Abstract
To evaluate the clinical significance of chestnut as a food allergen in Korea, skin prick test and ELISA were done in 1,738 patients with respiratory allergies. To identify the IgE binding components, IgE-immunoblotting, 2D IgE-immunoblotting and MALDI-TOF were performed. To observe the effects of digestive enzymes and a boiling treatment, simulated gastric fluid (SGF) and simulated intestinal fluids (SIF) were incubated with chestnut extracts, and IgE-immunoblotting were then repeated. Skin prick test revealed that 56 (3.2%) patients showed more than 2+ of allergen to histamine ratio to chestnut. Among the 21 IgE binding components, 9 bands were found in more than 50% of the sera tested and the 24 kDa protein had the highest binding intensity. The amino acid sequence of the 24 kDa protein (pI 6.3) had homology with legume protein of oak tree. SGF, SIF and boiling treatment were able to suppress the IgE binding components. In conclusion, chestnut ingestion was shown to induce IgE mediated responses with a 3.2% sensitization rate. Twenty one IgE binding components and one new allergen (the 24 kDa protein) were identified. Digestive enzymes and boiling treatment were able to decrease the allergenic potency.
Keywords: Chestnut, Food Hypersensitivity, Allergens, Digestive Enzyme
INTRODUCTION
Allergic reactions to tree nuts can be a serious and life threatening condition. Fresh and boiled chestnuts are commonly ingested food in Korea. A previous study demonstrated that chestnuts were accounted for the third greatest prevalence among the food allergens and it is the most prevalent tree nut allergen for both adult and pediatric allergy patients in Korea (1). Chestnut food allergy has also been reported in those patients presenting with latex-fruit or oral allergy syndrome (2). To date, there has been no published study to address the actual prevalence of IgE sensitization to chestnuts as a food allergen and its major allergic components in patients sensitized to this food (3).
It has been proposed that food allergens that can provoke systemic allergic reactions tend to be stable to digestion by the gastrointestinal tract (4, 5). Furthermore, resistance to pepsin digestion has been recently incorporated to the Food and Agriculture Organization/World Health Organization decision-making approach to the safety assessment of foods produced through agricultural biotechnology (6, 7).
In this study, we evaluated the clinical significance of chestnuts as a food allergen by using skin prick test and by performing serum specific IgE antibody determination via ELISA. To characterize the IgE-binding components, 10% SDS-PAGE, IgE-immunoblot and 2D-IgE-immunoblot followed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis were performed. The stability of chestnut to digestive enzymes and to a boiling treatment was also evaluated.
MATERIALS AND METHODS
Subjects
Patients who complained of respiratory allergy symptoms were recruited during one year from the Allergy Clinic of Ajou University Hospital in Suwon, Korea. Among the 1,738 patients, ELISA was performed on the sera from 56 patients showing ≥2+ positive reactions to chestnuts on skin prick test for detecting serum specific IgE to homegrown chestnuts. Among them, the sera from 9 patients showing high specific IgE levels on ELISA were used for the ELISA inhibitions testing and for IgE-immunoblotting.
Preparation of allergen extracts
Chestnut food allergen extracts were prepared according to the method described previously (8). Briefly, fresh chestnuts (the most commonly ingested one in this country, 1:5 wt/vol) were homogenized in phosphate-buffered saline (PBS, pH 7.5). The homogenate was kept at 4℃ overnight followed by centrifugation at 3,500 rpm for 40 min. The supernatant was dialyzed (the cut-off molecular weight was 6,000 Da; Spectrum Medical Industries, Houston, TX, U.S.A.) against 4 L of PBS at 4℃ for 48 hr. The supernatant was then passed through a Nalgen syringe filter (Rochester, New York, NY, U.S.A.) and lyophilized until ELISA, ELISA inhibition, immunoblotting analysis, and digestibility tests were performed. For skin prick test, the 1:5 wt/vol extract was mixed with an equal amount of glycerin (Sigma Co., St. Louis, MO, U.S.A.).
Skin prick test
Routine skin prick tests were performed for 30 food allergens using 26-gage needles on the backs of the patients. The results were read 15 min later. The size of the wheal produced by each allergen and histamine was expressed as the mean diameter of maximum length and vertical length at the midportion of the maximal length. Skin reactivity was expressed as the ratio of the wheal size of the allergen to the histamine (A/H). If the A/H ratio was from 0.1 to 1, but the erythema was >21 mm, it was read as 2+. If the A/H ratio was 1 to 2, it was read as 3+. If the A/H ratio was from 2 to 3, it was read as 4+. If the A/H ratio was greater than 3, it was read as 5+. A positive was defined as equal to or greater than 2+ on skin prick test (9).
ELISA for serum specific IgE to chestnut
ELISA was performed to determine the presence of specific IgE to chestnut as previously described (10). Microtiter plates (Costar, New York, NY, U.S.A.) were coated with chestnut extract at 100 µL/well (diluted in carbonated buffer, pH 9.6, 10 µg/well) and the plates were left at 4℃ for 24 hr. Each well was washed three times with 0.05% PBS-Tween (PBST), and was blocked by incubation with 3% BSA-PBST at 200 µL/well for 1 hr at room temperature (RT). The wells were then incubated for 2 hr at RT with either 50 µL of the patient's sera or the control sera (all the sera was diluted 1 to 5). After washing three times with PBST, biotin-labeled goat antihuman IgE antibody (Vector Co, Burlingame, CA, U.S.A.) was added to the each well and incubated for 1 hr. After washing, the wells were incubated with 100 µL of 1:1,000 vol/vol streptavidin-peroxidase (Sigma Co., St. Louis, MO, U.S.A.) for 30 min before ano- ther washing step. The colorimetric reaction was developed with B (3,3',5,5'-tetramethylbenzidine) substrate solution for 10 min at RT. The reaction was stopped by the addition of 100 µL of 2 N sulfuric acid and the absorbance was read at 450 nm by an automated microplate reader (Benchmark, Bio-Rad, Hercules, CA, U.S.A.). All assays were performed in duplicate. The amounts of specific antibodies in the samples were calculated from control curves that were created with the optical densities from serial dilutions of the positive control samples that showed higher antibody titer by using a spline fit program (Microplate Manager, Bio-Rad), and they were expressed as arbitrary units (A.U.). The chestnut-specific IgE was considered positive if the absorbance value was higher than the cutoff value, which was derived from the mean+3×S.D. of the absorbance values from the 15 control subjects.
SDS-PAGE and IgE-immunoblot
Electrophoresis in a 10% polyacrylamide separation gel with 4% stacking gel was performed and the proteins were stained with colloidal blue (Novex, San Diego, CA, U.S.A.) or the proteins were transferred onto a PVDF membrane (Millipore, Bedford, MA, U.S.A.), and this was then incubated with the pooled serum of patients with high serum specific IgE that had been diluted 1:5 vol/vol with PBST. Bound specific IgE was detected by biotin-labeled goat antihuman IgE antibody (Sigma). This was followed by a treatment with 0.66% 4-nitroblue tetrazolium and 0.33% 5-bromo-4-chloro-3-indolyl-phosphate (Sigma), which was used as a substrate.
Effect of simulated gastric fluid and intestinal fluid on the IgE binding components
The fresh chestnut extracts were prepared by heating the crude extracts at 100℃ for 5 min. The digestibility of the crude extract per se and the digestibility of the preheated extract that was incubated in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were examined as previously described (4). Briefly, for the SGF digestibility test, a protein sample (680 µg of naive crude protein or the preheated extract) was dissolved in 200 µL of prewarmed 100 mM/L HCl (pH 1.2), along with 30 mM/L NaCl containing a 0.32 wt/vol percentage of pepsin A (Sigma Chemical Co). Digestion was carried out at 37℃ with continuous shaking, and aliquots of this digest solution (20 µL) were withdrawn at 0, 0.5 and 60 min. These aliquots were quickly mixed with 26 µL of a sample buffer (containing 2.5% 2-mercaptoethanol and 1% SDS) together with 6.0 µL of Na2CO3 solution (200 mM/L). The mixture was then boiled for 5 min and stored at -20℃ until further analyses. For the evaluation of SIF and SGF, a sample (680 µg of the naive crude protein or the preheated extract) was dissolved in 260 µL of prewarmed intestinal control solution (0.05 M KH2SO4, pH 6.8) containing a 1.0 wt/vol percentage of pancreatin (Pancreatin USP, Sigma). This was then incubated at 37℃ with continuous shaking. Aliquots of this digest solution (26 µL) were withdrawn at 1, 90 and 240 min and quickly boiled for 5 min after mixing it with 26 µL of a sample buffer (containing 2.5% 2-mercaptoethanol and 1% SDS), and then 12% SDS-PAGE and IgE immunoblot analysis were repeated as described above.
RESULTS
Skin prick test and ELISA for detecting specific IgE to chestnut extracts
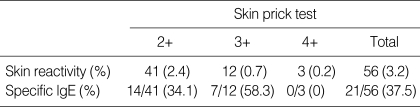
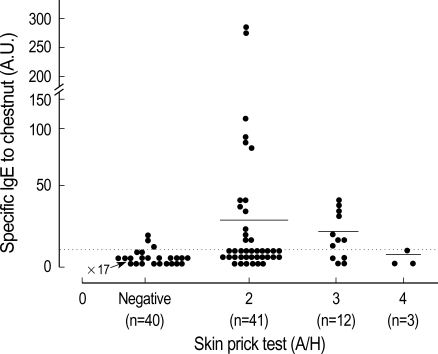
The results of the skin prick test revealed that 56 (3.2%) of 1,738 patients showed more than 2+ A/H response to the chestnut extracts, as is shown in Table 1. Among them, 15 (0.9%) showed more than 3+ A/H response. The prevalence of serum specific IgE, as determined by ELISA, to the chestnut extracts were 34.1% in the 2+ reactors, 58.3% in the 3+ reactors and 0% in the 4+ reactors. The absorbance value of specific IgE to chestnut, according to skin reactivity, is shown in Fig. 1.
Table 1.
Skin prick test and specific lgE bindings to chestnut extracts by ELISA
Patients (positive rate on specific lgE bindings, %).
Fig. 1.
Specific IgE bindings to chestnut by ELISA according to Allergen/Histamine (A/H) ratio of chestnut on skin prick test. Horizontal bars indicate the mean values.
ELISA inhibition test
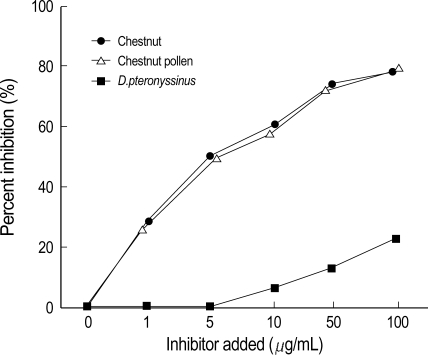
The ELISA inhibition test for wild chestnut using the pooled sera from subjects with high serum specific IgE level showed significant dose-dependent inhibitions by the chestnut and chestnut pollen extracts, and both extracts had similar potencies, while negligible inhibition was noted for D. pteronyssinus, as is shown in Fig. 2.
Fig. 2.
Chestnut-ELISA inhibition tests with the additions of chestnut (●), chestnut pollen (△) and D. pteronyssinus (■) extracts.
SDS-PAGE and IgE-immunoblot
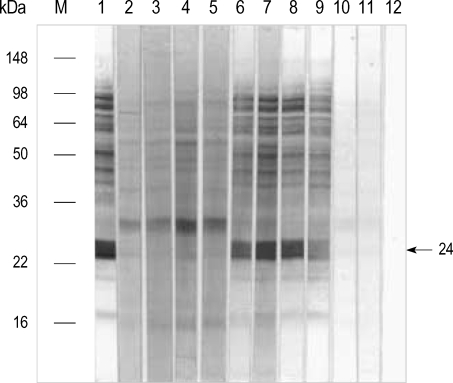
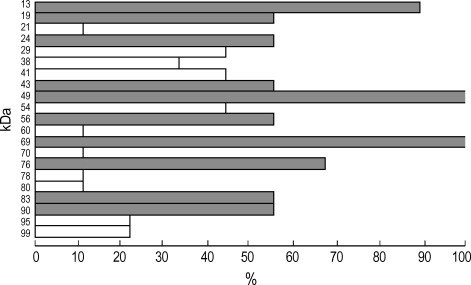
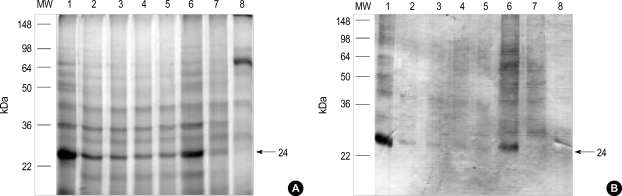
To observe the protein components of the fresh chestnut extracts, they were analyzed by using 10% SDS-PAGE and IgE-immunoblotting with the patients' sera. As shown in Fig. 3, 21 IgE-binding components were detected within the chestnut extracts, while no bindings were noted with the control sera. The 90, 83, 76, 69, 56, 49, 43, 24 and 13 components were noted in more than 50% of the sensitized patients (Fig. 4).
Fig. 3.
IgE-immunoblot analysis of chestnut extracts in the sera from the sensitized patients. M: standard molecular marker. Lane 1-9: subjects of the sensitized patients. Lane 10, 11: control. Lane 12: buffer. Arrow indicates the major IgE binding component (24 kDa).
Fig. 4.
Result of IgE-immunoblot analysis of the chestnut extracts using the sensitized sera. Shadow bar indicate 10 major auergeric components.
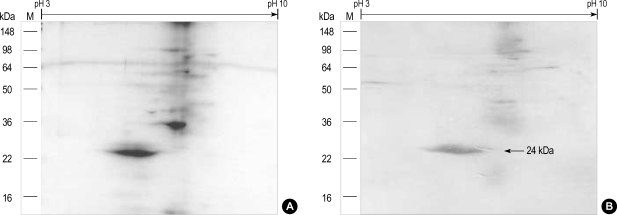
Two-dimensional SDS-PAGE, immunoblotting and N-terminal amino acid sequencing
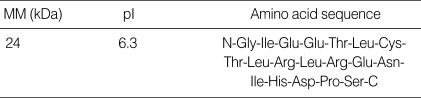
Two dimensional SDS-PAGE (Fig. 5A) and immunoblotting (Fig. 5B) of the fresh chestnut extracts using the pooled sera of two patients with high levels of specific IgE to chestnut extract showed the 24 kDa IgE binding component and its iso-electric point was 6.3, while a 29 kDa component was not detectable. Table 2 shows the result of amino acid sequencing for this major allergen having a 24 kDa of molecular weight, which has homology with legume protein from oak trees (11).
Fig. 5.
Two dimensional SDS-PAGE (A) and the IgE binding component (B) of the fresh chestnut extracts using the pooled sera of two patients with high levels of specific IgE. The iso-electric point was 6.3.
Table 2.
Amino acid sequence of the major allergen of chestnut
MW, Molecular weight; pI, Iso-electric point.
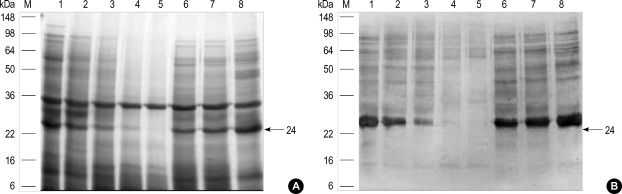
Effects of digestive enzymes on IgE-binding components
Immunoblotting using the pooled sera showed that most of the IgE-binding components were substantially digested in 10 to 30 min by SGF in the preheated and heated conditions. After the treatment with SIF, although most of the proteins within the chestnut extracts were not changed, IgE binding components were suppressed in the preheated and heated conditions (Fig. 6, 7).
Fig. 6.
Effect of simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) treatment on chestnut allergens as analyzed by 12% SDS-PAGE (A) and IgE-immunoblotting (B). 1, Standard; 2, SGF-30 sec; 3, SGF-2 min; 4, SGF-10 min; 5, SGF-30 min; 6, SIF-15 min; 7, SIF-2 hr; 8, SIF-16 hr.
Fig. 7.
Effect of simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) treatment on the boiled chestnut allergen as analyzed by SDS-PAGE (A) and IgE-immunoblotting (B). 1, Standard; 2, SGF-2 min; 3, SGF-15 min; 4, SGF-30 min; 5, SGF-1hr; 6, SIF-15 min; 7, SIF-2 hr; 8, SIF-16 hr.
DISCUSSION
Castanea crenata var. dulcis, is a member of the beech family (Fagaceae) that blooms with yellow flowers in spring and its nuts ripen in September and October, and the chestnut pollen has been found in the air of Korea. Chestnut is a commonly ingested food in this country in both the fresh and boiled form. A nationwide study on prevalence to commonly ingested food allergens by skin prick test in Korea has demonstrated chestnut to be the third most prevalent sensitizing food allergen in both adult and pediatric allergy patients (1). In this study, we demonstrated that the sensitization rate to homemade chestnut extracts was 3.2% in the adult allergy patients, and the serum specific IgE antibody was detected by ELISA in the positive reactors on a skin prick test. The ELISA inhibition tests demonstrated that significant inhibitions were noted with the addition of the chestnut extracts. These findings suggest that chestnut should be considered as one of the prevalent food allergens in this country.
There have been a few studies to identify the IgE binding components within chestnut allergen extracts (12). Previous studies reported two allergens named Cas s 5 (chitinase Ib, molecular weight was not identified) and Cas s 8 (lipid transfer protein, 9.7 kDa) from the chestnut. In this study, 21 IgE binding components were identified and 9 of them could be potential major allergens as they bound to ≥the 50% of the subjects' sera that we tested. Although the number of study subjects was not large enough to fully evaluate all the allergen components, the 24 kDa component had the highest of the IgE binding intensities. The amino acid sequencing of the 24 kDa protein demonstrated a homology with the legumelike protein of oak tree. Further studies with a larger group of study subjects will be needed to investigate the IgE binding components to differentiate between the symptomatic and asymptomatic sensitizers, and this identification of the symptomatic sensitizers is an important issue for food allergy patients.
Rico et al. reported that one third of the chestnut-allergic patients experience severe anaphylactic episodes upon ingestion of chestnuts (3). Chestnut reactivity has also been frequently associated to actual clinical allergies not only to fruits, but also to other tree nuts. Also, allergy to chestnuts has been widely reported in the latex-fruit syndrome (13-18), The percentage of individuals with latex allergy who have IgE antibodies to a chestnut was estimated to be 34.6% (13). An increasing number of plant sources has been associated with this syndrome, but avocado, banana, chestnut and kiwi are those most frequently reported foods (13, 19, 20). The relevant 32 kDa allergens of avocado and chestnut could be the pan-allergens responsible for the latex-fruit syndrome. In this study, among the 9 subjects having high levels of specific IgE antibody to chestnut, none had a history of latex allergy.
Typical food allergens are per oral sensitizers and, at the same time, they are per oral inducers of allergic symptoms. Complete food allergens are proteins having both the ability to sensitize and the ability to induce symptoms (21). They were also reported to be stable to digestion (19, 20), because they must be absorbed from the intestine and be recognized by the immune system for a person to be sensitized. In contrast, incomplete food allergens are those allergens having the capacity to induce allergic symptoms per orally, even though they are digestible and cannot per orally sensitize people (21). Indigestibility is not a prerequisite for a food protein to induce allergic symptoms. Digestible food allergens, as well as indigestible food allergens, have the ability to elicit allergic reactions per orally (22, 23). In this study, we evaluated changes of the allergenic components after they were incubated with digestive enzymes and a boiling process in vitro. Gastric enzyme treatment alone, and both the gastric enzyme treatment and the boiling treatment could decrease the IgE binding components of most major allergens, while intestinal enzyme treatment had minimal effects on the allergenicity. There has been a previous study suggesting that the possible lack of detection of SGF-digested products from an allergen by protein staining or IgE-immunoblotting after SDS-PAGE fractionation does not necessarily mean that it is inactivated in SGF (24). Further studies with a larger group of sensitized patients will be needed to make any definite conclusion.
In conclusion, variable IgE binding components were found within the chestnut extracts and ingestion of chestnut could induce IgE mediated response. A new major allergen (24 kDa, pI 6.3) that has homology with legume protein of oak tree was identified, and digestive enzyme treatment could decrease its allergenic potency.
Footnotes
This study was supported by ARPC Grant (204070-301).
References
- 1.Kim SH, Kang HR, Kim KM, Kim SS, Chang YS, Kim CW, Bahn JW, Kim YK, Cho SH, Park HS, Lee JM, Min KU, Hong CS, Kim NS, Kim YY. The sensitization rates of food allergens in a Korean population: a multi-center study (in Korean) J Asthma Allergy Clin Immunol. 2003;23:502–514. [Google Scholar]
- 2.Yagame T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000;106:752–762. doi: 10.1067/mai.2000.109171. [DOI] [PubMed] [Google Scholar]
- 3.Rico P, Crespo JF, Feliu A, Rodriguez J. Chestnut allergy: Beyond the latex-fruit syndrome. J Allergy Clin Immunol. 2004;113(Suppl 2) [Google Scholar]
- 4.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–1273. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 5.Van Ree R. Clinical importance of non-specific lipid transfer proteins as food allergens. Biochem Soc Trans. 2002;30:910–913. doi: 10.1042/bst0300910. [DOI] [PubMed] [Google Scholar]
- 6.FAO/WHO. Evaluation of allergenicity of genetically modified foods. Rome: FAO; 2001. pp. 1–26. [Google Scholar]
- 7.Taylor SL. Protein allergenicity assessment of foods produced through agricultural biotechnology. Annu Rev Pharmacol Toxicol. 2002;42:99–112. doi: 10.1146/annurev.pharmtox.42.082401.130208. [DOI] [PubMed] [Google Scholar]
- 8.Lee SK, Jee YK, Kim YK, Suh JH, Lee MH, Park HS. Identification of IgE binding components of Tetranychus urticae: species-specific and cross-reacting allergens with house dust mite (in Korean) Korean J Asthma Allergy Clin Immunol. 2000;20:879–886. [Google Scholar]
- 9.Park HS. Allergy skin tests in clinical practice. Korean J Asthma Allergy Clin Immunol. 2001;21:115–119. [Google Scholar]
- 10.Kim YK, Park HS, Kim HA, Lee MH, Choi JH, Kim SS, Lee SK, Nahm DH, Cho SH, Min KU, Kim YY. Two-spotted spider mite allergy: immunoglobulin E sensitization and characterization of allergenic components. Ann Allergy Asthma Immunol. 2002;89:517–522. doi: 10.1016/S1081-1206(10)62091-1. [DOI] [PubMed] [Google Scholar]
- 11.NCBI BLAST. http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi.
- 12.Roux KH, Teuher SS, Sathe SK. Tree nut allergens. Int Arch Allergy Immunol. 2003;131:234–244. doi: 10.1159/000072135. [DOI] [PubMed] [Google Scholar]
- 13.Brehler R, Theissen U, Mohr C, Luger T. Latex-fruit syndrome: frequency of cross-reacting IgE antibodies. Allergy. 1997;52:404–410. doi: 10.1111/j.1398-9995.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez de Corres L, Moneo I, Munoz D, Bernaola G, Fenandez E, Audicana M, Urrutia I. Sensitization from chestnuts and bananas in patients with urticaria and anaphylaxis from contact with latex. Ann Allergy. 1993;70:35–39. [PubMed] [Google Scholar]
- 15.Rodriguez M, Vega F, Garcia MT, Panizo C, Laffond E, Montalvo A, Cuevas M. Hypersensitivity to latex, chestnut, and bananas. Ann Allergy. 1993;70:31–34. [PubMed] [Google Scholar]
- 16.Diaz-Perales A, Coliada C, Blanco C, Sanchez-Monge R, Carrilio T, Aragoncillo C, Salcedo G. Class I chitinases with hevein-like domain, but not class II enzyme, are relevant chestnut and avocado allergens. J Allergy Clin Immunol. 1998;102:127–133. doi: 10.1016/s0091-6749(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 17.Blanco C, Diaz-Perales A, Collada C, Sanchez-Monge R, Aragoncillo C, Castillo R, Ortega N, Alvarez M, Carrillo T, Salcedo G. Class I chitinase as potential panallergens involved in the latex-fruit syndrome. J Allergy Clin Immunol. 1999;103:507–513. doi: 10.1016/s0091-6749(99)70478-1. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Monge R, Blanco C, Diaz-Perales A, Collada C, Carrillo T, Aragoncillo C, Salcedo G. Class I chitinase, the panallergens responsible for the latex-fruit syndrome, are induced by ethylene treatment and inactivated by heating. J Allergy Clin Immunol. 2000;106:190–195. doi: 10.1067/mai.2000.107599. [DOI] [PubMed] [Google Scholar]
- 19.Blanco C, Carrillo T, Castillo R, Quiralte J, Cuevas M. Latex allergy: clinical features and cross-reactivity with fruits. Ann Allergy. 1994;73:309–314. [PubMed] [Google Scholar]
- 20.Chen Z, Posch A, Cremer R, Raulf-Heimsoth M, Baur X. Identification of hevein (Hev b 6.02) in Hevea latex as a major cross-reacting allergen with avocado fruit in patients with latex allergy. J Allergy Clin Immunol. 1998;102:476–481. doi: 10.1016/s0091-6749(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 21.Aalberse RC. Food allergens. Environ Toxicol Pharmacol. 1997;4:55–60. doi: 10.1016/s1382-6689(97)10042-4. [DOI] [PubMed] [Google Scholar]
- 22.Hefle SL. The chemistry and biology of food allergens. Food Technol. 1996;50:86–92. [Google Scholar]
- 23.Veith S. Allergenic cross-reactivity, food allergy and pollen. Environ Toxicol Pharmacol. 1997;4:61–70. doi: 10.1016/s1382-6689(97)10043-6. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Perales A, Blanco C, Sanchez-Monge R, Varela J, Carrillo T, Salcedo G. Analysis of avocado allergen (Prs a 1) IgE-binding peptides generated by simulated gastric fluid digestion. J Allergy Clin Immunol. 2003;112:1002–1007. doi: 10.1016/j.jaci.2003.07.006. [DOI] [PubMed] [Google Scholar]