Abstract
Purpose
Pelvic pyomyositis in children is a rare infectious condition, although it is increasingly reported in temperate climates. Often considered a primary disease, new diagnostic methods are able to identify additional foci of infection. The purpose of this study is to review our patients and to analyze the imaging studies to determine its pathogenesis.
Methods
A retrospective study of the clinical charts and imaging records of 11 patients was made, noting the number and location of muscles involved, as well as bone and joint involvement.
Results
Besides the classical form of pelvic pyomyositis, i.e., iliopsoas pyomyositis, other muscular groups were frequently affected, often with multiple involvement. Bone involvement is also frequent. Magnetic resonance imaging (MRI) gives the most useful information.
Conclusion
MRI is the diagnostic procedure of choice for diagnosing pelvic pyomyositis in children. It may also have an elucidating role in the debated pathogenesis of this condition. In most of the cases, pelvic pyomyositis in children could be secondary.
Keywords: Pyomyositis, Children, Infection, MRI, Pelvis
Introduction
Pyomyositis is a bacterial infection of skeletal muscles which has more frequently been described in tropical areas, but it is becoming increasingly recognized in temperate climates, up to 1 case every 3,000–4,000 annual hospital admissions [1–4]. Its rare occurrence, paucity of symptoms on initial presentation, difficult differential diagnosis, differences in the diagnostic tools, common late diagnosis, and uncertain pathogenesis make it a very interesting clinical condition. The lack of familiarity of this entity may lead to delay in the diagnosis [5] and to inadequate treatment [6].
Pelvic muscles, thighs, and calves are the most frequent locations [7]. Although some authors have described potential underlying causes of pyomyositis as previous trauma [3, 8, 9], hematogeneous source [2, 9–12], skin penetration [11, 13], previous viral illness [14], appendicitis, bowel disease or retroperitoneal lymphadenitis [11, 15], and, in older patients, systemic processes, such as AIDS, leukemia, diabetes, chronic steroid usage, and renal failure [3, 4, 16, 17], it has largely been considered as a primary pyogenic bacterial infection [13, 17]. More recently, there has been some controversy about the apparent bone involvement seen on magnetic resonance imaging (MRI) and bone scintigraphy and its role as either the cause or effect of associated muscle involvement [4, 7, 18, 19].
The purpose of this paper is to review our experience in pelvic pyomyositis in the pediatric population in temperate climates and, particularly, to evaluate its relation to other neighboring septic or inflammatory conditions, in order to elucidate its pathogenesis.
Methods
All cases of pelvic pyomyositis and their records at a pediatric hospital over the period 1996–2003 were reviewed. Pelvic pyomyositis was defined by computed tomography (CT) and MRI criteria: the CT definition of pyomyositis was the presence of an enlarged muscle with heterogeneous attenuation and a central fluid collection with rim contrast enhancement. The MRI criteria were a slightly higher signal intensity on T1-weighted images with a higher signal intensity rim surrounding an iso/hypointense central area and a diffuse increase in signal intensity on T2-weighted images.
The clinical presentation was recorded, including duration from the onset of symptoms to diagnosis, pain, fever, previous trauma, and limp, as well as physical findings, which included general symptoms, local tenderness, and erythema. Laboratory studies, complete and differential blood cell count, erythrocyte sedimentation rate, and values of C-reactive protein were also evaluated. All of these parameters were recorded at admission as well as after treatment. The infecting organisms that had been isolated from blood and/or purulent material were documented. The imaging studies that had been performed during the work-up were recorded and reviewed for relevant findings, noting the location of muscles involved and of additional sites of infection. The clinical course, treatment modalities, and final outcome were also assessed.
Results
Eleven cases of pelvic pyomyositis were identified for this review. Their clinical data are summarized in Table 1.
Table 1.
Clinical findings of the patients studied
| Case | Age (years) | Sex | Duration (days) | Initial diagnosis | Temp (°C) | WBC (/ml) | ESR (mm/h) | CRP (mg/l) | Organism | Surgery | Antibiotics |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.6 | F | 10 | Septic arthr. hip | 38.1 | 15.2 | 70 | 3.5 | S. aureus | N | Cloxacillin |
| 2 | 10.8 | M | 5 | Septic arthr. hip | 38.7 | 16.8 | 32 | 1.1 | S. aureus | N | Cloxacillin |
| 3 | 12.4 | M | 5 | Trochanteric pain | 38.5 | 13.4 | 78 | 18 | Neg | Y | Cloxacillin |
| 4 | 5.2 | F | 7 | Invasive g/e | 39.3 | 18.7 | 32 | 9.8 | B. fragilis | Y | Clindamycin |
| 5 | 15.3 | F | 15 | Sacroiliitis | 39 | 12.7 | 110 | 6.2 | S. aureus | Y | Tobramycin |
| 6 | 5.4 | F | 8 | Sacroiliitis | 39.8 | 14 | 65 | 25 | S. aureus | Y | Amoxicillin |
| 7 | 16.0 | M | 5 | Crohn disease | 37.8 | 24.5 | 58 | 6.5 | Neg | Y | Meropenem |
| 8 | 12.4 | M | 10 | Septic arthr. hip | 37.8 | 20.8 | 45 | 1.3 | Neg | N | Cefotaxime |
| 9 | 8.1 | F | 2 | Septic arthr. hip | 39.3 | 14.6 | 97 | 17 | Neg | N | Cloxacillin + cefotaxime |
| 10 | 2.9 | M | 8 | Sacroiliitis | 38 | 14.06 | 52 | 12.6 | Neg | N | Cefotaxime |
| 11 | 6.3 | F | 12 | Appendicitis | 39 | 17 | 35 | 27.6 | Neg | N | Cefotaxime + clindamycin |
F female, M male, g/e gastroenteritis, WBC white blood cell count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, neg negative culture
The mean age at presentation was 9 years and 3 months (range, 2 years 9 months to 16 years). There were six females and five males. Three patients had a preceding history of Crohn disease, invasive intestinal infection, and local trauma, respectively. The mean duration of symptoms before diagnosis was 7.9 days (range, 2–15 days). On admission, nine patients were unable to bear weight and two patients could walk with limping. Five patients complained of abdominal pain, two patients of pain from the posterior aspect and gluteal area, and four patients of pain from the groin region. One patient with abdominal pain showed some signs of acute abdomen and she underwent a negative appendectomy.
At admission, all patients were febrile. The average leukocyte count was 16.5 × 106/ml with a left shift. The erythrocyte sedimentation rate was uniformly elevated and averaged 63.9 mm/h (range 32–110). C-reactive protein had a mean initial value of 10.1 mg/l (range, 1.1–25). Cultures of the blood and wound specimens revealed Staphylococcus aureus in four cases, a mixture of Bacteroides fragilis and Gram-negative bacteria in one case, from intestinal source, and a negative culture occurred in six cases.
The imaging studies findings are summarized in Table 2. Plain radiographs were available in all cases, but without remarkable information. Ultrasound examination was performed in nine cases, eight cases had CT scans, and eight cases had MRI examinations. A bone scan was performed in one case, with a positive increased uptake in sacral bone. Concerning muscle involvement, ultrasound was useful in three cases, CT scan in seven cases, and MRI in all eight cases. We had one false-negative with CT scanning in one case presenting a psoas abscess. Ultrasound and CT scan were not useful for demonstrating osteomyelitis in any case, and MRI demonstrated bone involvement in all cases. We have observed that there are five cases in this series with simultaneous CT and MRI studies, and CT had false-negatives in all of them for evaluating bone involvement. However, MRI was useful in all cases for muscle and bone involvement (Fig. 1). The muscles involved were the iliopsoas muscle in six cases, obturator internus muscle in four cases, obturator externus in three cases, adductor muscles in two cases, gluteus medius muscle in three cases, and quadratus femoris muscle in one case. In seven patients, there was multiple muscle involvement (Fig. 2). Bone involvement was observed on the ischiopubic area in four cases, the ischial bone in one case, the sacroiliac joint in two cases, the iliac bone in one case, and the sacral bone in another case.
Table 2.
Imaging data and findings of the patients studied
| Case | US | Joint aspiration | CT | MRI | Muscle site | Additional site |
|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | Y | OI, OE, add | Ischpub |
| 2 | Y | N | N | Y | OI, OE | Ischpub |
| 3 | N | N | N | Y | Quad, glut | Ischpub |
| 4 | N | N | Y | N | Iliopsoas | Intestine |
| 5 | Y | N | Y | N | Iliopsoas | s/i |
| 6 | Y | N | Y | Y | Il, glut med | Iliac bone |
| 7 | Y | N | Y | N | Iliopsoas | Intestine |
| 8 | Y | Y | Y | Y | OI, add | Ischpub |
| 9 | Y | Y | Y | Y | OI, OE | Ischpub |
| 10 | Y | N | Y | Y | Psoas, glut | s/i |
| 11 | Y | N | N | Y | Iliopsoas | Sacrum |
US ultrasound examination, CT computed tomography, MRI magnetic resonance imaging, OI obturator internus muscle, OE obturator externus muscle, add adductors muscles, quad quadratus femoris muscle, glut gluteus medius muscle, il iliacus muscle, ischpub ischiopubic area, isch ischial bone, s/i sacroiliac joint
Fig. 1.
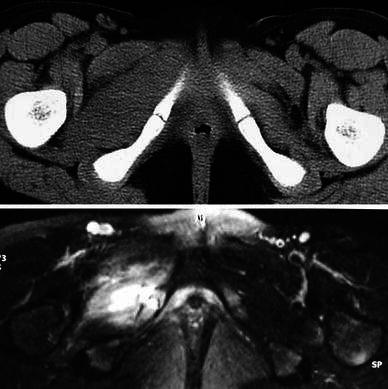
Case 9. Top computed tomography (CT) image showing isolated enlargement of the obturator muscle. Bottom the magnetic resonance imaging (MRI) image shows additional bone involvement in the ischiopubic area
Fig. 2.
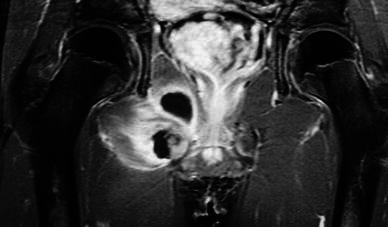
Case 8. MRI imaging showing multiple muscle involvement and abscess formation in the obturator and adductor muscle groups
Open surgical drainage was performed on five patients. The indication for open surgery was based on the treating surgeon’s own decision. The purulent material obtained was processed for cultures, but no bone biopsies were performed. All patients were treated with intravenous antibiotics, starting with an empiric coverage and according to culture results later. Cloxacillin was the most frequent antibiotic used, followed by cefotaxime, gentamicin, tobramycin, clindamycin, metronidazole, and meropenem. The duration of intravenous treatment and conversion to oral medication depended on the return to normal values of C-reactive protein and a decrease of the erythrocyte sedimentation rate (ESR). The total duration of antibiotic treatment was 3 weeks.
The outcome was assessed clinically, since routine imaging control was not done. The clinical course was excellent in all cases, with normalization of clinical status and laboratory parameters. A full range of motion was regained at an average of 35.4 days (range, 4–79). No recurrences were observed.
Discussion
Pyomyositis is becoming increasingly recognized in temperate climates [3, 4, 7, 10]. It is thought that the increasing use of MRI might be partially responsible for this recent increased reporting of cases of pyomyositis in children [19].
The typical age of presentation ranges between 5 and 9 years [3–5, 13, 20], and it is more frequent in males. Pelvic muscles, thighs, and calves are common locations [7], but multifocal location has been published in up to 43% of cases [2]. The microbiologic profile is similar in the majority of the previous reports, and S. aureus is present in as many as 85–90% of cases [1, 3–5, 8, 16, 17, 21]. Streptococcal species complicating primary varicella, gonococcal pyomyositis, and Mycobacterium tuberculosis have also been found to be causative in other reports [12, 14]. Pain, impaired ambulation, non weight-bearing, localized swelling, limp, and fever are the most common signs [3, 7], and it could be accepted that pain, fever, and limp are present in almost 100% of cases [3]. Increasing pain in internal rotation and extension, abdominal, genitourinary or spinal complaints, and fixed flexion deformity are more specific signs of psoas pyomyositis [11, 15]. In the present series, abdominal and groin pain and te inability to bear weight were the most common referring symptoms.
Blood test results are usually anemia, leukocytosis, shift to the left, elevation of sedimentation rate, elevation of C-reactive protein [5, 11, 12, 15], and blood cultures are positive in almost one-third of cases [4]. Five of our cases, i.e., 45%, had positive cultures. Some reports describe an elevation of muscle enzyme creatinine kinase level and urine myoglobin [1].
In order to differentiate pelvic pyomyositis in children from other septic regional conditions in the pelvis and/or inflammatory, a careful clinical evaluation must be considered as the first step: a distinctive combination of painful active motion and pain-free passive motion of the hip is common [6]; typical laboratory findings are helpful; plain films have a limited benefit in these cases, but they can be diagnostic in others, such as in pelvic osteomyelitis, Perthes’ disease, etc. [10]. Although a hip aspiration was performed in three of our patients with the aim of ruling out a hip joint infection, we do not agree with performing it at this moment for distinguishing extracapsular to intracapsular infection [6], because there would be a high risk of secondary infection of the hip joint in cases of adductor pyomyositis. Ultrasound examination, however, is a safe, quick, and cheap diagnostic tool, which is very sensitive for late presentation cases with muscle abscesses [9, 10] and can differentiate septic arthritis, subperiosteal abscesses, etc. [11, 15, 22, 23]. So, ultrasound examination should be considered as the next step [9], although it may not to be helpful in detecting early stages of pyomyositis [8].
CT, bone scan, and MRI are other diagnostic tools. Bone scan has been described as being very useful for the diagnosis of pelvic osteomyelitis as the first option of extracapsular infection around the hip [6], but it is not useful for detecting soft tissue inflammation [19, 24]. CT scan with contrast may define the extent of abscesses [12], although it cannot distinguish abscesses from some muscle hematomas [25]. CT is also useful for identifying joint effusions [14] and initial stages of pyomyositis. Nevertheless, some false-negatives have been described in CT and pelvic pyomyositis [4]. MRI is the preferable diagnostic tool after negative ultrasound and/or bone scan for many authors [6, 8, 10, 12, 18, 19]. MRI is ideal for evaluating soft tissue details and making early diagnosis, for distinguishing coexisting arthritis and/or osteomyelitis in T2-weighted signal, for differentiating between invasive or purulent stages with gadolinium, and delineating between involved muscle and edema [5, 7, 13, 17, 20, 24]. Briefly, MRI is a valuable diagnostic study for evaluating several different septic entities around the hip and allowing a final diagnosis. However, it is not always available and it needs sedation in younger patients. Early diagnosis of this condition is heavily dependent upon diagnostic imaging, and MRI is the best tool to accomplish it.
Abnormal bone marrow signal on MRI as well as on bone scintigraphy has been increasingly reported, and its significance is debated. For some authors, these correspond to reactive changes [2, 4, 26], but, for others, they represent a true osteomyelitis, so the bone infection would be the primary infection, spreading to the muscle, rather than vice versa [19, 24]. The pathogenesis of pelvic pyomyositis is still controversial regarding its primary or secondary condition. Some cases have been described secondary to cutaneous source, pyoderma or penetrating trauma [11, 13], previous viral [2, 4, 14, 16], systemic processes, such as AIDS, leukemia, renal failure, and diabetes, inducing leukocyte dysfunction and associated with adult age [3, 13, 16, 18], but, more often, no previous cause or coexisting condition could be detected or recognized, so some authors have entitled their reports of these cases as primary pyomyositis [15, 26]. The prevalent hypothesis has been that muscle tissue was infected in the course of a transient bacteremia, although muscle infection is a rare complication, even in children with septicemia [2, 4]. Muscle tissue has an inherent resistance to bacterial infection, so a previous trauma has been considered as a predisposing factor and it has been variably reported in 25–35% of cases [3, 11, 27]. In our series, only one patient had a consistent history of previous trauma. It is currently accepted by most authors that iliopsoas pyomyositis, the classical form of pelvic pyomyositis, is actually a secondary infection associated with gastrointestinal, urinary tract, or spinal infection [2, 26]. Five of our cases were iliopsoas pyomyositis, two of them had an associated gastrointestinal focus and in the remaining three cases, an abnormal signal in MRI was observed in the sacroiliac joint in two patients and in the sacral bone in one patient. All cases of perisciatic pyomyositis (i.e., obturator, adductor, quadratus, etc.) showed multiple involvement and an abnormal bone signal, usually located at their shared bone insertion point, so we also feel that these bone changes are actually a true osteomyelitis and we suggest that contiguous bone infection may play a relevant and more rational role in this controversial pathogenesis (Fig. 3). This would better explain why multiple site involvement can occur in an intrinsically resistant-to-infection tissue (Fig. 2) and, particularly, why non-contiguous compartments are also involved (Fig. 4).
Fig. 3.
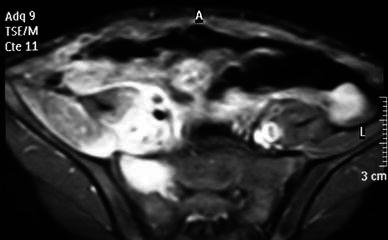
Case 11. A case of iliopsoas pyomyositis with an associated abnormal signal in the sacral bone marrow
Fig. 4.
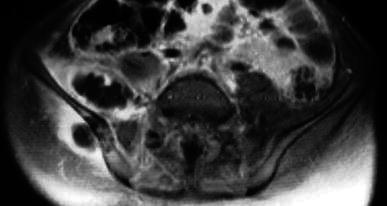
Case 6. MRI image of a case of multiple and non-contiguous involvement, in the iliac and gluteus medius muscles, with an associated involvement of the iliac bone
The limitations of this study are the small number of patients and the lack of objective evidence of bone infection by a bone biopsy. This could well be a matter of further investigation.
In conclusion, pelvic pyomyositis must be included in the diagnostic process of a child with fever, hip region pain, and limping. If diagnosed during the early stages, the prognosis is good, with a frequent non-surgical treatment. MRI is the most valuable diagnostic tool because it allows diagnosis in the early stages of disease and it is able to detect several different conditions. Furthermore, multiple muscle involvement as well as additional bone lesions are frequent, so the term ‘primary’ should be used with caution when referring to pelvic pyomyositis in the pediatric population.
Conflict of interest statement
The authors have not received funding from any organization or institution.
Footnotes
This study was conducted at Hospital Infantil Niño Jesús, Madrid, Spain.
References
- 1.Armstrong DG, D’Amato CR, Strong ML. Three cases of staphylococcal pyomyositis in adolescence, including one patient with neurologic compromise. J Pediatr Orthop. 1993;13(4):452–455. doi: 10.1097/01241398-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bickels J, Ben-Sira L, Kessler A, Wientroub S. Primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277–2286. doi: 10.2106/00004623-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Gubbay AJ, Isaacs D. Pyomyositis in children. Pediatr Infect Dis J. 2000;19:1009–1012. doi: 10.1097/00006454-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel DA, Meyer JS, Dormans JP, Flynn JM, Drummond DS. Pyomyositis in children and adolescents: report of 12 cases and review of the literature. J Pediatr Orthop. 1999;19(2):143–150. doi: 10.1097/01241398-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Renwick SE, Ritterbusch JF. Pyomyositis in children. J Pediatr Orthop. 1993;13:769–772. doi: 10.1097/01241398-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 6.De Boeck H, Noppen L, Desprechins B. Pyomyositis of the adductor muscles mimicking an infection of the hip. Diagnosis by magnetic resonance imaging: a case report. J Bone Joint Surg Am. 1994;76:747–750. doi: 10.2106/00004623-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez RJ, Strouse PJ, Craig CL, Farley FA. Focal pyomyositis of the perisciatic muscles in children. Am J Roentgenol. 2002;179:1267–1271. doi: 10.2214/ajr.179.5.1791267. [DOI] [PubMed] [Google Scholar]
- 8.Orlicek SL, Abramson JS, Woods CR, Givner LB. Obturator internus muscle abscess in children. J Pediatr Orthop. 2001;21:744–748. [PubMed] [Google Scholar]
- 9.Parbhoo A, Govender S. Acute pyogenic psoas abscess in children. J Pediatr Orthop. 1992;12:663–666. doi: 10.1097/01241398-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Hall RL, Callaghan JJ, Moloney E, Martinez S, Harrelson JM. Pyomyositis in a temperate climate. Presentation, diagnosis, and treatment. J Bone Joint Surg Am. 1990;72:1240–1244. [PubMed] [Google Scholar]
- 11.Song J, Letts M, Monson R. Differentiation of psoas muscle abscess from septic arthritis of the hip in children. Clin Orthop Relat Res. 2001;391:258–265. doi: 10.1097/00003086-200110000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Birkbeck D, Watson JT. Obturator internus pyomyositis. A case report. Clin Orthop Relat Res. 1995;316:221–226. [PubMed] [Google Scholar]
- 13.Peckett WR, Butler-Manuel A, Apthorp LA. Pyomyositis of the iliacus muscle in a child. J Bone Joint Surg Br. 2001;83:103–105. doi: 10.1302/0301-620X.83B1.11095. [DOI] [PubMed] [Google Scholar]
- 14.Mills WJ, Mosca VS, Nizet V. Orthopaedic manifestations of invasive group A streptococcal infections complicating primary varicella. J Pediatr Orthop. 1996;16:522–528. doi: 10.1097/01241398-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra R, Singh KD, Bhan S, Dave PK. Primary pyogenic abscess of the psoas muscle. J Bone Joint Surg Am. 1992;74:278–284. [PubMed] [Google Scholar]
- 16.Patel SR, Olenginski TP, Perruquet JL, Harrington TM. Pyomyositis: clinical features and predisposing conditions. J Rheumatol. 1997;24:1734–1738. [PubMed] [Google Scholar]
- 17.Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology. 1995;197:279–286. doi: 10.1148/radiology.197.1.7568838. [DOI] [PubMed] [Google Scholar]
- 18.Highland TR, LaMont RL. Osteomyelitis of the pelvis in children. J Bone Joint Surg Am. 1983;65:230–234. doi: 10.2106/00004623-198365020-00013. [DOI] [PubMed] [Google Scholar]
- 19.Karmazyn B, Kleiman MB, Buckwalter K, Loder RT, Siddiqui A, Applegate KE. Acute pyomyositis of the pelvis: the spectrum of clinical presentations and MR findings. Pediatr Radiol. 2006;36:338–343. doi: 10.1007/s00247-005-0082-1. [DOI] [PubMed] [Google Scholar]
- 20.Thomas S, Tytherleigh-Strong G, Dodds R. Pyomyositis of the iliacus muscle in a child. J Bone Joint Surg Br. 2001;83:619–620. doi: 10.1302/0301-620X.83B2.11404. [DOI] [PubMed] [Google Scholar]
- 21.Andrew JG, Czyz WM. Pyomyositis presenting as septic arthritis. A report of 2 cases. Acta Orthop Scand. 1988;59:587–588. doi: 10.3109/17453678809148792. [DOI] [PubMed] [Google Scholar]
- 22.Tong CW, Griffith JF, Lam TP, Cheng JC. The conservative management of acute pyogenic iliopsoas abscess in children. J Bone Joint Surg Br. 1998;80:83–85. doi: 10.1302/0301-620X.80B1.8005. [DOI] [PubMed] [Google Scholar]
- 23.Abernethy LJ, Lee YC, Cole WG. Ultrasound localization of subperiosteal abscesses in children with late-acute osteomyelitis. J Pediatr Orthop. 1993;13:766–768. doi: 10.1097/01241398-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Karmazyn B, Loder RT, Kleiman MB, Buckwalter KA, Siddiqui A, Ying J, Applegate KE. The role of pelvic magnetic resonance in evaluating nonhip sources of infection in children with acute nontraumatic hip pain. J Pediatr Orthop. 2007;27:158–164. doi: 10.1097/01.bpb.0000248563.18595.6b. [DOI] [PubMed] [Google Scholar]
- 25.Perry J, Barrack RL, Burke SW, Haddad RJ., Jr Psoas abscess mimicking a septic hip. Diagnosis by computed tomography. J Bone Joint Surg Am. 1985;67:1281–1283. [PubMed] [Google Scholar]
- 26.Ovadia D, Ezra E, Ben-Sira L, Kessler A, Bickels J, Keret D, Yaniv M, Wientroub S, Lokiec F. Primary pyomyositis in children: a retrospective analysis of 11 cases. J Pediatr Orthop B. 2007;16:153–159. doi: 10.1097/BPB.0b013e3280140548. [DOI] [PubMed] [Google Scholar]
- 27.King RJ, Laugharne D, Kerslake RW, Holdsworth BJ. Primary obturator pyomyositis: a diagnostic challenge. J Bone Joint Surg Br. 2003;85:895–898. [PubMed] [Google Scholar]


