Abstract
Neural stem cells (NSCs) are undifferentiated neural cells characterized by their high proliferative potential and the capacity for self-renewal with retention of multipotency. Over the past two decades, there has been a huge effort to identify NSCs morphologically, genetically, and molecular biologically. It is still controversial, however, what bona fide NSCs are. To define and characterize NSCs more systematically, it is crucial to explore novel cell-surface marker molecules of NSCs. In this study, we focused on GD3, a b-series ganglioside that is enriched in the immature brain and the subventricular zone (SVZ) of the postnatal and adult brain, and evaluated the usefulness of GD3 as a cell-surface biomarker for identifying NSCs. We demonstrated that GD3 was expressed in more than 80% of NSCs prepared from embryonic, postnatal, and adult mouse brain tissue by the neurosphere culture method. The percentage of GD3-expressing NSCs in neurospheres was nearly the same as it was in neurospheres derived from embryonic, postnatal, and adult brains but decreased drastically to about 40% after differentiation. GD3+ cells isolated from embryonic mouse striata, postnatal, and adult mouse SVZs by fluorescence-activated cell sorting with an R24 anti-GD3 monoclonal antibody efficiently generated neurospheres compared with GD3− cells. These cells possessed multipotency to differentiate into neurons, astrocytes, and oligodendrocytes. These data indicate that GD3 is a unique and powerful cell-surface biomarker to identify and isolate NSCs.
Keywords: development, FACS, glycoconjugate, glycosphingolipid, neurospheres
Introduction
Neural stem cells (NSCs) are undifferentiated neural cells characterized by their high proliferative potential and the capacity for self-renewal with retention of multipotency to differentiate into neurons and glial cells (Weiss et al. 1996; McKay 1997; Gage 2000; Zhao et al. 2008). In postnatal and adult mammalian brains, NSCs are located mainly in the subventricular zone (SVZ) of the lateral ventricles and the subgranular layer of the dentate gyrus in the hippocampus (Doetsch et al. 1999; Seri et al. 2001). The identity of NSCs in those regions has been revealed, but it still remains controversial. First, it was reported that NSCs are ependymal cells (Johansson et al. 1999). However, it has been later demonstrated that NSCs are a kind of astrocyte positive for glial fibrillary acidic protein (GFAP) (Doetsch et al. 1999), but not ependymal cells (Capela and Temple 2002). On the other hand, a certain population of ependymal cells in the SVZ has been recently reported to possess the characteristics of NSCs (Coskun et al. 2008). Thus, there is an urgent need to define and characterize bona fide NSCs in this region.
At present, several marker molecules to define NSCs, such as nestin (Lendahl et al. 1990), Sox2 (Zappone et al. 2000), and Musashi-1 (Sakakibara et al. 2002), have been proposed. Those marker molecules, however, are intracellular proteins or transcription factors not suitable for isolating native NSCs by fluorescence-activated cell sorting (FACS). Although CD133 (prominin-1) and stage-specific antigen-1 (SSEA-1) localized on the cell surface have been used to isolate NSCs (Uchida et al. 2000; Capela and Temple 2002), those antigens are expressed not only in NSCs but also in other somatic stem cells, cancer stem cells, and embryonic stem cells (Muramatsu and Muramatsu 2004; Huhn et al. 2005; Tong et al. 2008). Moreover, it has been reported that CD133+/SSEA-1+ and CD133+/SSEA-1− cells derived from embryonic stem cells formed neurospheres, floating aggregates of NSCs, at similar frequencies (Peh et al. 2009). In addition to these antigens, CD24a, peanut agglutinin ligand, syndecan-1, Notch-1, β1 integrin, and biantennary complex-type N-glycans recognized by Phaseolus vulgaris erythroagglutinating lectin have been reported as cell-surface marker molecules to identify and isolate NSCs (Rietze et al. 2001; Nagato et al. 2005; Hamanoue et al. 2009). However, it is still desirable to explore other novel cell-surface marker molecules with defined structures of NSCs to characterize NSCs more systematically.
Gangliosides are sialic acid-containing glycosphingolipids abundantly expressed in the plasma membrane. The quantity and species of gangliosides in the brain drastically change during development; predominant gangliosides are simple GM3 and GD3 in the embryonic brain, but more complex GM1, GD1a, GD1b, and GT1b in the adult brain (Yu et al. 2009). Because of the characteristic expression patterns, some gangliosides have been used as developmental marker molecules (Yanagisawa and Yu 2007); for instance, c-series gangliosides (A2B5 antigens) are well-known markers of glial precursor cells (Zhang 2001). It suggests that certain gangliosides can be useful as specific NSC markers.
GD3 (NeuAcα2-8NeuAcα2-3Galβ1-4Glcβ1-1′Cer; CD60a), a b-series disialoganglioside, is known to be highly expressed in embryonic brains, but its concentration rapidly decreases after birth (Ngamukote et al. 2007). It has been reported that GD3 is expressed in the rat SVZ (Goldman et al. 1984), in a small population of human astrocytes with a high proliferation capacity (Satoh and Kim 1995), in mouse radial glia, bipolar cells transiently appearing in the neuroepithelium and playing roles as NSCs at the embryonic stage (Cammer and Zhang 1996), and in mouse neuroepithelial cells known to be rich in embryonic NSCs (Yanagisawa et al. 2004). Therefore, we hypothesized that GD3 is expressed preferentially in NSCs and useful as a marker molecule. In this study, we have evaluated whether GD3 is suitable as a cell-surface biomarker for identifying NSCs in the embryonic, postnatal, and adult brains.
Results
Expression of GD3 in mouse brains
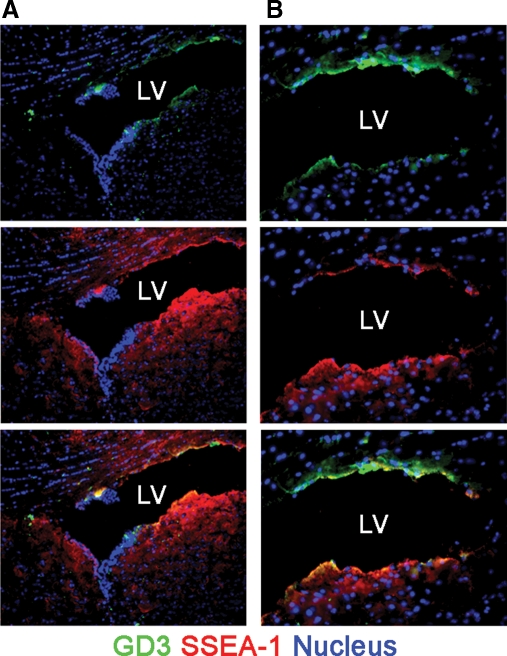
First, we confirmed the expression of GD3 in the SVZ of the lateral ventricle where NSCs robustly exist in adult mouse brains (Goldman et al. 1984) by immunohistochemistry with an R24 anti-GD3 antibody (Pukel et al. 1982). As shown in Figure 1, GD3 was found to be exclusively localized in the SVZ of the lateral ventricle. The GD3 signals in the SVZ were found in cells positive for SSEA-1, a cell-surface carbohydrate antigen expressed in NSCs (Klassen et al. 2001; Capela and Temple 2002). This result indicates the possibility that GD3 is expressed preferentially in NSCs. Thus, we then evaluated the expression pattern of GD3 in isolated mouse NSCs.
Fig. 1.
GD3 expression in mouse brains. (A and B) Cryosections of adult mouse brains were stained with the R24 anti-GD3 antibody and AK97 anti-SSEA-1 antibody. Panel (B) is higher magnification view of panel (A). Nuclei were stained with Hoechst 33258 (H33258). “LV” indicates lateral ventricles.
Preparation of NSCs from embryonic, postnatal, and adult mouse brains
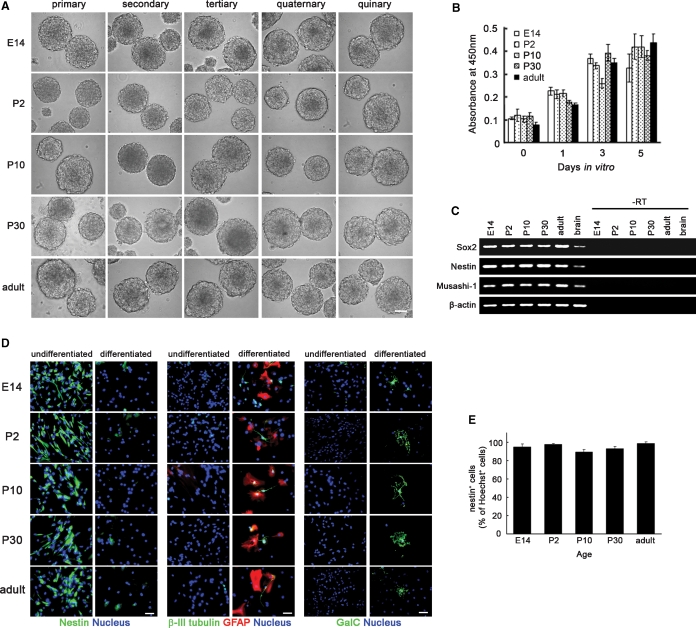
In this study, NSCs were isolated from mouse striata [embryonic day 14 (E14)] and SVZs [postnatal day 2 (P2), P10, P30, and adult] in the form of neurospheres, floating aggregates formed by NSCs in vitro (Reynolds and Weiss 1992) (Figure 2A). The size of obtained neurospheres was more than 100 μm. Single cells prepared from the neurospheres could also regenerate secondary, tertiary, quaternary, and quinary neurospheres (Figure 2A). There was no clear difference in the size of the neurospheres cultured within five passages or 1 month. The cells forming these embryonic, postnatal, and adult neurospheres proliferate at the similar rates (Figure 2B). The neurosphere-forming cells expressed NSC-specific genes such as Sox2, nestin, and Musashi-1 (Figure 2C). The percentages of nestin+ cells were more than 85% (Figure 2D and E). Neurons positive for β-III tubulin, astrocytes positive for GFAP, and oligodendrocytes positive for galactosylceramide (GalC) were not detected in the neurosphere-forming cells (Figure 2D). In contrast, neurosphere-forming cells cultured in the presence of 1% fetal bovine serum and the absence of fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF) to induce differentiation were negative for nestin but positive for β-III tubulin (10–20%), GFAP (80–90%), and GalC (<1%) (Figure 2D). These results clearly demonstrate that the neurosphere-forming cells prepared from embryonic, postnatal, and adult mouse brains in this study are NSCs.
Fig. 2.
NSCs prepared from mouse striata (E14) and SVZs (P2, P10, P30, and adult) in the form of neurospheres. (A) Primary, secondary, tertiary, quaternary, and quinary neurospheres prepared from mouse striata (E14) or SVZs (P2, P10, P30, and adult). Scale bar represents 100 μm. (B) The proliferation rates of neurosphere-forming cells estimated by WST-8 assay at 0, 1, 3, and 5 days in vitro (n = 3). (C) The expression of NSC-specific genes in neurosphere-forming cells prepared from mouse striata (E14) or SVZs (P2, P10, P30, and adult) was analyzed by reverse transcription-polymerase chain reaction (RT-PCR). “Brain” indicates the control cDNA prepared from the total RNA of adult mouse brains. “–RT” indicates negative controls without reverse transcription. β-Actin was detected as the control. (D) Expressions of nestin (NSC marker), β-III tubulin (neuron marker), GFAP (astrocyte marker), and GalC (oligodendrocyte marker) in neurosphere-forming cells and the differentiated cells cultured in the presence of 1% fetal bovine serum and the absence of FGF-2 and EGF. Nuclei were stained with H33258. Scale bar represents 100 μm. (E) The percentages of nestin+ cells in neurosphere-forming cells (n = 10).
Expression of GD3 in embryonic, postnatal, and adult mouse NSCs
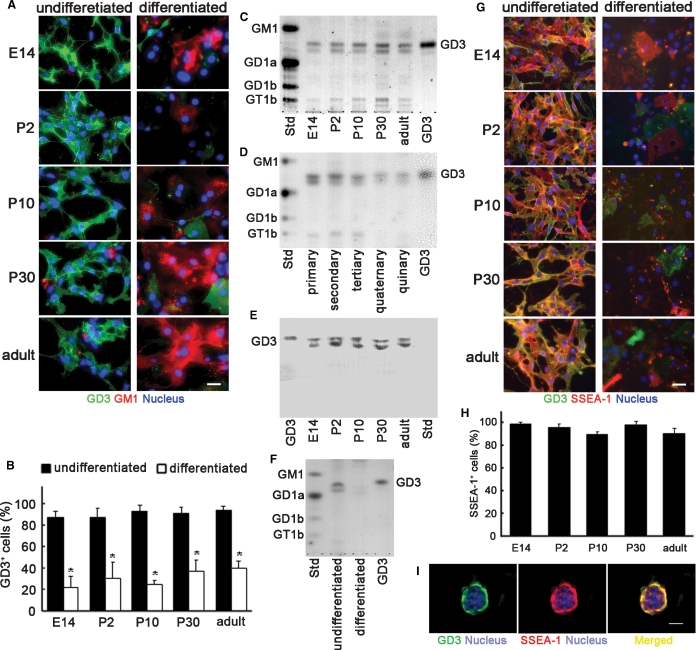
To evaluate the GD3 expression in NSCs, we stained NSCs prepared as above with the R24 anti-GD3 monoclonal antibody without permeabilization. In the embryonic, postnatal, and adult NSCs, GD3 was abundantly expressed on the cell surface (Figure 3A). The percentages of GD3+ cells were almost at the same levels in NSCs prepared from embryonic, postnatal, and adult brains (>80%), but drastically decreased after differentiation (<40%) (Figure 3A and B). To confirm the GD3 expression level in NSCs qualitatively and quantitatively, we analyzed the total lipids extracted from neurospheres by thin-layer chromatography (TLC). As shown in Figure 3C, in the total lipids extracted from embryonic, postnatal, and adult neurospheres, two bands having the same Rf values as that of authentic GD3 were detected with a resorcinol-HCl reagent that can visualize gangliosides. Those two bands were detected in primary, secondary, tertiary, quaternary, and quinary neurospheres (Figure 3D). By TLC-immunostaining with the R24 anti-GD3 monoclonal antibody, the bands were identified as GD3 (Figure 3E). Those results indicate that two GD3 species having different ceramide components are expressed in NSCs. The expression of O-acetyl GD3 including 9-O-acetyl GD3 (CD60b), a derivative of GD3 and a marker of neuroglial progenitor cells (Maric et al. 2003, 2007), in NSCs was less than the limit of chemical detection by a resorcinol-HCl reagent and barely detectable by TLC-immunostaining (data not shown).
Fig. 3.
Expression of GD3 in NSCs. (A) Expression of GD3 and GM1 in NSCs and the differentiated cells. (B) The percentages of GD3+ cells in NSCs and the differentiated cells (n = 8–11). (C) TLC of total lipids extracted from embryonic, postnatal, and adult neurospheres. (D) TLC of total lipids extracted from primary, secondary, tertiary, quaternary, and quinary neurospheres. (E) TLC-immunostaining of total lipids extracted from neurospheres with an R24 anti-GD3 monoclonal antibody. (F) TLC of total lipids extracted from differentiated cells derived from neurospheres. In panels (C)–(F), bovine brain gangliosides (Std; GM1, GD1a, GD1b, and GT1b) and authentic GD3 were used as standard gangliosides. In panels (C), (D), and (F), gangliosides were visualized with the resorcinol-HCl reagent. Gangliosides prepared from the same number of cells were applied to each lane. (G) Double-immunostaining of NSCs prepared from embryonic, postnatal, and adult neurospheres with the R24 anti-GD3 monoclonal antibody and AK97 anti-SSEA-1 monoclonal antibody. (H) The percentage of SSEA-1+ cells in GD3+ NSCs prepared from embryonic, postnatal, and adult neurospheres (n = 10). (I) Double-immunostaining of a neurosphere from adult mouse brain tissue with the anti-GD3 antibody and anti-SSEA-1 antibody. In panels (A), (G), and (I), scale bars represent 100 μm. Nuclei were stained with H33258.
During mammalian brain development, it has been known that the expression levels of simple gangliosides such as GM3 and GD3 drastically decrease, and those of complex gangliosides such as GM1 and GD1a show a concomitant increase (Ngamukote et al. 2007). Similarly, GD3 was found to decrease drastically in the total lipids extracted from differentiated cells derived from NSCs (Figure 3F). In contrast, GM1 and the related gangliosides recognized by cholera toxin B subunit were detected in differentiated cells but not in NSCs (Figure 3A). Therefore, the expression patterns of GM1 and the related gangliosides in differentiating NSCs were contrary to those of GD3, reflecting the change of ganglioside expressions during brain development, as previously reported (Ngamukote et al. 2007).
To confirm that the GD3+ cells co-express another well-known NSC marker, we double-immunostained NSCs with antibodies to GD3 and SSEA-1. As shown in Figure 3G, the NSCs prepared from embryonic, postnatal, and adult neurospheres were intensely stained with an anti-SSEA-1 antibody. The percentage of SSEA-1+ cells (>90%) was almost at the same levels in the NSCs prepared from embryonic, postnatal, and adult neurospheres (Figure 3H). Most cells positive for SSEA-1 were stained with the R24 anti-GD3 monoclonal antibody (Figure 3G). In the neurospheres, both antigens were also intensely expressed (Figure 3I). In contrast, the expression levels of those antigens in the differentiated cells derived from NSCs were less than those in undifferentiated NSCs (Figure 3G). Those results clearly indicate that NSCs derived from embryonic, postnatal, and adult brains express GD3.
FACS of GD3+ cells in embryonic, postnatal, and adult mouse brains
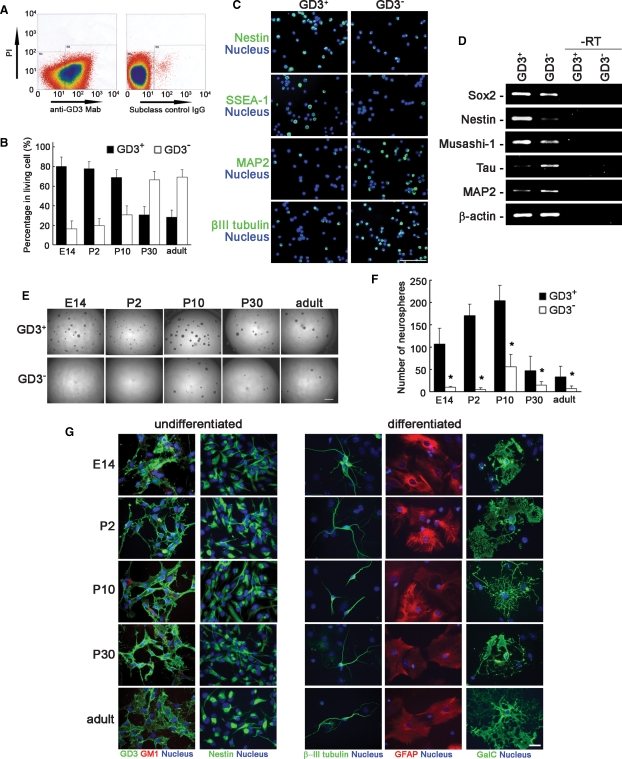
The above-mentioned results suggest the possibility that GD3 can be used as a novel and more well-defined cell-surface biomarker for identifying NSCs. However, co-expression of GD3 with other NSC marker proteins such as nestin, Sox2, and Musashi-1 could not be confirmed because GD3 is a lipid antigen which is easily washed out by permeabilization with a detergent or an organic solvent essential for staining intracellular proteins. Therefore, to demonstrate the possibility, we directly sorted GD3+ cells from cell suspensions prepared from mouse striata (E14) or SVZs (P2, P10, P30, and adult) and evaluated the characteristics as NSCs. In E14 striata and P2 and P10 SVZs, most living cells were positive for GD3 (Figure 4A and B). In contrast, in P30 and adult SVZs, fewer cells were positive for GD3. Most GD3+ cells isolated by FACS were positive for nestin and SSEA-1, but negative for microtubule-associated protein 2 (MAP2) and β-III tubulin (Figure 4C). The expression of NSC-specific genes such as Sox2, nestin, and Musashi-1 was confirmed in the GD3+ cell population (Figure 4D). The expression of neuronal marker genes, Tau and MAP2, in GD3+ cells was considerably lower than that in GD3− cells (Figure 4D). The GD3+ cells isolated by FACS could more efficiently generate neurospheres as compared with GD3− cells (Figure 4E and F). The cells nonspecifically recognized with a subclass control IgG had practically no neurosphere-forming capability (data not shown). The cells forming neurospheres generated in the GD3+ cell population were also positive for nestin and GD3 but negative for GM1 (Figure 4G). Under proper conditions to induce the differentiation, the cells forming neurospheres generated in the GD3+ population were capable of differentiating into all types of neural cell lineages, such as neurons positive for β-III tubulin, astrocytes positive for GFAP, and oligodendrocytes positive for GalC (Figure 4G). Those results clearly suggest that the GD3+ cells isolated from brain tissues are NSCs possessing high proliferative potential, the capacity for self-renewal, and multipotency. Thus, we conclude that GD3 is a novel useful cell-surface marker for isolating NSCs, especially from adult brain tissue in which NSCs are less abundant (Figures 1, 4A and B).
Fig. 4.
Isolation of GD3+ cells from mouse brains by FACS with an R24 anti-GD3 monoclonal antibody. (A) Dot plots of E14 mouse brain cells stained with the R24 anti-GD3 monoclonal antibody (anti-GD3 Mab) or subclass control mouse IgG. (B) The percentages of GD3+ and GD3− cells in the living cells prepared from striata (E14) or SVZs (P2, P10, P30, and adult). (C) Immunostaining of GD3+ and GD3− cell populations from E14 striata with antibodies to nestin, SSEA-1, MAP2, and β-III tubulin at 3 h after cell sorting. Nuclei were stained with H33258. Scale bar represents 100 μm. (D) Expression of NSC-specific genes (Sox2, nestin, and Musashi-1) and neuron-specific genes (tau and MAP2) in GD3+ and GD3− cells from E14 striata analyzed by RT-PCR. “–RT” indicates negative controls without reverse transcription. β-Actin was detected as the control. (E) Phase views of neurospheres generated by GD3+ and GD3− cell populations that were sorted from E14 striata and P2, P10, P30, and adult SVZs. Scale bar represents 1 mm. (F) The number of neurospheres formed in GD3+ and GD3− cell populations (n = 5–10). The number of neurospheres was counted at 5 days after cell sorting. (G) Cell staining of NSCs and differentiated cells derived from neurosphere formed by GD3+ cells. Nuclei were stained with H33258. Scale bar represents 100 μm.
Discussion
In the present investigation, we have examined the expression of GD3 in NSCs prepared from mouse brain tissues in the form of neurospheres and found the usefulness of GD3 as a cell-surface biomarker for identifying and isolating NSCs. In embryonic, postnatal, and adult NSCs, the percentage of GD3+ cells was more than 80%. The expression levels of GD3 were similar in embryonic, postnatal, and adult NSCs, but drastically decreased after the differentiation. Those results suggest that GD3 expression is maintained in NSCs during development to adulthood and is synchronous with the stemness. To identify NSCs, cell-surface marker molecules such as GD3 are quite suitable because they can be used for FACS. In fact, it was demonstrated in this study that GD3+ cells isolated by FACS possessed all the characteristics of NSCs such as marker expression (nestin, Sox2, Musashi-1, and SSEA-1), a neurosphere-forming ability and a multipotency to differentiate into neurons, astrocytes, and oligodendrocytes. These findings strongly suggest that GD3 is useful for isolating NSCs, especially from postnatal and adult brain tissues in which GD3 is rare but GM1 and GD1a are abundantly expressed. On the other hand, as is obvious from the percentage of GD3+ cells present in the SVZ (0.5–2.0%), not all GD3+ cells are expected to be NSCs. There is a strong possibility that some differentiated cells, such as neuronal precursor cells and glial precursor cells, also express GD3. Therefore, to purify NSCs and/or remove non-NSCs, a combination of GD3 with other cell-surface markers is important. So far, CD133, CD24a, peanut agglutinin ligand, SSEA-1, syndecan-1, Notch-1, β1 integrin, and Phaseolus vulgaris erythroagglutinating lectin ligand have been reported as cell-surface marker molecules to identify and isolate NSCs (Uchida et al. 2000; Klassen et al. 2001; Rietze et al. 2001; Capela and Temple 2002; Nagato et al. 2005; Hamanoue et al. 2009). The molecular structures of those carbohydrate epitopes such as SSEA-1 and Phaseolus vulgaris erythroagglutinating lectin ligand carrying compounds remain largely undefined, which hampers the study of their function. In addition, a JONES monoclonal antibody recognizing a derivative of GD3, 9-O-acetyl GD3, has been used to stain neuroglial progenitor cells (Maric et al. 2003, 2007). GD3 in conjunction with other marker molecules should contribute to the systematic classification of the neural lineages of cells, including NSCs, and in the identification of bona fide NSCs.
So far, numerous studies have been attempted to identify bona fide NSCs morphologically, genetically, and molecular biologically, and it was found that the NSC is a type of cell existing in SVZs. SVZs are known to be composed of at least four types of cells: type A migrating neuroblasts; type B GFAP+ astrocytes; type C rapidly dividing transit-amplifying cells, and ependymal cells (Zhao et al. 2008). Among them, type B cells have been identified as NSCs (Doetsch et al. 1999). Type A and type C cells possess a proliferative ability but are considered as the more differentiated because they have less multipotency as compared with NSCs (Zhao et al. 2008). Ependymal cells have been considered to have no properties of NSCs (Doetsch et al. 1999; Capela and Temple 2002). On the other hand, a recent study has revealed that CD133+/GFAP+ ependymal cells possess the characteristics of NSCs in the SVZ (Coskun et al. 2008). Thus, it is unclear whether NSCs are astrocytes, ependymal cells, and/or yet other cells in SVZs. Moreover, there is a possibility that the characteristics of NSCs are different in brain tissues at different developmental stages. The above confusion underlies the difficulty in defining bona fide NSCs. GD3, whose expression level reflects the immaturity of cells and the change of brain development in vivo, can be useful as a suitable and reasonable biomarker for identifying NSCs more systematically. Further characterization of the NSCs positive for GD3 will be the subject of future investigation.
Material and methods
Animals
ICR mice (Harlan, Indianapolis, IN) used in this study were treated in accordance with the guidelines of the Laboratory Animal Service Committee of the Medical College of Georgia.
Immunohistochemistry
Mouse brains fixed in phosphate-buffered saline containing 4% paraformaldehyde were embedded in O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) and frozen in liquid nitrogen. Cryosections (12 μm thick) were prepared from the embedded brains and then stained with the R24 anti-GD3 monoclonal antibody (IgG3) (Pukel et al. 1982) and AK97 anti-SSEA-1 monoclonal antibody (IgM) (Yanagisawa et al. 1999). As the secondary antibody, Alexa Fluor 488-conjugated anti-mouse IgG antibody (Invitrogen, Carlsbad, CA) or Alexa Fluor 546-conjugated anti-mouse IgM antibody (Invitrogen) was used. Nuclei were stained with H33258 (1 μg/mL; Sigma-Aldrich, St. Louis, MO). Stained sections were photographed under a Nikon Eclipse TE300 fluorescent microscope (Nikon Instruments, Melville, NY) equipped with a Magnafire digital charge-coupled device camera (Optronics, Goleta, CA).
Neurosphere culture
Neurospheres were prepared from mouse striata (E14) or SVZs (P2, P10, P30, and adult). In brief, the tissues dissected from mouse brains under a stereo microscope were dissociated with 0.15% trypsin (Sigma-Aldrich) and 0.1% DNase I (Sigma-Aldrich) at 37°C for 10 to 60 min. The tissues were gently triturated using a fire-polished Pasteur pipette every 20 min. After being centrifuged, the tissues were mechanically triturated in 0.07% trypsin inhibitor (Invitrogen) and 0.1% DNase I. The obtained single-cell suspensions were used for neurosphere culture or cell sorting. For neurosphere culture, cell suspensions were grown in Neurobasal-A medium (Invitrogen) supplemented with B27 (Invitrogen), 2 mM l-glutamine (Invitrogen), FGF-2 (20 ng/mL; Peprotech, Rocky Hill, NJ), and EGF (20 ng/mL; Peprotech) according to the previously described method with slight modifications (Reynolds and Weiss 1992; Weiss et al. 1996). Neurospheres formed after 5–7 days were collected for passage or further analyses. To avoid transformation, neurospheres were cultured within 1 month or five passages.
WST-8 assay
NSC proliferation was analyzed by a WST-8 assay using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). In brief, cells were plated onto uncoated 96-well plates at the density of 100 cells/μL (1 × 104 cells/well). After 0, 1, 3, and 5 days, the cells were incubated with WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] solution at 37°C for 4 h. The spectrophotometric absorbance of WST-8-formazan produced by the dehydrogenase activity in the living cells was measured at the wavelength of 450 nm using a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA). The spectrophotometric absorbance measured by this assay is highly correlative with the number of living cells.
RT-PCR
RT-PCR was performed as previously described (Ngamukote et al. 2007). Total RNAs were isolated from cultured neurospheres using a Trizol reagent (Invitrogen). cDNAs were synthesized from the total RNAs as templates using SuperScript III reverse transcriptase (Invitrogen). PCR was performed with the following setting: 24–36 cycles of 94°C for 20 s, 60°C for 20 s, and 72°C for 40 s. The sequences of primer sets were as follows: 5′-AAC GCC TTC ATG GTA TGG TC-3′ and 5′-CCG GGA AGC GTG TAC TTA TC-3′ for Sox2; 5′-TGG AAG TGG CTA CAT ACA GGA C-3′ and 5′-GGT ATT AGG CAA GGG GGA AG-3′ for nestin; 5′-ATG GTG GAA TGC AAG AAA GC-3′ and 5′-ATA CCC AGC ATG AAG GCA TC-3′ for Musashi-1; 5′-AGC CAT GTA CGT AGC CAT CC-3′ and 5′-TCT CAG CTG TGG TGG TGA AG-3′ for β-actin; 5′-AAA GCC AAG GGC GCT GAT GG-3′ and 5′-ATG GAT GTT CCC TAA CGA GCC-3′ for Tau; 5′-CCT CAG CTG ACA GAG AAA CAG-3′ and 5′-CTT GGT TCT GTG CTC TGT TTT C-3′ for MAP2. The PCR products were analyzed by agarose gel electrophoresis using 2% agarose gels containing SYBR Safe DNA Gel stain (Invitrogen).
Cell staining
Cells forming neurospheres were plated onto Lab-Tek Chamber Slides (Nalge Nunc International, Naperville, IL) that had been coated with poly-l-ornithine (Sigma-Aldrich) and fibronectin (Sigma-Aldrich) as previously described (Nakashima et al. 1999). To induce differentiation, the cells were cultured in the presence of 1% fetal bovine serum and the absence of both FGF-2 and EGF. After 5 days in culture, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline. To stain intracellular proteins, cells were permeabilized with 0.2% Triton X-100 in phosphate-buffered saline. Then, cells were incubated with the anti-nestin monoclonal antibody (BD Biosciences, San Jose, CA), anti-β-III tubulin monoclonal antibody (Sigma-Aldrich), anti-GFAP polyclonal antibody (DAKO, Glostrup, Denmark), anti-GalC monoclonal antibody (Millipore, Billerica, MA), R24 anti-GD3 monoclonal antibody, AK97 anti-SSEA-1 monoclonal antibody, biotin-conjugated cholera toxin B subunit (Gibco BRL, Grand Island, NY) or anti-MAP2 monoclonal antibody (Sigma-Aldrich). As the secondary antibody, Alexa Fluor 488-conjugated anti-mouse IgG antibody, Alexa Fluor 546-conjugated anti-mouse IgM antibody, or rhodamine-conjugated anti-rabbit IgG antibody was used. To detect biotin-conjugated cholera toxin B subunit, rhodamine-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA) was used. Nuclei were stained with H33258. Stained cells were photographed under a Nikon Eclipse TE300 fluorescent microscope equipped with a Magnafire digital charge-coupled device camera. The number of cells positive for GD3, nestin, and SSEA-1 was counted using ImageJ image analysis software (National Institutes of Health, Bethesda, MD). A minimum of eight areas were randomly chosen for counting.
TLC and TLC-immunostaining
Total lipids were extracted from neurospheres according to the previously described method with slight modifications (Ngamukote et al. 2007). In brief, from neurospheres or differentiated cells dispersed into single cells, the total lipids were extracted with chloroform/methanol (2:1, v/v) and then chloroform/methanol/water (1:2:0.8, v/v/v) by sonication for 15 min. The extracted total lipids were redissolved in chloroform/methanol (2:1, v/v) with the amount corresponding to 1 × 105 cells/μL and then developed on a high-performance TLC plate (silica gel 60; Merck Chemicals, Darmstadt, Germany) using a solvent system of chloroform/methanol/0.2% CaCl2 in water (55:45:10, v/v/v). Gangliosides on the TLC plate were visualized with a resorcinol-HCl reagent (Svennerholm 1956). For TLC-immunostaining, the TLC plate was coated with 0.4% polyisobutylmethacrylate in n-hexane after development and then incubated with the R24 anti-GD3 monoclonal antibody. As a secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG antibody (GE Healthcare, Piscataway, NJ) was used. GD3 reacted with the primary antibody was detected with a Western Lightning Western blot chemiluminescence reagent plus (Perkin Elmer Life and Analytical Sciences, Waltham, MA).
FACS
Cell suspensions prepared from mouse striata (E14) or SVZs (P2, P10, P30, and adult) were incubated with the R24 anti-GD3 monoclonal antibody or subclass control IgG3 (BD Biosciences) on ice for 30 min after blocking with 1% fetal bovine serum albumin in Hanks’ buffered salt solution (Invitrogen). Then, the cells were incubated with the Alexa Fluor 488-conjugated anti-mouse IgG antibody (Invitrogen) on ice for 30 min. Dead cells were stained with 10 μg/mL of propidium iodide (BD Biosciences) in Hanks’ buffered salt solution. GD3+ and GD3− cells were separated using a MoFlo cell sorter (DakoCytomation, Fort Collins, CO), and then plated onto uncoated 96-well plates at the density of 100 cells/μL. After 5 days, the number of formed neurospheres was counted under a phase contrast microscope. The neurospheres were collected and replated for cell staining.
Statistics
The comparison of data was performed using one-way ANOVA followed by Tukey's post hoc multiple comparison test or Student's paired t-test. Data were represented as mean ± SD. Significant differences were as considered at P < 0.05.
Funding
National Institutes of Health (NS11853, AG027199, and NS26994) and the Children's Medical Research Foundation.
Acknowledgments
We thank the Flow Cytometry Core Laboratory, Medical College of Georgia, for technical support, and Drs. Toshio Ariga, Chandramohan Wakade, and Fung-Chow Chiu, Medical College of Georgia, for their technical assistance and helpful discussion throughout this study.
Conflict of interest statement
None declared.
Abbreviations
- E
embryonic day
- EGF
epidermal growth factor
- FACS
fluorescence-activated cell sorting
- FGF-2
fibroblast growth factor-2
- GalC
galactosylceramide
- GFAP
glial fibrillary acidic protein
- H33258
Hoechst 33258
- MAP2
microtubule-associated protein 2
- NSCs
neural stem cells
- P
postnatal day
- RT-PCR
reverse transcription-polymerase chain reaction
- SSEA-1
stage-specific antigen-1
- SVZ
subventricular zone
- TLC
thin-layer chromatography
References
- Cammer W, Zhang H. Ganglioside GD3 in radial glia and astrocytes in situ in brains of young and adult mice. J Neurosci Res. 1996;46(1):18–23. doi: 10.1002/(SICI)1097-4547(19961001)46:1<18::AID-JNR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35(5):865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr., Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105(3):1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Hirano M, Yu RK, Seyfried TN. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7(2–3):179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Matsuzaki Y, Sato KI, Okano HJ, Shibata S, Sato I, Suzuki S, Ogawara M, Takamatsu K, Okano H. Cell surface N-glycans mediated isolation of mouse neural stem cells. J Neurochem. 2009;110(5):1575–1584. doi: 10.1111/j.1471-4159.2009.06256.x. [DOI] [PubMed] [Google Scholar]
- Huhn SL, Yung Y, Cheshier S, Harsh G, Ailles L, Weissman I, Vogel H, Tse V. Identification of phenotypic neural stem cells in a pediatric astroblastoma. J Neurosurg. 2005;103(5 Suppl):446–450. doi: 10.3171/ped.2005.103.5.0446. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Klassen H, Schwartz MR, Bailey AH, Young MJ. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312(3):180–182. doi: 10.1016/s0304-3940(01)02215-7. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Maric D, Fiorio Pla A, Chang YH, Barker JL. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci. 2007;27(8):1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23(1):240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276(5309):66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muramatsu H. Carbohydrate antigens expressed on stem cells and early embryonic cells. Glycoconj J. 2004;21(1–2):41–45. doi: 10.1023/B:GLYC.0000043746.77504.28. [DOI] [PubMed] [Google Scholar]
- Nagato M, Heike T, Kato T, Yamanaka Y, Yoshimoto M, Shimazaki T, Okano H, Nakahata T. Prospective characterization of neural stem cells by flow cytometry analysis using a combination of surface markers. J Neurosci Res. 2005;80(4):456–466. doi: 10.1002/jnr.20442. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19(13):5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103(6):2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- Peh GS, Lang RJ, Pera MF, Hawes SM. CD133 expression by neural progenitors derived from human embryonic stem cells and its use for their prospective isolation. Stem Cells Dev. 2009;18(2):269–282. doi: 10.1089/scd.2008.0124. [DOI] [PubMed] [Google Scholar]
- Pukel CS, Lloyd KO, Travassos LR, Dippold WG, Oettgen HF, Old LJ. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412(6848):736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, Ueda S, Uchiyama Y, Noda T, Okano H. RNA-binding protein Musashi family: Roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 2002;99(23):15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Kim SU. Ganglioside markers GD3, GD2, and A2B5 in fetal human neurons and glial cells in culture. Dev Neurosci. 1995;17(3):137–148. doi: 10.1159/000111282. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Tong QS, Zheng LD, Tang ST, Ruan QL, Liu Y, Li SW, Jiang GS, Cai JB. Expression and clinical significance of stem cell marker CD133 in human neuroblastoma. World J Pediatr. 2008;4(1):58–62. doi: 10.1007/s12519-008-0012-z. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, Van Der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19(9):387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kojima H, Kawakami Y, Iriko H, Nakamura T, Nakamura K, Uchida A, Murata Y, Tamai Y. A monoclonal antibody against a glycolipid SEGLx from Spirometra erinaceieuropaei plerocercoid. Mol Biochem Parasitol. 1999;102(2):225–235. doi: 10.1016/s0166-6851(99)00102-4. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 2004;9(9):801–809. doi: 10.1111/j.1365-2443.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J Lipid Res. 2009;50(Suppl):S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127(11):2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2(11):840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]