data support that SRPK2 triggers apoptosis through up-regulating cyclin D1-Cdk4 complex activity in cortical neurons, which can be inhibited by 14-3-3.
FIGURE 5.
SRPK2 provokes neuronal apoptotic through up-regulating cyclin D1-Cdk4. A, wild-type SRPK2 and T492D provoke cyclin D1and Cdk4 expression and trigger apoptosis in cortical neurons. Various lentivirus expressing SRPK2 wild type and mutants were infected into cortical neurons and incubated for 4 days. The lysates were assessed by immunoblotting using antibodies against indicated proteins. Tubulin was included as a loading control. DNA fragmentation was also conducted to verify apoptosis (bottom panel). B, 14-3-3 suppresses cyclin D1-Cdk4 expression and prevents apoptosis in neurons. Various SRPK2 were co-infected with 14-3-3β into cortical neurons and incubated for 4 day. The lysates were assessed by immunoblotting using antibodies against indicated proteins. DNA fragmentation was also conducted to verify apoptosis (bottom panel). C, INKp16 blocks cyclin D1-Cdk4 kinase activity and prevents apoptosis. Various SRPK2 lentiviruses were co-infected with INKp16 into cortical neurons and incubated for 4 days. The lysates were assessed by immunoblotting using antibodies against indicated proteins. DNA fragmentation was also conducted to verify apoptosis (bottom panel).
Characterization of Akt/SRPK2/Cyclin D1 Cascade in Stroke
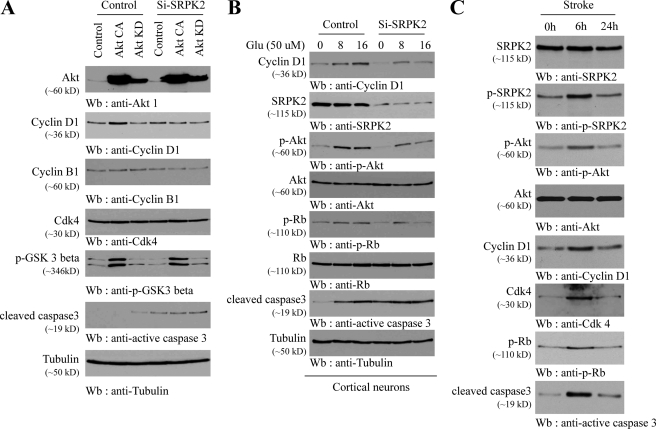
Our data support the paradigm that Akt phosphorylates SRPK2 and enhances its stimulatory activity on cyclin D1 expression. To further test this signaling cascade, we depleted SRPK2 in cortical neurons that were infected with active or kinase-dead Akt. Active Akt markedly enhanced cyclin D1 expression levels, which was significantly blocked in SRPK2-depleted neurons. Noticeably, cyclin B1 and Cdk4 levels remained the same regardless of Akt or SRPK2 status (Fig. 6A, second, third, and fourth panels). Remarkably, depletion of SRPK2 induced caspase-3 activation even in the presence of active Akt (sixth panel), indicating that SRPK2 is required for neuronal survival. Furthermore, depletion of SRPK2 by its short hairpin RNA dramatically decreased cyclin D1 expression in cortical neurons provoked by glutamate. Consequently, Rb phosphorylation was also evidently attenuated (Fig. 6B). It is noteworthy that glutamate provoked Akt phosphorylation that was diminished in SRPK2-depleted neurons (third panel), suggesting that SRPK2 somehow regulates Akt activation by glutamate. Once again, elimination of SRPK2 provoked caspase-3 activation even in the absence of glutamate (seventh panel). Together, these results suggest that the Akt/SRPK2/cyclin D1 pathway exists in primary neurons under the stress of neuroexcitotoxicity.
FIGURE 6.
Characterization of Akt/SRPK2/cyclin D1 cascade in ischemia-attacked brain. A, SRPK2 depletion blocks Akt-provoked cyclin D1 expression in neurons. Primary cortical neurons were infected with adenovirus expressing Akt-CA or KD followed by infection with lentivirus expressing short hairpin RNA of SRPK2. The cell lysates were analyzed by immunoblotting with indicated antibodies. Interestingly, SRPK2 knocking down alone initiated caspase-3 activation (sixth panel). B, SRPK2 depletion decreases glutamate-provoked cyclin D1 expression. Control and short hairpin RNA-SRPK2 lentivirus-infected cortical neurons were treated with 50 μm glutamate and then harvested at the indicated time. Extracts were separated by SDS-PAGE and analyzed by Western blotting using antibodies against indicated proteins. C, stroke triggers Akt/SRPK2/cyclin D1 signaling cascade. The stroke-attacked mouse brain tissues at the indicated times were analyzed by Western blotting using antibodies against indicated proteins.
Cyclin D1, cyclin B1, and Cdk4 levels are up-regulated in brains during stroke/excitotoxic damage. To further test whether the Akt/SRPK2/cyclin D1 pathway occurs under pathophysiological conditions, we monitored SRPK2 phosphorylation by Akt and cyclin D1-Cdk4 complex expression in mouse brain treated by transient middle cerebral artery occlusion. At 6 h Akt was evidently activated, which in turn potently phosphorylated SRPK2. Concurrently, cyclin D1 and Cdk4 were strongly up-regulated, which was accompanied by robust Rb phosphorylation. Accordingly, caspase-3 was keenly activated (Fig. 6C). This finding was in alignment with a previous report that inhibition of Cdk4 activity in ischemic stroke is highly neuroprotective (30). Therefore, our data support that Akt/SRPK2/cyclin D1 signal cascade is activated in ischemia-attacked brain.
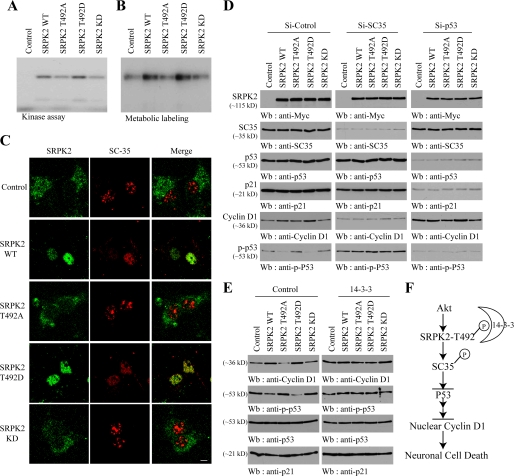
SRPK2 Phosphorylates SC35 and Blocks p53 Activation, Up-regulating Cyclin D1 Expression
Overexpression of SRPK2 disassembles the nuclear speckles, leading to the nucleoplasmic translocation of SC35 and acinus, critical SR splicing factors (17, 31). To examine whether acinus mediates the SRPK2 effect on cyclin D1, we infected cortical neurons with various acinus constructs including wild type, S422D, and S422A and found that cyclin D1 expression was not altered by any of the proteins (supplemental Fig. 5). Depletion of acinus from cortical neurons also failed to influence cyclin D1 expression (data now shown), indicating that SRPK2 regulates cyclin D1 expression through other substrates than acinus. A previous study shows that SC35 is essential for cell proliferation, which is negatively regulated by p53. Depletion of SC35 results in G2/M arrest, which is associated with p53 activation and hyperphosphorylation (32). Furthermore, it has been shown before that p53 represses cyclin D1 transcription (33). Thus, we hypothesize that SRPK2 provokes cyclin D1 expression through phosphorylating SC35 and inhibition of p53, resulting in up-regulation of cyclin D1. To test this hypothesis, we conducted an in vitro kinase assay with purified GST-SC35. Wild-type SRPK2 and T492D strongly phosphorylated SC35, whereas T492A and SRPK2-KD weakly phosphorylated SC35 (Fig. 7A). This finding confirms a previous observation that SRPK1 can phosphorylate SC35 (15). To explore whether SRPK2 can phosphorylate SC35 in intact cells, we conducted a metabolic labeling assay with [32P]orthophosphate. We virtually made the similar observation as in vitro phosphorylation, supporting that SRPK2 robustly phosphorylates SC35 in neurons (Fig. 7B). Infection of SRPK2 wild type and T492D in cortical neurons significantly relocalized SC35 from the nuclear speckles into the nucleoplasm, whereas T492A and SRPK-KD failed (Fig. 7C), confirming a previous report that SRPK2 dissembles the nuclear speckle (17, 31). These data suggest that SRPK2 phosphorylates SC35 and provokes its relocation within the nucleus of primary neurons. We next sought to determine the biological roles of SC35 and p53 in mediating SRPK2-regulated cyclin D1 expression. Compared with control, depletion of SC35 led to prominent suppression of cyclin D1 expression. Interestingly, p53 Ser-15 phosphorylation, which decreases the interaction between p53 and its negative regulator MDM2 (34), was markedly inhibited in SRPK2 wild-type and T492D-infected neurons (Fig. 7D, left bottom panel). Conversely, cyclin D1 was evidently up-regulated in these neurons, verifying the previous report that p53 represses cyclin D1 expression (33). Remarkably, p53 was strongly phosphorylated in SC35-depleted samples (Fig. 7D, middle bottom panel), fitting with the previous observation that p53 is hyperphosphorylated in SC35-null cells (32). As predicted, knocking down p53 highly enhanced cyclin D1 expression in all samples, but the increment was higher in SRPK2 wild-type and T492D-infected neurons (Fig. 7D, right fifth panel). As a positive control, p21 but not SC35 was selectively diminished in p53-depleted neurons. Because 14-3-3 binds Akt-phosphorylated SRPK2 and blocks its stimulatory effect on cyclin D1, we reasoned that 14-3-3 might suppress SRPK2-triggered cyclin D1 expression through augmenting p53 inhibitory activity. Immunoblotting demonstrated that the diminished p53 phosphorylation in SRPK2 wild-type and T492D-infected neurons was significantly reverted in 14-3-3 co-overexpressed neurons, supporting that 14-3-3 antagonizes SRPK2 inhibitory effect on p53 activation (Fig. 7E). Collectively, these data support that SRPK2 phosphorylates SC35 and inactivates p53 through blocking its phosphorylation, relieving inhibition of cyclin D1 transcription by p53.
FIGURE 7.
SRPK2 phosphorylates SC35, inhibits p53 activation, and up-regulates cyclin D1. A, in vitro SRPK2 kinase assay with SC35 recombinant protein. The reaction mixture was resolved on 10% SDS-PAGE. Wild-type SRPK2 and T492D mutant strongly phosphorylated SC35, but T492A and KD mutants were unable to phosphorylate it. B, SRPK2 phosphorylates SC35 in neurons. Various SRPK2 lentivirus were infected into cortical neurons and followed by metabolic labeling in [32P]H3PO4. SC35 was pulled down and resolved on SDS-PAGE. Wild-type SRPK2 and T492D mutant strongly phosphorylated SC35, whereas other mutants displayed weak effect. C, SRPK2 redistributes SC35 in the nucleus. Cortical neurons were infected with various SRPK2 lentivirus. Localization of various SRPK2 and endogenous SC35 was visualized by indirect immunofluorescent staining using anti-Myc polyclonal antibody and anti-SC35 antibody. Wild-type SRPK2 and T492D mutant strongly redistributed SC35 from the nuclear speckles into the nucleoplasm. D, depletion of SC35 abolishes SRPK2-regulated cyclin D1 expression. Various SRPK2 constructs co-transfected with si-control, si-SC35, or si-p53 into cortical neurons. The lysates were analyzed by Western blotting using antibodies against indicated proteins. E, 14-3-3 inhibits the SRPK2 effect on p53 activation. Various SRPK2 constructs were co-infected with control and 14-3-3 into cortical neurons. The lysates were analyzed by immunoblotting with various antibodies. Wild-type SRPK2 and T492D repressed p53 phosphorylation and increased cyclin D1 expression, which was abolished by 14-3-3. F, a schematic model for Akt/SRPK2/sc35/p53/cyclin D1 signaling in neuronal cell cycle and cell death.
DISCUSSION
In the present study we identify SRPK2 as a physiological substrate for Akt. Akt phosphorylation of SRPK2 enhances its SR protein kinase activity toward acinus and SC35, two pre-mRNA splicing factors in the nuclear speckles. Moreover, Akt phosphorylation provokes SRPK2 nuclear translocation, which can be blocked by the co-expressed 14-3-3. We show that SRPK2 selectively interacts with three 14-3-3 isoforms, β, σ, and ϵ, but the association between SRPK2 and14-3-3β and -ϵ isoforms are Akt phosphorylation-dependent. Thus, it appears that 14-3-3 is a negative regulator against Akt-dependent induction of SRPK2. Undoubtedly, in most cases 14-3-3 is a positive regulator of Akt-mediated events. To reconcile this finding, we propose that endogenous local SRPK2 levels might overwhelm endogenous 14-3-3 (specifically, β and ϵ isoforms) protein levels. If SRPK2 and 14-3-3β and -ϵ are not uniformly distributed in the cells, Akt-phosphorylated SRPK2, which might be locally enriched and outnumber 14-3-3, evades the sequestration by14-3-3β and -ϵ translocates into the nucleus. Alternatively, phosphorylation of SRPK2 elicits its conformational change and promotes its association with importins, which might shield the access by 14-3-3, resulting in p-SRPK2 nuclear translocation. Nevertheless, when 14-3-3 is overexpressed, a tremendous amount of 14-3-3 competes with importins for binding to p-SRPK2, tethering p-SRPK2 in the cytoplasm. On the other hand, 14-3-3 binding to SRPK2 depends on its own phosphorylation status as well. For instance, cellular stresses induce c-Jun N-terminal kinase (JNK)-mediated 14-3-3ζ phosphorylation at Ser-184, and phosphorylation of 14-3-3 by JNK releases the proapoptotic proteins BAD and FOXO3a from 14-3-3 and antagonizes the effects of Akt signaling (35, 36). Conceivably, under ischemia conditions, Akt is activated, and it provokes SRPK2 phosphorylation. Concomitantly, 14-3-3 is phosphorylated by JNK and loses its affinity to p-SRPK2. Subsequently, the nuclear translocated p-SRPK2 promotes cyclin D1 expression and triggers neuronal cell death in post-mitotic neurons.
Interestingly, SRPK2 robustly triggers cyclin D1 expression, BrdUrd incorporation, and neuronal apoptosis in post-mitotic neurons, for which SRPK2 kinase activity is required. Accordingly, Akt phosphorylation mimetic mutant T492D displays the strongest effect, whereas SRPK2 T492A, an unphosphorylatable mutant, exhibits a substantially decreased activity. The cell cycle progression and programmed cell death effects by SRPK2 can be inhibited by14-3-3β wild type but not K50E mutant, underscoring that the association between SRPK2 and 14-3-3 is necessary for 14-3-3 to suppress the cell proliferation and cell death activity by SRPK2. We provide compelling evidence to demonstrate that SRPK2 exerts its apoptotic effect through cyclin D1-dependent kinases including Cdk4. Both INKp16, an endogenous cyclin D1-Cdk4,6 inhibitory protein, and small pharmacological Cdk4 inhibitor potently inhibit the neuronal apoptosis triggered by SRPK2. Although both Cdk4 inhibitor and Cdk2 inhibitor can block cyclin B1 expression and cell cycle progression, only Cdk4 inhibitor can strongly block cyclin D1-Cdk4 kinase activity and repress Rb phosphorylation, leading to neuronal cell survival. This finding fits with a previous report that dominant negative forms of the Cdk4 and Cdk6 and G1/S blockers prevent neuronal cell death (13).
Cell cycle and cell death are intimately related and use many similar mechanisms for their execution. In addition, the cell cycle machinery is molecularly linked to the cell death signaling cascade. For instance, Cdk1-cyclin B1 directly phosphorylates caspase-9 and protects mitotic cells from apoptosis (37). Thus, phosphorylation of caspase-9 determines the balance between the control of cell division and apoptosis and sets the threshold for activation of the intrinsic apoptotic pathway during cell cycle, restrains apoptosis during mitosis, and determines sensitivity to anti-mitotic drugs. Cdc2 is expressed in post-mitotic granule neurons of the developing rat cerebellum, and Cdc2 mediates apoptosis of cerebellar granule neurons upon the suppression of neuronal activity. Cdc2 catalyzes the phosphorylation of BAD at serine 128 and induces BAD-mediated apoptosis in primary neurons (38). Furthermore, cleavage of p21Cip1/Waf1 and p27Kip1 by caspase-3 results in a substantial reduction in their association with nuclear cyclin-Cdk2 complexes, leading to a dramatic induction of Cdk2 activity. Dominant-negative Cdk2 as well as a mutant of p21Cip1/Waf1 resistant to caspase cleavage partially suppresses apoptosis (39, 40). SRPK1 was first identified as a serine kinase regulating intracellular localization of splicing factors in the cell cycle (15). It translocates to the nucleus before initiation of M phase, indicating that it may play a role in cell cycle progression (41). A previous study shows that both SRPK1 and SRPK2 are the substrates of caspases. SRPKs are activated early during apoptosis followed by caspase-mediated proteolytic inactivation at later time points, suggesting that SRPKs are involved in apoptosis progression (42). Therefore, these observations support that SRPKs implicate in both cell cycle advance and programmed cell death. Here, we provide strong evidence demonstrating that SRPK2 provokes the post-mitotic neurons to re-enter the cell cycle by triggering cyclin D1, Cdk4, and cyclin B1 expression and elevating DNA synthesis. Subsequently, the augmented cyclin D1-Cdk4 elicits prominent neuronal cell death (Figs. 3, 4, and 5).
SR proteins are a family of splicing factors that are important component of spliceosomes. Phosphorylation of SR proteins is a key event for the regulation of pre-mRNA splicing. Previous studies indicate that pre-mRNA splicing is regulated by phosphorylation in a cell cycle-dependent manner (15, 43). This notion is supported by numerous findings. For example, SF2/ASF (alternative splicing factor/splicing factor 2), a prototype of SR protein, is phosphorylated by Cdc2 kinase in a cell cycle-dependent manner (44). Recently, numerous Cdk-cyclin complexes have been implicated in transcription and mRNA processing (45, 46). Sequential phosphorylation of ASF/SF2 by SRPK and Clk/Sty induces specific redistribution of splicing factors in the nucleus (14, 47). The common substrates, SR proteins in the nuclear speckles, provide a cross-talk platform between SRPK and cell cycle-dependent kinases (Cdks). SRPKs kinase activity is regulated in a cell cycle-dependent manner. Thus, it is tempting to speculate that SRPKs may be also phosphorylated by some Cdks in addition to Akt (Fig. 1).
PI 3-kinase/Akt signaling is a major pathway mediating survival signals in neuronal cells. Thus, PI 3-kinase/Akt signaling is generally considered neuroprotective, acting against stress conditions that occur during neurodegeneration. Surprisingly, Akt phosphorylates SRPK2 and enhances its catalytic activity, leading to promotion of neuronal cell death. Akt signaling triggers a network that positively regulates G1/S cell cycle progression through inactivation of glycogen synthase kinase 3β, leading to increased cyclin D1 (48). Infection of cortical neurons with active Akt selectively enhances cyclin D1 but not Cdk4 or cyclin B1 expression. Of course, no apoptosis occurs in the neurons (Fig. 6A). However, Akt-phosphorylated SRPK2 strongly up-regulates cyclin D1, Cdk4, and cyclin B1, resulting in BrdUrd incorporation and cell cycle progression and cell death in neurons (Figs. 3, 4, and 5). Hence, SRPK2 can switch the cell cycle advance into the cell death session, which is facilitated by Akt phosphorylation; in addition to the neurons under pathophysiological conditions, Akt/SRPK2/cyclin D1 signaling cascade also occurs in other cell type. For instance, SRPK2 expression level was substantially decreased in Akt1- or Akt2-depleted cells, and it was almost not detectable in double knock-out cells compared with wild-type mouse embryonic fibroblast cells. Accordingly, cyclin D1 was dramatically attenuated in both Akt1 and Akt2 null cells, and it was completely repressed in double knock-out cells, correlating with SRPK2 expression pattern (supplemental Fig. 5).
Previously, we show that Akt phosphorylates acinus and prevents its proteolytic cleavage in vitro and in vivo, blocking chromatin condensation and apoptosis (26). Most recently, we show that SRPK2 binds and phosphorylates acinus and redistributes it from the nuclear speckles to the nucleoplasm, resulting in cyclin A1 but not A2 up-regulation and leukemia cell proliferation (18). Nevertheless, acinus does not implicate in SRPK2-mediated cyclin D1 expression in cortical neurons (supplemental Fig. 6). Instead, we show that SC35 plays a critical role in this process. SC35 is also a substrate of SRPK2 in post-mitotic neurons. Overexpression of SRPK2 triggers SC35 redistribution from the nuclear speckles into the nucleoplasm (Fig. 7C), reflecting the function of the SRPK family of kinases in spliceosome assembly and trafficking of splicing factors in mammalian cells. Depletion of endogenous SC35 substantially blocks SRPK2-triggered cyclin D1 in cortical neurons, which is accompanied with markedly augmentation of p53 phosphorylation. Moreover, we found that p53 phosphorylation was significantly decreased in wild-type SRPK2 and T492D-infected neurons (Fig. 7D). These data suggest that SRPK2 phosphorylates and activates SC35, leading to the suppression of p53 phosphorylation and relieving the repressive effect of p53 on cyclin D1 expression (Fig. 7F). This concept is supported by a previous report that SC35 negatively regulates p53 (32). The involvement of p53 in the SRPK2-mediated cell cycle and cell death interplay is not surprising at all, as a large body of evidence demonstrates that p53 plays a key role in regulating cell cycle progression and cell death in a variety of organs (49, 50).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 NS060680 (to K. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- AD
- Alzheimer disease
- SR
- serine/arginine
- PI
- phosphatidylinositol
- BrdUrd
- bromodeoxyuridine
- Cdk
- cyclin-dependent kinase
- JNK
- c-Jun N-terminal kinase
- HA
- hemagglutinin
- EGF
- epidermal growth factor
- GST
- glutathione S-transferase
- Rb
- retinoblastoma
- PBS
- phosphate-buffered saline
- KD
- kinase dead
- Wb
- Western blot.
REFERENCES
- 1.Yang Y., Herrup K. (2007) Biochim. Biophys. Acta 1772, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husseman J. W., Nochlin D., Vincent I. (2000) Neurobiol. Aging 21, 815–828 [DOI] [PubMed] [Google Scholar]
- 3.Arendt T., Rödel L., Gärtner U., Holzer M. (1996) Neuroreport 7, 3047–3049 [DOI] [PubMed] [Google Scholar]
- 4.Busser J., Geldmacher D. S., Herrup K. (1998) J. Neurosci. 18, 2801–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McShea A., Harris P. L., Webster K. R., Wahl A. F., Smith M. A. (1997) Am. J. Pathol. 150, 1933–1939 [PMC free article] [PubMed] [Google Scholar]
- 6.Smith T. W., Lippa C. F. (1995) J. Neuropathol. Exp. Neurol. 54, 297–303 [DOI] [PubMed] [Google Scholar]
- 7.Vincent I., Jicha G., Rosado M., Dickson D. W. (1997) J. Neurosci. 17, 3588–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashidian J., Iyirhiaro G. O., Park D. S. (2007) Biochim. Biophys. Acta 1772, 484–493 [DOI] [PubMed] [Google Scholar]
- 9.Farinelli S. E., Greene L. A. (1996) J. Neurosci. 16, 1150–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park D. S., Farinelli S. E., Greene L. A. (1996) J. Biol. Chem. 271, 8161–8169 [DOI] [PubMed] [Google Scholar]
- 11.Park D. S., Levine B., Ferrari G., Greene L. A. (1997) J. Neurosci. 17, 8975–8983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park D. S., Morris E. J., Greene L. A., Geller H. M. (1997) J. Neurosci. 17, 1256–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park D. S., Obeidat A., Giovanni A., Greene L. A. (2000) Neurobiol. Aging 21, 771–781 [DOI] [PubMed] [Google Scholar]
- 14.Wang H. Y., Lin W., Dyck J. A., Yeakley J. M., Songyang Z., Cantley L. C., Fu X. D. (1998) J. Cell Biol. 140, 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gui J. F., Lane W. S., Fu X. D. (1994) Nature 369, 678–682 [DOI] [PubMed] [Google Scholar]
- 16.Gui J. F., Tronchère H., Chandler S. D., Fu X. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10824–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroyanagi N., Onogi H., Wakabayashi T., Hagiwara M. (1998) Biochem. Biophys. Res. Commun. 242, 357–364 [DOI] [PubMed] [Google Scholar]
- 18.Jang S. W., Yang S. J., Ehlén A., Dong S., Khoury H., Chen J., Persson J. L., Ye K. (2008) Cancer Res. 68, 4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 20.Fu H., Subramanian R. R., Masters S. C. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 617–647 [DOI] [PubMed] [Google Scholar]
- 21.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. (1997) Cell 91, 231–241 [DOI] [PubMed] [Google Scholar]
- 22.Hsu S. Y., Kaipia A., Zhu L., Hsueh A. J. (1997) Mol. Endocrinol. 11, 1858–1867 [DOI] [PubMed] [Google Scholar]
- 23.Biggs W. H., 3rd, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 25.Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. (1999) Nature 398, 630–634 [DOI] [PubMed] [Google Scholar]
- 26.Hu Y., Yao J., Liu Z., Liu X., Fu H., Ye K. (2005) EMBO J. 24, 3543–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z., Jang S. W., Liu X., Cheng D., Peng J., Yepes M., Li X. J., Matthews S., Watts C., Asano M., Hara-Nishimura I., Luo H. R., Ye K. (2008) Mol. Cell 29, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumrejkanchanakij P., Tamamori-Adachi M., Matsunaga Y., Eto K., Ikeda M. A. (2003) Oncogene 22, 8723–8730 [DOI] [PubMed] [Google Scholar]
- 29.Appert-Collin A., Hugel B., Levy R., Niederhoffer N., Coupin G., Lombard Y., André P., Poindron P., Gies J. P. (2006) Life Sci. 79, 484–490 [DOI] [PubMed] [Google Scholar]
- 30.Wen Y., Yang S., Liu R., Simpkins J. W. (2005) FEBS Lett. 579, 4591–4599 [DOI] [PubMed] [Google Scholar]
- 31.Koizumi J., Okamoto Y., Onogi H., Mayeda A., Krainer A. R., Hagiwara M. (1999) J. Biol. Chem. 274, 11125–11131 [DOI] [PubMed] [Google Scholar]
- 32.Xiao R., Sun Y., Ding J. H., Lin S., Rose D. W., Rosenfeld M. G., Fu X. D., Li X. (2007) Mol. Cell. Biol. 27, 5393–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha S., Martin A. M., Meek D. W., Perkins N. D. (2003) Mol. Cell. Biol. 23, 4713–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh S. Y., Ikeda M., Taya Y., Prives C. (1997) Cell 91, 325–334 [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta F., Sunayama J., Mori Y., Hattori S., Shimizu S., Tsujimoto Y., Yoshioka K., Masuyama N., Gotoh Y. (2004) EMBO J. 23, 1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunayama J., Tsuruta F., Masuyama N., Gotoh Y. (2005) J. Cell Biol. 170, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allan L. A., Clarke P. R. (2007) Mol. Cell 26, 301–310 [DOI] [PubMed] [Google Scholar]
- 38.Konishi Y., Lehtinen M., Donovan N., Bonni A. (2002) Mol. Cell 9, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 39.Levkau B., Koyama H., Raines E. W., Clurman B. E., Herren B., Orth K., Roberts J. M., Ross R. (1998) Mol. Cell 1, 553–563 [DOI] [PubMed] [Google Scholar]
- 40.Jin Y. H., Yoo K. J., Lee Y. H., Lee S. K. (2000) J. Biol. Chem. 275, 30256–30263 [DOI] [PubMed] [Google Scholar]
- 41.Ding J. H., Zhong X. Y., Hagopian J. C., Cruz M. M., Ghosh G., Feramisco J., Adams J. A., Fu X. D. (2006) Mol. Biol. Cell 17, 876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamachi M., Le T. M., Kim S. J., Geiger M. E., Anderson P., Utz P. J. (2002) J. Exp. Med. 196, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector D. L., Fu X. D., Maniatis T. (1991) EMBO J. 10, 3467–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto Y., Onogi H., Honda R., Yasuda H., Wakabayashi T., Nimura Y., Hagiwara M. (1998) Biochem. Biophys. Res. Commun. 249, 872–878 [DOI] [PubMed] [Google Scholar]
- 45.Seghezzi W., Chua K., Shanahan F., Gozani O., Reed R., Lees E. (1998) Mol. Cell. Biol. 18, 4526–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loyer P., Trembley J. H., Katona R., Kidd V. J., Lahti J. M. (2005) Cell. Signal. 17, 1033–1051 [DOI] [PubMed] [Google Scholar]
- 47.Ngo J. C., Chakrabarti S., Ding J. H., Velazquez-Dones A., Nolen B., Aubol B. E., Adams J. A., Fu X. D., Ghosh G. (2005) Mol. Cell 20, 77–89 [DOI] [PubMed] [Google Scholar]
- 48.Diehl J. A., Cheng M., Roussel M. F., Sherr C. J. (1998) Genes Dev. 12, 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kastan M. B., Canman C. E., Leonard C. J. (1995) Cancer Metastasis Rev. 14, 3–15 [DOI] [PubMed] [Google Scholar]
- 50.Canman C. E., Chen C. Y., Lee M. H., Kastan M. B. (1994) Cold Spring Harbor Symp. Quant. Biol. 59, 277–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.