Abstract
Children with spina bifida develop a wide variety of congenital and acquired orthopedic deformities. Among these are hip deformities such as contracture, subluxation, or dislocation. Patients may also have problems with the knee joint, such as knee flexion or extension contracture, knee valgus deformity, or late knee instability and pain. In addition, rotational deformities of the lower extremities, either internal or external torsion, are common as well. This paper will review both the overall orthopedic care of a patient with spina bifida and provide a focused review of the diagnosis and management of the above deformities. In addition, this paper will review the incidence, etiology, classification, and prognosis of spina bifida. The use of gait analysis and orthoses will be covered as well. The forthcoming Part II will cover foot and ankle deformities in spina bifida.
Keywords: Spina bifida, Myelomeningocele, Hip deformity, Knee valgus, Rotational deformity
Introduction
Neural tube defects (NTDs) result from failure of the neural tube to close during embryogenesis and are the cause of chronic disability of between 70,000 and 100,000 individuals in the United States [1]. Although the incidence of NTDs has declined in recent decades, spina bifida (also known as myelomeningocele) remains the most common NTD and is the most severely disabling birth defect compatible with survival [1].
Spina bifida is a myelodysplasia of the neural elements which manifests in the vertebrae as a defect in the posterior elements. Dysplasia of the spinal cord and nerve roots leads to bowel, bladder, motor, and sensory paralysis below the level of the lesion [2]. Patients with spina bifida may often have other associated spinal cord lesions, such as diastematomyelia or hydromyelia, or structural abnormalities of the brain, such as hydrocephalus, which may also compromise neurological function [2].
Both congenital and acquired orthopedic deformities are seen in patients with spina bifida. Examples of congenital deformities, which are present at birth, are kyphosis, hemivertebrae, teratologic hip dislocation, clubfoot, and vertical talus. Acquired developmental deformities are related to the level of involvement [3] and are caused by muscle imbalance, paralysis, and decreased sensation in the lower extremities [4]. Additionally, orthopedic problems may also be caused by iatrogenic injury such as postoperative tethered cord.
Incidence
The incidence of infants born with NTDs shows regional variations but is decreasing overall. The birth–prevalence rate of spina bifida from 1983 to 1990 in the United States was 4.6 per 10,000 [3]. Since that time, there has been a decrease in the number of new cases of spina bifida. This decrease can be attributed to two main factors: prenatal screening with elective termination of affected pregnancies and increased awareness of the importance of administration of folate to women before and during pregnancy, as recommended by the US Public Health Service.
An estimated 50–70% of NTDs can be prevented through the daily consumption of 400 μg of folic acid [5]. The US Food and Drug Administration mandated adding folic acid to all enriched grain products by January 1998 [6]. From October 1998 to December 1999, the birth–prevalence rate of spina bifida in the Unites States decreased 22.9% compared with 1995–1996 [6]. Furthermore, comparing the incidence during the period from 1999 to 2000 to the period from 2003 to 2005, the prevalence of spina bifida in the United States decreased 6.9%, from 2.04 to 1.90 per 10,000 live births [6].
In a population-based study examining the effect of folic acid supplementation on the prevalence of NTDs in 16 European countries, a 32% decrease in NTDs was found when comparing the periods from 1989–1991 and 1999–2001 in the United Kingdom and Ireland, where supplementation was introduced in the early 1990s [7]. A reduction in prevalence of NTDs of 17% was found in countries with folic acid supplementation introduced by 1999. In contrast, a decrease of 9% was seen in countries with no supplementation policy by 1999.
Etiology
Spina bifida results from failure of the neural tube to close during the fourth week of embryogenesis. The cause of this embryonic failure is not known but is suspected to be multifactorial in origin, involving both genetic and environmental factors. Folate deficiency is an important contributor to the cause of NTDs as evidenced by the decrease in incidence observed after folate supplementation. Other environmental factors have also been examined for a potential role in NTDs, including temperature, drug exposure, substance abuse, maternal infection, and other nutritional factors, such as vitamin B12 and zinc [8].
Genetic factors seem to play an important role in the development of spina bifida. Association with single gene defects, increased recurrence risk among siblings, and a higher frequency in twins than in singletons indicate a genetic contribution to the etiology [8]. A recent study investigated the role of cell adhesion molecules which are involved in cell–cell interactions as a possible factor in the formation and closure of the neural tube [9]. Animal studies have shown as many as 100 mutant genes which affect neurulation, and almost all have homologs in humans [8]. However, the low frequency of families with a significant number of NTD cases makes research into genetic causation difficult.
Overview of orthopedic care
The overall care of children with spina bifida has changed substantially over the past 30 years with regards to all specialties, including neurosurgery, urology, rehabilitation, orthotics, and orthopedics. In the realm of orthopedics, these changes have occurred as a result of a better understanding of deformities and their affect on function. The advent of gait analysis in the late 1980s has played a major role in shifting the focus of orthopedic treatment from radiological changes to functional improvement [10].
The main goal of orthopedic care of a patient with spina bifida is to correct deformities that may prevent the patient from using orthoses to ambulate during childhood [2]. In addition, the orthopedic surgeon must monitor spinal balance and deformity and assist in monitoring the neurologic status. The newborn examination should include identification of the level of paralysis for each extremity, as well as identify associated problems such as clubfoot or hip or knee contractures. The follow-up periodic orthopedic examination should include assessment of motor and sensory function, range of motion, spinal deformity, and integrity of skin. Changes should alert the physician to the possibility of tethered cord. In addition, mobility and bracing needs should be addressed to ensure that orthoses are appropriate, in good shape, and not causing any pressure points on the skin.
Orthopedic care of the child with spina bifida is made challenging by the presence of multiple medical comorbidities which must be taken into account in any treatment plan. These comorbidities include central nervous system involvement such as hydrocephalus, syringomyelia, and tethering, insensate skin, latex allergy, renal anomalies, and bowel and bladder incontinence [3]. In addition, patients with spina bifida may have precocious puberty, cognitive learning difficulties, or depression [2]. For this reason, whenever possible, orthopedic care should be rendered as part of a multi-disciplinary team, working together with neurosurgery, urology, and physiatry.
Providers should also be aware that patients with spina bifida are at increased risk for certain complications, hence, special precautions may be indicated. Complications in this population may relate to latex allergy or increased risk of post-operative infection. Infection of the urinary tract may result from bladder paralysis, and wound infection can result from the lack of protective pain sensation. In addition, special care must be taken to avoid pressure sores. Patients with spina bifida are also at increased risk for pathologic fractures due to joint contracture and post-surgical immobilization, especially spica casting. Pathologic fractures are more prevalent in patients with a higher level of neurologic involvement due to the presence of osteopenia related to their relative lack of mobility [4].
Prognosis for ambulation
Attaining early ambulation can provide physiological and psychological benefits to a child with spina bifida, even if that child will later become a sitter. A study comparing patients with high-level spina bifida who had participated in a walking program with those who had been prescribed a wheelchair early in life found patients who walked early had fewer fractures and pressure sores, were more independent, and were better able to transfer [11]. Of note, not all orthopedic surgeons support this idea, and another school of thought does exist which disputes the benefits of early ambulation.
Various factors affect the potential for ambulation in an individual patient with spina bifida. Among these are neurologic level of involvement, hip deformity, scoliosis, foot and ankle deformity, age, and obesity [12]. Multiple studies have demonstrated the crucial role that neurologic level of involvement and resulting muscle group strength plays in achieving and maintaining ambulation. In a study of 98 patients aged 5–31 years, 20 of 21 patients with L5 or sacral level of involvement were community ambulators [12]. In the same study, most L4 patients were also community ambulators, but patients with L3 or above level of involvement were mostly non-functional ambulators. Similarly, a review of 29 adult spina bifida patients aged 20–43 years found that 19 of 20 patients with L3 or lower level of involvement were ambulatory, while only 2 of 9 patients with L2 or above level of involvement remained ambulatory [13]. In this study, the status of the hip did not correlate with the ability to ambulate.
One of the most important physical factors for maintaining ambulation in adulthood is the strength of the quadriceps and hamstrings muscles [2, 12, 14]. In a study of 109 patients, quadriceps strength correlated strongly with ambulatory ability. Eighty-two percent of patients with grade 4 or 5 quadriceps power became community ambulators, and 98% were at least household ambulators [15]. Including grade 3 power, 89% were at least household ambulators. In contrast, 88% of patients with grade 0, 1, or 2 quadriceps power were not functional ambulators. Iliopsoas strength has also been shown to play an important role in ambulation. In a study of 291 patients with average age of 14.5 years at the last examination, 77 of 87 patients with iliopsoas strength grade 3 or less were non-ambulatory [16]. In the same study, 163 patients with symmetrical grade 4 or 5 iliopsoas strength were all ambulatory. In addition, one study found that achieving sitting balance was an important predictor of ambulatory potential in patients with higher levels of involvement [17].
Classification
The most widely used classification of spina bifida is based on the neurologic level of the lesion [17, 18] (see Table 1). Patients can be divided into three groups based on lesion level and accompanying functional and ambulatory capacity. Group 1 consists of thoracic and high-lumbar level patients. The functional hallmark of this group is the lack of quadriceps function. Consequently, in order to ambulate children requires orthoses which span the hip, and the majority of patients require a wheelchair for mobility in adulthood.
Table 1.
Spina bifida classification
| Group | Level of lesion | Functional hallmark | Ambulatory capacity | FMS classification |
|---|---|---|---|---|
| 1 | Thoracic, high-lumbar | Lack quadriceps function | As children, require hip-spanning orthosis for ambulation (RGO, HKAFO) In adulthood, majority require wheelchair for mobility |
FMS 1,1,1 |
| 2 | Low-lumbar | Lack gluteus medius and maximus function Retain quadriceps and medial hamstring function |
Require crutches and AFOs for ambulation Most retain community ambulation as adults |
FMS 3,3,1 |
| 3 | Sacral | Retain quadriceps and gluteus medius function | FMS 6,6,6 | |
| High-sacral | Lack gastrocnemius-soleus function | Ambulate with AFOs and no support | ||
| Low-sacral | Retain gastrocnemius-soleus function | Ambulate without braces or support |
Group 2 patients have low-lumbar level of involvement. These patients retain quadriceps and medial hamstring function but lack function of the gluteus medius and maximus. Patients in this group require crutches and braces to control foot and ankle position in order to ambulate. Approximately 80% of the patients in this group maintain community ambulation as adults [17]. Since medial hamstring function is needed for community ambulation, there is a notable difference in ambulatory ability between children with L4 and L3 level lesions [12]. For this reason, children with L4 level of involvement have the most potential benefit from proper orthopedic care of musculoskeletal deformities [18].
Patients in Group 3 have sacral level of involvement and demonstrate both quadriceps and gluteus medius function. Group 3 can be further subdivided into high-sacral and low-sacral level of involvement, distinguished by the presence of gastrocnemius-soleus strength in low-sacral patients. Patients with high-sacral level walk without support but with the use of braces for the foot and ankle. These children have a characteristic gluteus lurch during gait. Low-sacral level patients walk without braces with a gait that is close to normal.
Functional Mobility Scale
The Functional Mobility Scale (FMS) was initially described in 2004 as a useful tool to describe functional mobility in children with cerebral palsy [19]. Recently, it has also been applied to children with spina bifida [20]. The FMS is unique because it allows quick, practical scoring of mobility over three distinct distances representing mobility in the home (5 m), at school (50 m), and in the community (500 m). In this way, it is effective for distinguishing between groups of children with varying levels of disabilities and provides a means for standardized communication between health professionals [19].
For each of the three distances assessed, a child is given a score from one to six based on their walking ability. A score of one is used when a child uses a wheelchair, two for a walker, three for the use of two crutches, four for the use of one crutch, five for a child who is independent on level surfaces, and six for a child who is independent on all surfaces. Two additional ratings used are C for a child who crawls for mobility in the home and N for a child who does not complete the given distance. For example, a child who uses a wheelchair for long distances but ambulates with crutches at home and school would be an FMS 3,3,1 (see Table 1).
Gait analysis
Computerized gait analysis is a valuable component of the comprehensive orthopedic evaluation of patients with spina bifida. It is especially useful in pre-operative planning for ambulatory patients and in quantitatively assessing surgical results. Its use has been reported in the literature for analyzing many manifestations of spina bifida, including hip subluxation/ dislocation, lower extremity contractures, and rotational abnormalities [21–25]. Clinical evaluation of patients with spina bifida is complex and involves multiple inter-related levels of deformity. Often, a patient’s true functional status is different from what would be expected based on information obtained during the static clinical examination [23]. This was demonstrated in a study examining crouched gait in patients with spina bifida [23]. The authors found a significantly greater amount of dynamic knee flexion during ambulation using gait analysis than what was measured on clinical examination. Computerized gait analysis should be employed as an important part of the examination of patients with spina bifida, especially when surgical treatment is being considered.
Orthoses
Almost all children with spina bifida, with the exception of some patients with low-sacral level involvement, will require the use of orthoses for ambulation. There are many indications for the use of orthoses in the management of children with spina bifida, including maintenance of alignment, prevention of deformity, correction of flexible deformity, facilitation of independent mobility, and protection for the insensate limb.
Children with thoracic and high-lumbar level involvement often have hip flexor and adductor function, but not quadriceps function, hence, orthoses are needed for upright weight-bearing and mobility. This begins with a standing frame, often prescribed around 12 months of age or once the child demonstrates development of head and neck control. Patients will then require an orthosis which crosses and controls the hip in order to control the trunk over the pelvis and lower limbs, such as a hip–knee–ankle–foot orthosis (HKAFO) or reciprocating gait orthosis (RGO) (Fig. 1). The RGO is usually introduced around 24 months of age. An important factor in the ability to walk with an RGO is sitting balance without hand support. If a child is unable to do this, he or she will be better served with a parapodium. It is important for providers to recognize that most patients with higher levels of involvement will eventually choose a wheelchair as a more energy-efficient way to achieve mobility [2].
Fig. 1.
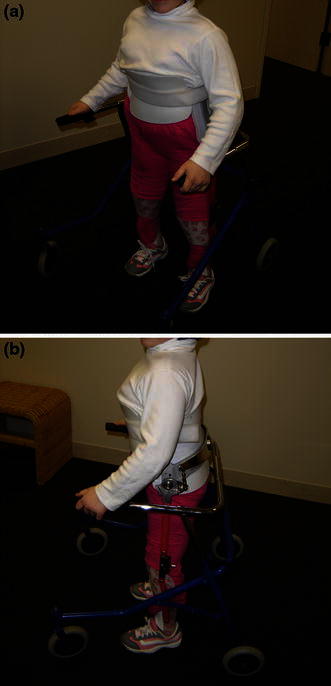
a Reciprocating gait orthosis (RGO), frontal view. b RGO, side view
Patients with sacral or low-lumbar level involvement require a solid ankle–foot orthosis (AFO) to address the muscle weakness present below the knee [24]. The solid AFO substitutes for weak or absent ankle plantar flexors and dorsiflexors. Because these patients also have weakness of hip extensors and abductors, the use of forearm crutches should be considered to improve pelvic and hip kinematics. Crutches allow the upper extremities to share in weight-bearing, hence, decreasing the demand on the lower extremity musculature, allowing a more functional gait pattern [24].
Patients with rotational deformities, either internal or external, will benefit from AFOs with twister cables (Fig. 2) to help control alignment until an appropriate age for surgical correction is reached, usually around 6 years [18]. AFOs with twister cables can be introduced as early as 2 years of age. In addition, in patients who are too young for osteotomies who develop excessive valgus stress at the knee joint due to rotational malalignment, a knee–ankle–foot orthosis (KAFO) with a free knee joint may be used [18].
Fig. 2.
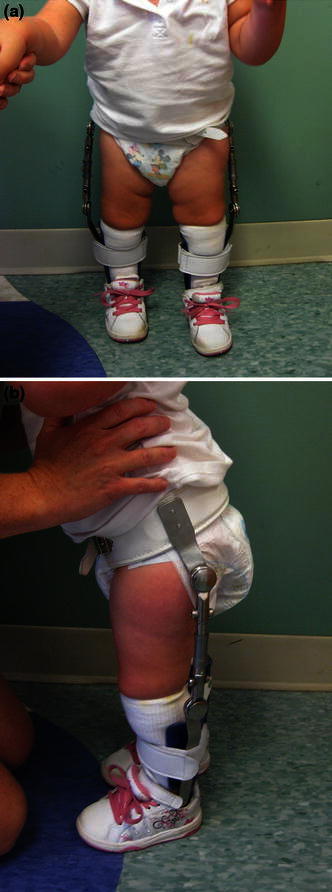
a Ankle–foot orthosis (AFOs) with twister cables, frontal view. b AFO with twister cables, side view
Hip
Hip deformity in patients with spina bifida results from muscle imbalance and paralysis around the hip joint and may present as contracture, subluxation, or dislocation [4]. If not treated properly, contractures can lead to pelvic obliquity and compensatory spinal abnormality [4]. Paralytic hip dislocation is a common and complicated problem. In the past, transfer of the iliopsoas tendon along with open reduction and capsular plication was used in patients with spina bifida in order to achieve and maintain reduction of paralytic hip dislocations. However, current treatment goals based on studies of functional results focus on maintaining hip range of motion with contracture release only [26].
Many of the earlier studies reporting on the results of major hip reconstruction in spina bifida patients classified success or failure solely on the basis of radiological results demonstrating the maintenance of hip reduction. The functional consequences of such an extensive surgical procedure for patients with spina bifida were often not assessed. Concerns developed regarding whether the radiological success of hip reduction led to restricted range of motion and pathologic fractures, thus, compromising the functional result [27].
Subsequent studies of functional results found that the presence of a concentric reduction did not lead to improved hip range of motion or the ability to ambulate. One series of 76 patients compared the functional results in those who had undergone surgical treatment to reduce the hip to those who had not [28]. The authors found that the presence of a concentric reduction did not lead to improved hip range of motion or ability to ambulate, nor did it provide a decrease in pain or need for bracing.
Another series examined 20 patients with low-lumbar spina bifida who walked with crutches and underwent three-dimensional gait analysis to determine the influence of unilateral hip dislocation on gait [22]. The authors reported that the walking speed of patients with dislocated hips was 60% of normal, which corresponds to the walking speed in low-lumbar patients without hip dislocation in previous studies from the same center. The authors also noted that gait symmetry corresponded to either the absence of hip contractures or bilateral symmetrical contractures. They found no relationship between gait symmetry and hip dislocation and concluded that there is no indication for surgical relocation of the unilaterally unstable hip. Rather, they recommend correcting unilateral soft tissue contractures in order to restore gait symmetry.
Many authors have noted a high rate of complications leading to decrease in ambulatory function in patients treated surgically for the reduction of hip dislocation. One series reported a high rate of complications, including loss of motion (29%) and pathologic fractures (17%), in a surgically treated group [28]. In another series, 36% of patients had worsened ambulatory capacity as a result of surgical complications [29]. Another series reported that 11% of patients had a worsening of their neurological deficit after surgery to reduce the hip [27]. Although surgical treatment may allow reduction of the dislocated hip, this result must be weighed in terms of the potential for complications and functional decline. Another factor is the likely need for subsequent procedures to maintain reduction and the effects of prolonged treatment on the patient and family [30].
Multiple authors have concluded that the most important factor in determining the ability to walk is level of neural involvement and not the status of the hip [22, 27–29, 31]. In a review comparing 30 patients with no surgical treatment of hip dislocation with 11 patients who had surgical treatment, the authors found that the ability to walk was independent of hip reduction, but, instead, depended on neurological level [29]. The authors of the previously reported comparative review came to the same conclusion [28]. Correspondingly, authors have stressed that the preservation of muscle strength, specifically of the iliopsoas and quadriceps, is more relevant to determining potential for adult ambulation than the status of the hip joint. Treatment goals should include a level pelvis and free motion of the hips rather than radiographic reduction of the hip [28]. Especially in high dislocations and older children, the only recommended surgical treatment is contracture release [22, 28].
An important consideration not addressed by any of the currently existing studies is the question of how to treat the rare sacral-level patient with a dislocated hip who walks without support. These patients may demonstrate an increased lurch due to the loss of a fulcrum resulting from the dislocated hip. Consequently, these patients may benefit from surgical reduction, and further studies are necessary to examine this issue.
Knee
Patients with spina bifida may have involvement of the knee joint in the form of knee flexion or extension contracture, knee valgus deformity, or late knee instability and pain. Contractures occur most commonly in patients with lower thoracic and high lumbar lesions, and less often in patients with lower lumbar lesions [4]. Deformity at the knee joint results from many factors, including static forces of positioning, fibrosis of surrounding muscles, muscle imbalance around the knee joint, and fracture malunion.
Knee flexion contractures may occur in both ambulatory and non-ambulatory patients, but tend to be of greater magnitude in thoracic-level as opposed to lumbar-level patients. Many factors contribute to the development of knee flexion contracture, including gradual contracture of the hamstrings with contracture of the posterior knee capsule due to quadriceps weakness and prolonged sitting, spasticity of the hamstrings due to tethered cord, and quadriceps weakness with paralysis of the gastrocnemius-soleus and gluteus muscles [18]. In ambulatory patients, flexion deformity of greater than 20° can interfere with orthotic fitting and ambulation [32]. Increased knee flexion during gait causes increased oxygen cost and less efficient ambulation [23]. Non-ambulatory patients can usually tolerate a greater degree of contracture without severe limits on mobility and transfer. Flexion contractures that interfere with ambulation or with transfers or sitting balance in non-ambulatory patients respond well to radical knee flexor release including the hamstrings, gastrocnemius, and posterior capsule. In a prospective review of 45 knees with flexion contracture in patients with spina bifida, the mean knee flexion contracture decreased from 39° before surgery to 5° after surgical release with an average follow-up of 13 years [33]. The authors noted improvement in gait after surgical release, especially in low-lumbar level patients, and noted recurrence most commonly in thoracic-level patients.
Knee extension contracture is less common than knee flexion contracture and may occur secondary to unopposed quadriceps function with weak hamstrings, extensive bracing in extension, or surgical treatment for flexion contracture [2]. However, most cases are congenital and occur bilaterally. Often, there are associated congenital anomalies such as an ipsilateral teratologic hip dislocation or clubfoot [18]. Initial treatment consists of serial casting attempting to achieve at least 90° of knee flexion, which is successful in most patients. For patients with persistent extension contracture that interferes with gait, a VY quadriceps plasty has been shown to successfully improve gait and sitting. One study reported that 13 of 15 patients treated with VY quadriceps plasty maintained at least 90° of flexion at 43 months follow-up [32]. In non-ambulatory patients in whom extension contracture causes difficulty with sitting and transfers, tenotomy of the patellar tendon is an option. In a series of eight patients without active quadriceps function, the authors noted 50–70° of knee flexion with tenotomy of the patellar tendon and 90° or more of flexion with division of the medial and lateral retinacula as well [34]. A successful result was achieved in five out of eight patients without further surgery required at follow-up of at least 4 years. The authors stress that this technique is recommended only for patients without normal quadriceps function and they would, otherwise, would perform a formal quadricepsplasty.
Another problem seen frequently in spina bifida, especially in low-lumbar and sacral-level patients, is valgus knee deformity leading to instability, pain, and arthritis in adulthood. A study of 72 community ambulators greater than 23 years of age found that 17 (24%) had significant knee symptoms [35]. Gait analysis has allowed the identification of multiple factors contributing to abnormal valgus stress, including rotational malalignment of the femur, femoral anteversion in association with excessive external tibial torsion, excessive trunk and pelvic movement, and knee flexion contractures [10, 24, 36, 37]. Surgical correction of excessive rotational deformities is indicated in patients over the age of 6 years and has been shown to lead to a significant improvement in knee stress and pain and may prevent the onset of late degenerative changes [21]. If knee valgus is associated with knee flexion contracture or hindfoot valgus, the surgical correction of these deformities is required at the same setting. In addition, the use of an AFO, forearm crutches, or a combination of both should be encouraged to increase stance-phase stability and decrease stress in the knee joint [24, 36].
Rotational deformities
Both ambulatory and non-ambulatory patients with spina bifida frequently develop torsional deformities of the lower extremities involving the femur and/or the tibia. The femoral torsion present at birth in all newborns does not decrease normally with growth in a child with spina bifida due to the presence of abnormal gait and activity levels [4]. Tibial torsion is even more common in patients with spina bifida than femoral torsion. Tibial torsion can be either external, which is acquired secondary to muscle imbalance, or internal, which is congenital and frequently associated with clubfoot deformity.
For non-ambulatory patients, torsional deformities are largely a cosmetic problem. Initially, ambulatory patients can be treated conservatively with orthoses such as AFOs with twister cables. However, many ambulatory patients will go on to develop severe femoral and tibial rotational deformities, which can lead to labored gait, difficulty with orthotic fitting resulting in skin ulceration, and pain [21]. For ambulatory patients, the goal is to minimize bracing requirements while achieving as normal a gait as possible [38]. A careful assessment of the patient’s gait, including computerized gait analysis if available, should be done to determine the extent of deformity correction needed before recommending rotational osteotomy.
Internal tibial torsion causes gait disturbance when the foot of one extremity catches on the contralateral side during swing [2]. Internal torsion can be treated by rotational osteotomy using the technique described previously [39]. We have found successful healing using this technique with a few modifications. We perform the osteotomy distally using a drill first to create multiple holes along the path of the intended osteotomy and then complete the osteotomy with a saw in the hope of decreasing thermal insult to the bone. In addition, we fix the osteotomy using a dynamic compression plate to provide stable fixation.
External rotation deformity may result from the hip but is more commonly due to external tibial torsion, which interferes with gait and causes difficulty with orthotic fit. The external rotation of the tibia places the medial malleolus in the line of progression and can cause rubbing against the AFO, leading to skin breakdown [2]. Treatment of this deformity with internal rotational osteotomy of the tibia should be considered when external torsion is greater than 20° [40]. We use the same technique as for correcting internal torsion. In addition, it is essential to examine the patient’s entire lower extremity, paying particular attention to the hindfoot, as we have noticed a correlation between external tibial torsion and hindfoot valgus. Often, both deformities require treatment in order to achieve a successful result. Treatment of the hindfoot valgus consists of a medial sliding osteotomy of the os calcis.
Studies have reported successful results in terms of gait parameters and range of motion in 80–90% of patients treated with lower extremity osteotomies [38, 41]. Additionally, derotation osteotomy may delay or prevent the onset of late degenerative changes about the knee for patients with excessive external tibial torsion [21]. One study found increased valgus knee stress in eight out of eight patients with external tibial torsion [21]. A significant improvement in the abnormal knee moment was seen after derotational osteotomy. However, serious consideration must be given to the increased risk of complications in patients with spina bifida undergoing tibial osteotomies, including delayed union and wound infection [41].
Post-operative care
For patients with spina bifida, care must be taken during the post-operative period to prevent known complications, such as skin breakdown and post-immobilization fractures. Unless absolutely necessary, the use of a spica total body cast should be avoided. A custom-molded total body splint may be used as an alternative to a spica cast, even in patients who have undergone bony surgical procedures (Fig. 3). Rigid internal fixation should be used rather than Kirschner wire fixation in order to decrease the risk of non-union, allow early mobilization, and allow early weight-bearing. Post-operative physical therapy should begin as soon as wounds are stable and signs of healing are present in order to work on active and passive range of motion and initiate early weight-bearing. Once immobilization is discontinued, crawling should be strictly forbidden for at least 3–4 weeks to prevent the risk of fracture.
Fig. 3.
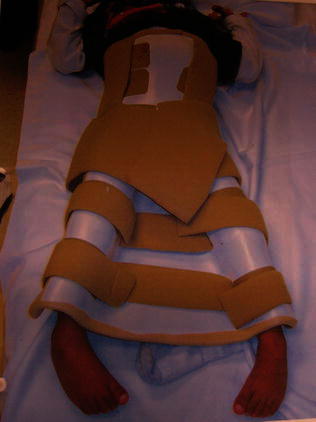
Custom-molded total body splint
Conclusion
The orthopedic care of patients with spina bifida is both challenging and rewarding. Due to the many medical comorbidities involved, careful evaluation and management of these patients should occur as part of a team approach involving members from multiple specialties, including urology, neurosurgery, pediatrics, physiatry, orthotics, physical therapy, and social work. As part of this team, the goal of the orthopedic surgeon should be to minimize deformity and maximize function and mobility, while limiting complications.
References
- 1.McLone DG, Bowman RM (2009) Overview of the management of myelomeningocele—I. In: Rose BD (ed) UpToDate. Waltham, MA
- 2.(2008) Neuromuscular disorders. In: Herring J (ed) Tachdjian’s pediatric orthopaedics. Saunders Elsevier, Philadelphia, pp 1405–1453
- 3.Guille JT, Sarwark JF, Sherk HH, Kumar SJ. Congenital and developmental deformities of the spine in children with myelomeningocele. J Am Acad Orthop Surg. 2006;14:294–302. doi: 10.5435/00124635-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Westcott MA, Dynes MC, Remer EM, Donaldson JS, Dias LS. Congenital and acquired orthopedic abnormalities in patients with myelomeningocele. Radiographics. 1992;12:1155–1173. doi: 10.1148/radiographics.12.6.1439018. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Racial/ethnic differences in the birth prevalence of spina bifida—United States, 1995–2005. MMWR Morb Mortal Wkly Rep. 2009;57(53):1409–1413. [PubMed] [Google Scholar]
- 7.Busby A, Abramsky L, Dolk H, Armstrong B, Eurocat Folic Acid Working Group Preventing neural tube defects in Europe: population based study. BMJ. 2005;330:574–575. doi: 10.1136/bmj.330.7491.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto) 2006;46:55–67. doi: 10.1111/j.1741-4520.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Deak KL, Boyles AL, Etchevers HC, Melvin EC, Siegel DG, Graham FL, Slifer SH, Enterline DS, George TM, Vekemans M, McClay D, Bassuk AG, Kessler JA, Linney E, Gilbert JR, Speer MC. SNPs in the neural cell adhesion molecule 1 gene (NCAM1) may be associated with human neural tube defects. Hum Genet. 2005;117:133–142. doi: 10.1007/s00439-005-1299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias L. Orthopaedic care in spina bifida: past, present, and future. Dev Med Child Neurol. 2004;46:579. doi: 10.1111/j.1469-8749.2004.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazur JM, Shurtleff D, Menelaus M, Colliver J. Orthopaedic management of high-level spina bifida. Early walking compared with early use of a wheelchair. J Bone Joint Surg Am. 1989;71:56–61. [PubMed] [Google Scholar]
- 12.Asher M, Olson J. Factors affecting the ambulatory status of patients with spina bifida cystica. J Bone Joint Surg Am. 1983;65:350–356. [PubMed] [Google Scholar]
- 13.Barden GA, Meyer LC, Stelling FH., 3rd Myelodysplastics—fate of those followed for twenty years or more. J Bone Joint Surg Am. 1975;57:643–647. [PubMed] [Google Scholar]
- 14.Seitzberg A, Lind M, Biering-Sørensen F. Ambulation in adults with myelomeningocele. Is it possible to predict the level of ambulation in early life? Childs Nerv Syst. 2008;24:231–237. doi: 10.1007/s00381-007-0450-2. [DOI] [PubMed] [Google Scholar]
- 15.Schopler SA, Menelaus MB. Significance of the strength of the quadriceps muscles in children with myelomeningocele. J Pediatr Orthop. 1987;7:507–512. doi: 10.1097/01241398-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 16.McDonald CM, Jaffe KM, Mosca VS, Shurtleff DB. Ambulatory outcome of children with myelomeningocele: effect of lower-extremity muscle strength. Dev Med Child Neurol. 1991;33:482–490. doi: 10.1111/j.1469-8749.1991.tb14913.x. [DOI] [PubMed] [Google Scholar]
- 17.Swank M, Dias LS. Walking ability in spina bifida patients: a model for predicting future ambulatory status based on sitting balance and motor level. J Pediatr Orthop. 1994;14:715–718. doi: 10.1097/01241398-199414060-00005. [DOI] [PubMed] [Google Scholar]
- 18.Dias L (2002) Myelomeningocele and intraspinal lipoma. In: Sponseller PD (ed) Orthopaedic knowledge update: pediatrics 2nd edn. American Academy of Orthopaedic Surgeons, pp 249–259
- 19.Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS) J Pediatr Orthop. 2004;24(5):514–520. doi: 10.1097/01241398-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Battibugli S, Gryfakis N, Dias L, Kelp-Lenane C, Figlioli S, Fitzgerald E, Hroma N, Seshadri R, Sullivan C. Functional gait comparison between children with myelomeningocele: shunt versus no shunt. Dev Med Child Neurol. 2007;49:764–769. doi: 10.1111/j.1469-8749.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 21.Dunteman RC, Vankoski SJ, Dias LS. Internal derotation osteotomy of the tibia: pre- and postoperative gait analysis in persons with high sacral myelomeningocele. J Pediatr Orthop. 2000;20:623–628. doi: 10.1097/01241398-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Gabrieli APT, Vankoski SJ, Dias LS, Milani C, Lourenco A, Filho JL, Novak R. Gait analysis in low lumbar myelomeningocele patients with unilateral hip dislocation or subluxation. J Pediatr Orthop. 2003;23:330–334. [PubMed] [Google Scholar]
- 23.Moen T, Gryfakis N, Dias L, Lemke L. Crouched gait in myelomeningocele: a comparison between the degree of knee flexion contracture in the clinical examination and during gait. J Pediatr Orthop. 2005;25(5):657–660. doi: 10.1097/01.mph.0000165136.76238.23. [DOI] [PubMed] [Google Scholar]
- 24.Vankoski S, Moore C, Statler KD, Sarwark JF, Dias L. The influence of forearm crutches on pelvic and hip kinematics in children with myelomeningocele: don’t throw away the crutches. Dev Med Child Neurol. 1997;39:614–619. doi: 10.1111/j.1469-8749.1997.tb07497.x. [DOI] [PubMed] [Google Scholar]
- 25.Duffy CM, Hill AE, Cosgrove AP, Corry IS, Mollan RA, Graham HK. Three-dimensional gait analysis in spina bifida. J Pediatr Orthop. 1996;16:786–791. doi: 10.1097/01241398-199611000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Swaroop VT, Dias LS. What is the optimal treatment for hip and spine in myelomeningocele? In: Wright JG, editor. Evidence-based orthopaedics. Amsterdam: Elsevier Health Sciences; 2008. pp. 273–277. [Google Scholar]
- 27.Sherk HH, Ames MD. Functional results of iliopsoas transfer in myelomeningocele hip dislocations. Clin Orthop Relat Res. 1978;137:181–186. [PubMed] [Google Scholar]
- 28.Feiwell E, Sakai D, Blatt T. The effect of hip reduction on function in patients with myelomeningocele. Potential gains and hazards of surgical treatment. J Bone Joint Surg Am. 1978;60:169–173. [PubMed] [Google Scholar]
- 29.Sherk HH, Uppal GS, Lane G, Melchionni J. Treatment versus non-treatment of hip dislocations in ambulatory patients with myelomeningocele. Dev Med Child Neurol. 1991;33:491–494. doi: 10.1111/j.1469-8749.1991.tb14914.x. [DOI] [PubMed] [Google Scholar]
- 30.Swaroop VT, Dias LS. Strategies of hip management in myelomeningocele: to do or not to do. Hip Int. 2009;19:S53–S55. doi: 10.1177/112070000901906s09. [DOI] [PubMed] [Google Scholar]
- 31.Feiwell E. Surgery of the hip in myelomeningocele as related to adult goals. Clin Orthop Relat Res. 1980;148:87–93. [PubMed] [Google Scholar]
- 32.Dias LS. Surgical management of knee contractures in myelomeningocele. J Pediatr Orthop. 1982;2:127–131. doi: 10.1097/01241398-198202020-00002. [DOI] [PubMed] [Google Scholar]
- 33.Marshall PD, Broughton NS, Menelaus MB, Graham HK. Surgical release of knee flexion contractures in myelomeningocele. J Bone Joint Surg Br. 1996;78:912–916. doi: 10.1302/0301-620X78B6.1254. [DOI] [PubMed] [Google Scholar]
- 34.Sandhu PS, Broughton NS, Menelaus MB. Tenotomy of the ligamentum patellae in spina bifida: management of limited flexion range at the knee. J Bone Joint Surg Br. 1995;77:832–833. [PubMed] [Google Scholar]
- 35.Williams JJ, Graham GP, Dunne KB, Menelaus MB. Late knee problems in myelomeningocele. J Pediatr Orthop. 1993;13:701–703. doi: 10.1097/01241398-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Selber P, Dias L. Sacral-level myelomeningocele: long-term outcome in adults. J Pediatr Orthop. 1998;18:423–427. [PubMed] [Google Scholar]
- 37.Lim R, Dias L, Vankoski S, Moore C, Marinello M, Sarwark J. Valgus knee stress in lumbosacral myelomeningocele: a gait-analysis evaluation. J Pediatr Orthop. 1998;18:428–433. [PubMed] [Google Scholar]
- 38.Dias LS, Jasty MJ, Collins P. Rotational deformities of the lower limb in myelomeningocele. Evaluation and treatment. J Bone Joint Surg Am. 1984;66:215–223. [PubMed] [Google Scholar]
- 39.Dodgin DA, De Swart RJ, Stefko RM, Wenger DR, Ko JY. Distal tibial/fibular derotation osteotomy for correction of tibial torsion: review of technique and results in 63 cases. J Pediatr Orthop. 1998;18:95–101. [PubMed] [Google Scholar]
- 40.Vankoski SJ, Michaud S, Dias L. External tibial torsion and the effectiveness of the solid ankle-foot orthoses. J Pediatr Orthop. 2000;20:349–355. [PubMed] [Google Scholar]
- 41.Fraser RK, Menelaus MB. The management of tibial torsion in patients with spina bifida. J Bone Joint Surg Br. 1993;75:495–497. doi: 10.1302/0301-620X.75B3.8496230. [DOI] [PubMed] [Google Scholar]


