EMBO J 28 22, 3500–3513 (2009); published online 10 September 2009
SnoN was first identified based on its homology with the proto-oncogene c-Ski, and has since been implicated as a promoter of oncogenic transformation and cancer progression. Consistent with a role as proto-oncogene, SnoN negatively regulates TGF-β signalling, through its interactions with Smad complexes. Thus, SnoN inhibits the growth inhibitory effect of TGF-β, which is considered as the basis for the tumour suppressor activity of TGF-β signalling. In this issue of The EMBO Journal, Pan et al (2009) now demonstrate that SnoN also functions as a tumour suppressor, independently of its role in Smad signalling. The tumour suppressor role of SnoN results from its interaction with the promyelocytic leukaemia (PML) protein and the accumulation of SnoN in PML nuclear bodies, thus allowing SnoN to stabilize p53 and induce premature senescence.
c-Ski and SnoN are members of a small Ski family of transcription regulators defined by their homology with the viral transforming protein v-Ski. This homology defines structural domains that mediate dimerization and interactions with transcription co-regulators, including Smad4, the common mediator Smad of TGF-β family-induced transcription responses (Deheuninck and Luo, 2009). c-Ski has been shown to induce malignant transformation and enhance tumourigenesis (Colmenares et al, 1991; Kiyono et al, 2009). Consistent with the identity of c-Ski as proto-oncogene, SnoN was also shown to be oncogenic. Increased SnoN expression promotes the transformation of avian fibroblasts, and elevated SnoN levels are observed in many tumours (Boyer et al, 1993; Deheuninck and Luo, 2009). Further, silencing SnoN expression by RNA interference results in the inhibition of tumourigenic properties of cancer cells in vitro and in vivo (Zhu et al, 2007).
The oncogenic functions of c-Ski and SnoN have been reinforced by insights into their roles as inhibitors of TGF-β signalling. TGF-β signalling has a tumour suppressor role in the initiation and progression of carcinomas, resulting from autocrine inhibition of cell proliferation by TGF-β (Bierie and Moses, 2006). Increased c-Ski or SnoN expression, as observed in tumour cells, inhibits TGF-β signalling, alleviating TGF-β's growth control and tumour suppressor activities (Figure 1). The inhibition of TGF-β signalling by c-Ski or SnoN results from direct interactions with the TGF-β-activated Smad complexes, which mediate the TGF-β-induced gene expression responses, thus providing a scenario to explain the oncogenic activities of c-Ski and SnoN (Deheuninck and Luo, 2009).
Figure 1.
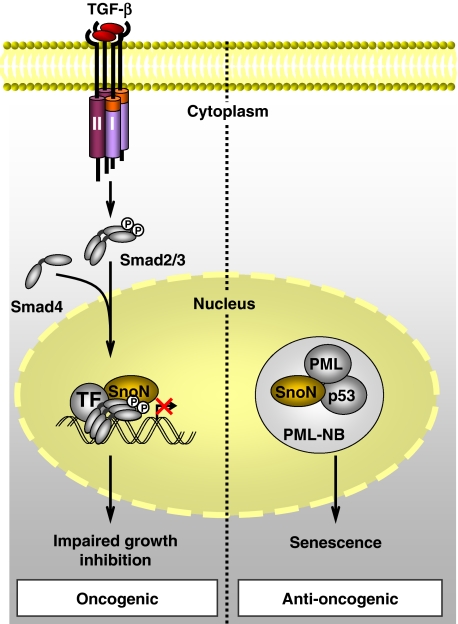
Oncogenic and anti-oncogenic roles of SnoN. In the nucleus, SnoN can bind the Smad complex with transcription factors (TF) and repress TGF-β-induced inhibition of proliferation, thus acting as an oncogene. Independently of the Smad interactions, SnoN can interact with the promyelocytic leukaemia (PML) protein in PML nuclear bodies (PML-NB), resulting in stabilization of p53 expression. This leads to premature senescence and defines an anti-oncogenic role for SnoN.
The present findings should be seen against the established perception that c-Ski and SnoN are bona fide oncogenes, and the knowledge of how SnoN (or c-Ski) functions as oncogene. Therefore, the authors' conclusion that SnoN acts as a tumour suppressor may come as a surprise. Using a mutant of SnoN that no longer interacts with TGF-β-activated Smad complexes and does not interfere with TGF-β signalling, the authors show that increased SnoN expression in mice decreases the susceptibility to carcinogen-induced skin tumourigenesis. Further, high SnoN levels inhibit the transformation of mouse fibroblasts induced by Ras and Myc in vitro (Pan et al, 2009). Considering the nature of the SnoN mutant, these anti-oncogenic properties of SnoN occur independently of its ability to regulate TGF-β signalling. That SnoN has anti-oncogenic properties is consistent with previous observations. Mice lacking one copy of the snoN gene are more susceptible to carcinogen-induced tumour formation than wild-type mice with two gene copies, thereby allowing the conclusion that SnoN acts as tumour suppressor in at least some cell types (Shinagawa et al, 2000). Previous studies in the authors' laboratory also revealed an ambivalence in the function of SnoN in tumourigenesis; increased SnoN expression promoted tumourigenesis but inhibited epithelial–to–mesenchymal transition, and decreased SnoN expression enhanced cancer metastasis in a mouse model (Zhu et al, 2007). Finally, in patients with Barrett's oesophageal disease, SnoN is expressed at high level in low-grade dysplasia, but is absent in high-grade dysplasia and adenocarcinoma, consistent with an anti-oncogenic role (Villanacci et al, 2008).
The key contribution of this paper is that the authors have linked the tumour suppressor effect of SnoN to the induction of senescence, through its effect on the promyelocytic leukaemia (PML) and p53 proteins, which have been implicated in senescence. Indeed, the lower susceptibility of mice expressing the SnoN mutant to carcinogen-induced tumourigenesis is accompanied with increased senescence of the tumour cells in vivo. Moreover, fibroblasts derived from these mice senesce prematurely in culture, when compared with wild-type cells, and the anti-oncogenic effect of SnoN on the transformation of these cells by Ras and Myc was again linked to senescence. It is important to note that the authors have demonstrated that SnoN-mediated senescence occurs in a Smad-independent and p53-dependent way. The increased expression of PML protein in SnoN expressing cells and the interaction of SnoN with PML protein, leading to the gradual accumulation of SnoN in PML nuclear bodies, result in p53 stabilization and induction of premature senescence (Figure 1). Thus, the induction of senescence by SnoN depends on its interaction with PML and consequent stabilization of p53 (Pan et al, 2009).
The fact that induction of senescence provides a mechanism for tumour suppression is well established, and that p53 has a key role in the induction of senescence is also well documented. As SnoN can function as an oncogene, one may propose that the induction of senescence resembles other scenarios of oncogene-induced senescence. However, oncogene-induced senescence involves increased expression of p19ARF and the cell-cycle inhibitor p16INK4A, and a p53-dependent DNA-damage response (Campisi, 2005). In contrast, Pan et al (2009) show that increased expression of a non-oncogenic SnoN mutant, which cannot associate with the Smad complex, induces an increase in p53 level independently of changes in p19ARF or p16INK4A expression, or DNA damage pathways, illustrating a different mechanism. Furthermore, the colocalization of SnoN with PML in the nuclear bodies raises the possibility that functions of PML and PML nuclear bodies are affected. These results therefore provide a novel mechanism for the anti-oncogenic function of SnoN, distinct from oncogene-induced cellular senescence.
In closing, how do we deal conceptually with a protein that can function both as oncogene and as tumour suppressor? As the oncogenic and anti-oncogenic functions result from different mechanisms that can be uncoupled, that is inhibitory interactions with TGF-β/Smad signalling versus stabilizing interactions with PML and p53, one can envision cell context-dependent scenarios in which one mechanism is favoured over the other (Figure 1). For example, functional inactivation of the interactions of SnoN with the TGF-β-activated Smad complexes should result in a potent tumour suppressor effects. Alternatively, downregulating p53 or PML expression will favour the oncogenic function of SnoN. Clearly, the current observations, in combination with the authors' previous findings on interference with Smad signalling by SnoN, now provide a solid basis for targeted studies to resolve as to how the oncogenic versus anti-oncogenic activities are balanced during cancer progression in different tumour types.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bierie B, Moses HL (2006) TGF-β and cancer. Cytokine Growth Factor Rev 17: 29–40 [DOI] [PubMed] [Google Scholar]
- Boyer PL, Colmenares C, Stavnezer E, Hughes SH (1993) Sequence and biological activity of chicken SnoN cDNA clones. Oncogene 8: 457–466 [PubMed] [Google Scholar]
- Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522 [DOI] [PubMed] [Google Scholar]
- Colmenares C, Sutrave P, Hughes SH, Stavnezer E (1991) Activation of the c-ski oncogene by overexpression. J Virol 65: 4929–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deheuninck J, Luo K (2009) Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res 19: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono K, Suzuki HI, Morishita Y, Komuro A, Iwata C, Yashiro M, Hirakawa K, Kano MR, Miyazono K (2009) c-Ski overexpression promotes tumor growth and angiogenesis through inhibition of transforming growth factor-β signaling in diffuse-type gastric carcinoma. Cancer Sci 100: 1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Zhu Q, Luo K (2009) SnoN functions as a tumour suppressor by inducing premature senescence. EMBO J 28: 3500–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa T, Dong HD, Xu M, Maekawa T, Ishii S (2000) The sno gene, which encodes a component of the histone deacetylase complex, acts as a tumor suppressor in mice. EMBO J 19: 2280–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanacci V, Bellone G, Battaglia E, Rossi E, Carbone A, Prati A, Verna C, Niola P, Morelli A, Grassini M, Bassotti G (2008) Ski/SnoN expression in the sequence metaplasia-dysplasia-adenocarcinoma of Barrett's esophagus. Hum Pathol 39: 403–409 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Krakowski AR, Dunham EE, Wang L, Bandyopadhyay A, Berdeaux R, Martin GS, Sun L, Luo K (2007) Dual role of SnoN in mammalian tumorigenesis. Mol Cell Biol 27: 324–339 [DOI] [PMC free article] [PubMed] [Google Scholar]