Abstract
The aim of our study was to investigate the correlation of the proliferative activity of pituitary neoplasms with clinical characteristics and recurrences. Tumor specimens were obtained from 44 consecutive patients with pituitary macroadenomas who underwent surgery between July 1998 and August 2003. Specimens were immediately fixed in 10% buffered formalin and then embedded in paraffin. The Ki-67 antigen was assessed by immumohistochemical analysis using the monoclonal antibody. We investigated the correlation of the Ki-67 labeling index with the following clinical and radiological characteristics: sex, age, presence or absence visual field defect, tumor classification, maximal tumor diameter, Hardy's classification, type of tumor, invasiveness, and recurrence. Our study suggests that the clinical characteristics such as visual field defect and recurrence are correlated with the high Ki-67 labeling index. No statistical differences were observed in the Ki-67 labeling index in relation to the following characteristics: sex, age, tumor classification, maximal tumor diameter, Hardy's classification, type of tumor, and invasiveness into the sphenoid sinus or cavernous sinus.
Keywords: Pituitary Neoplasms, Ki-67 Antigen, Recurrence
INTRODUCTION
Pituitary adenomas comprise 10-15% of all primary brain tumors and benign tumors usually of slow growth (1). The goal of management is to improve visual and other neurological deficits and to remove as much of the tumor as possible (2). Complete removal of tumors is infrequent because of the large size and invasive nature of these tumors. It has been estimated that approximately 50% of patients have tumor remnants after surgery (3). Some authors advocate adjunctive radiotherapy in the early postoperative period to prevent tumor recurrence (4, 5). Others prefer individualized treatment recommending additional therapy only after demonstration of unequivocal tumor recurrence or residual symptomatic disease (6, 7). Ki-67 antigen, which may be detected in all stages of the cell cycle except G0, represents a marker associated with tumor proliferation, invasiveness, and ultimately prognosis (8-10). However, it is still controversial whether Ki-67 is related to the aggressive behavior in pituitary adenomas (11). To assess the relationship between Ki-67 and recurrences, we used MIB-1 monoclonal antibody to detected the Ki-67 antigen in formalin-fixed, paraffin-embedded tissues (11, 12).
MATERIALS AND METHODS
Patient characteristics
Between July 1998 and August 2003, 44 patients with pituitary macroadenoma underwent surgery. In all cases the Ki-67 labeling index was measured in the surgical specimens using the MIB-1 monoclonal antibody. The male-to-female ratio was 1:1.44 (18 males and 26 females). The patients' age ranged from 23 to 76 yr (mean 48.9 yr). A functioning adenoma, with clinical manifestation of hormonal dysfunction, was present in 16 patients: 8 with prolactinoma and 8 with growth hormone (GH) secreting tumor. Visual field defect was present in 32 patients: 10 with functioning adenoma and 22 with non-functioning adenoma. All patients were classified according to the Hardy's classification and were analyzed in terms of maximal tumor diameter, type of tumor, invasiveness, and recurrence. In all cases the neuroradiological diagnosis was established.
Ki-67 immunostaining
To determine tumor characteristics at an early stage, we used MIB-1 antibody to identify the Ki-67 antigen. Surgically removed specimens were immediately fixed in 10% buffered formalin and then embedded in paraffin. Ki-67 immunostaining was performed using the avidin-biotin-peroxidase complex method. Five micrometer sections were mounted onto glass slides, dried, and were incubated with the MIB-1 antibody at 4℃ overnight. The regions with highest concentrations of MIB-1 positive nuclei and were selected and were analyzed at high power magnification (×400). On the basis of 1,000 neoplastic nuclei, the Ki-67 labeling index was calculated in each slide as the percentage of immunopositive nuclei. Vascular components and hematogenous cells were excluded. Only the dark brown stained nuclei were considered as immunopositive.
Statistical analysis
Computer assisted data analysis was performed with commercially available software (SPSS 12.0). The independent samples t-test and ANOVA were used to identify the statistical significance of difference of Ki-67 labeling index observed in relation to the following characteristics: sex, age, tumor classification, presence or absence of visual field defect, maximal tumor diameter, Hardy's classification, type of tumor, invasiveness to sphenoid or cavernous sinus, and recurrence. Values are expressed as the mean; for each comparison, a p-value was obtained and significance was assumed at p<0.05.
RESULTS
Relationship between clinical characteristics and Ki-67 labeling index
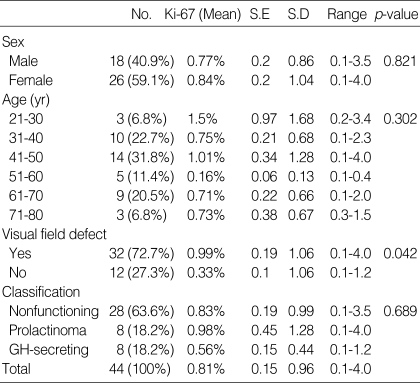
The Ki-67 labeling index in 44 pituitary macroadenomas as detected by using the MIB-1 antibody ranged from 0.1% to 4%. The mean Ki-67 labeling index was 0.81%. The Ki-67 labeling index was slightly higher in female than in male, but without a statistical significance (0.84% vs. 0.77%, p=0.821). The patients' age at surgery ranged from 23 to 76 yr. The mean age was 48.9 yr. For statistical analysis, patients' age at surgery was classified into six groups: from 21 to 30 yr (n=3), 31 to 40 yr (n=10), 41 to 50 yr (n=14), 51 to 60 yr (n=5), 61 to 70 yr (n=9), and 71 to 80 yr (n=3). The Ki-67 labeling index was 1.5%, 0.75%, 1.01%, 0.16%, 0.71%, and 0.73% in each of these age groups, respectively. The younger patients (21 to 30 yr) had a higher Ki-67 labeling index than older patients. However, the difference was not statistically significant (p=0.302). The Ki-67 labeling index was significantly higher in pituitary adenomas with visual field defect than in those without visual field defect (0.99% vs. 0.33%, p=0.042). As for the hormonal state of the tumors, prolactinomas had a higher Ki-67 labeling index than GH-secreting adenomas, but without a statistical significance (0.98% vs. 0.56%, p=0.689). The patients' clinical data are summarized in Table 1.
Table 1.
Correlation of clinical data and Ki-67 labeling index
No., Number; S.E, standard error; S.D, standard deviation; GH, growth hormone; p-value <0.05.
Relationship between radiological characteristics and Ki-67 labeling index
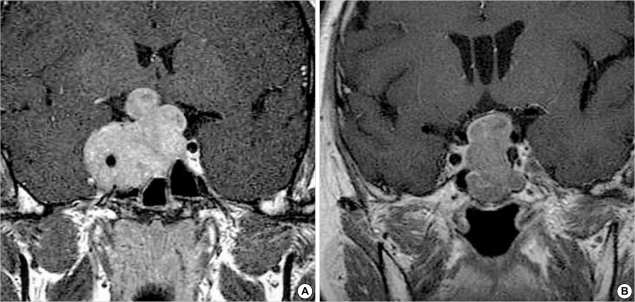
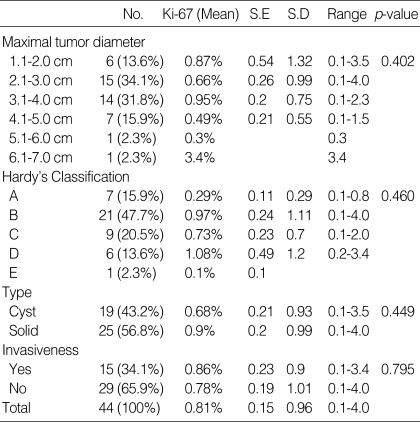
The maximal tumor diameter on magnetic resonance (MR) image was classified into six groups: from 1.1 to 2.0 cm (n=6), 2.1 to 3.0 cm (n=15), 3.1 to 4.0 cm (n=14), 4.1 to 5.0 cm (n=7), 5.1 to 6.0 cm (n=1), and 6.1 to 7.0 cm (n=1), with the Ki-67 labeling index 0.87%, 0.66%, 0.95%, 0.49%, 0.3%, and 3.4% respectively. The difference was not statistically significant (p=0.402). The Hardy's classification D (1.08%) had a higher Ki-67 labeling index than the others, but without a statistical significance (p=0.460). The cystic type was defined as magnetic resonance (MR) image, T2-weighted image shows more frequently hypertense signal. The Ki-67 labeling index was higher in solid type than in cystic type, but the difference was not statistically significant (p=0.449). In our cases, invasion was defined as radiological (MR image) invasion into sphenoid or cavernous sinus (Fig. 1). Adenomas with invasion had a higher Ki-67 labeling index (0.86%) than those without invasion (0.78%), but the difference was not statistically significant (p=0.795). The patients' radiological data are summarized in Table 2.
Fig. 1.
Coronal T1-weighted magnetic resonance (MR) images for radiological invasion of pituitary macroadenoma. Gadolinum-enhanced coronal image (A) shows a homogeneously enhanced intrasellar mass with invasion into the cavernous sinus. Gadolinum-enhanced coronal image (B) shows a homogeneously enhanced intrasellar mass without invasion. In adenomas with invasion, the Ki-67 labeling index was higher than those without invasion.
Table 2.
Correlation of radiological data and Ki-67 labeling index
No., Number; S.E, standard error; S.D, standard deviation; GH, growth hormone; p-value <0.05.
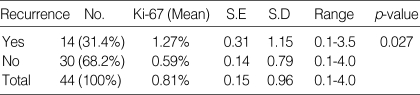
Relationship between recurrence and Ki-67 labeling index
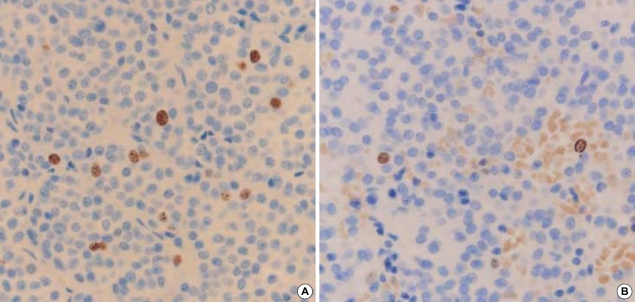
Recurrence was defined as the reappearance of hormonal hypersecretion after normalization of hormone values and radiological tumor growth. Adenomas with recurrence had a significantly higher Ki-67 labeling index (1.27%) than those without recurrence (0.56%) (p=0.027) (Fig. 2). The patients' recurrence data are summarized in Table 3.
Fig. 2.
Immunostaining for Ki-67 in pituitary macroadenoma. Only the dark brown stained nuclei were considered as immunopositive. (A) The Ki-67 labeling index in recurrent pituitary macroadenoma (3.5%) (original magnification, ×400). (B) The Ki-67 labeling index in non-recurrent pituitary macroadenoma (1%) (original magnification, ×400). The Ki-67 labeling index was higher in recurrent cases than in those without recurrence.
Table 3.
Correlation of recurrent data and the Ki-67 labeling index
No., Number; S.E, standard error; S.D, standard deviation; p-value <0.05.
Our study suggests that clinical characteristics such as visual field defect and recurrence in patients with pituitary macroadenomas are significantly correlated with the high Ki-67 labeling index. On the other hand, there were no statistical difference in the Ki-67 labeling index in relation to the following characteristics: sex, age at surgery, tumor classification, maximal tumor diameter, Hardy's classification, type of tumor, and invasiveness.
DISCUSSION
Most of the pituitary microadenomas are excised completely, but it is difficult to totally remove invasive pituitary macroadenomas (13). Although most of the pituitary adenomas grow slowly, some show aggressive or invasive growth (10, 13). Until now, no routine markers have been available to identify the aggressive behavior of pituitary adenoma. Recently, however, several cell cycle specific nuclear antigens have been recognized, using various immunohistochemical methods, which has aided reliable evaluation of tumor growth characteristics. Among these, Ki-67, a nuclear antigen readily identified by the monoclonal antibody MIB-1 (14), is typically expressed in proliferating cells during the G1, S, G2, and M phases of the cell cycle (15, 16). It has been found to be useful in assessing several brain tumors, providing information about cell proliferation and thus long-term prognosis (17, 18). Ki-67 antigen detectable at all stages of the cell cycle except G0 is considered a useful proliferation marker (12). The MIB-1 antibody permits detection of the Ki-67 antigen in formalin-fixed, paraffin-embedded tissues. In the studies by Knosp et al. (15), Zhao et al. (11) and Mastronardi et al. (17), the mean positive reaction to Ki-67 was 1.1%, 1.4%, and 2.64%, respectively. Generally the Ki-67 labeling index in pituitary adenomas is relatively low compared with other brain tumors (12). With respect to the patient gender and age, Yonezawa et al. (19) reported a significantly higher MIB-1 in nonfunctioning adenomas of patients younger than 30 yr than in patients older than 40 yr. Similarly, Jaffrain-Rea et al. (20) reported a significantly higher index in patients younger than 30 than in older patients. However, Mastronardi et al. (17) compared different age groups (<25, between 25 and 50, >50 yr) and found no significant difference in the Ki-67 index. In the present study, there was no significant difference in the Ki-67 index among the different age groups analyzed. Others reported no significant differences in the Ki-67 index regarding patients gender or tumor diameter (10, 21). In prolactinomas, Delgrange et al. (22) found that the indices were likely to be higher in males than in females, though such difference was not statistically significant. Landolt et al. (10) examined the proliferative activity of 31 pituitary adenomas and reported higher Ki-67 values for acromegalic patients. In the present series, there was no significant relationship between the proliferation and hormonal states of the adenomas (15). Mindermann and Wilson (23) analyzed the gender-specific incidence of different types of pituitary adenomas according to clinical phenotypes and found that prolactinomas occurred predominantly in females, while hormonally inactive adenomas and GH-secreting adenomas occurred mainly in males. In the present study, clinically GH-secreting adenomas were predominant in females (1:1.6), while nonfunctioning adenomas were predominant in males (1.2:1). Functioning pituitary tumors tend to be more common among younger adults, whereas nonfunctioning adenomas become more prominent with increasing age (24). The tumor size, expressed as the maximal diameter measured on preoperative MR image scans, did not correlate with the Ki-67 labeling index (7, 21). Hardy (25) classified them into five type: A) tumor bulging into the chiasmatic cistern; B) tumor reaching the anterior third ventricle; C) huge suprasellar extension filling the entire third ventricle; D) parasellar extension into the temporal, frontal, or posterior fossa; and E) lateral expansion towards the cavernous sinus. Lath et al. (26) reported a higher Ki-67 labeling index in Hardy's classification type E than tumors without extrasellar extension. The Ki-67 labeling index of the other Hardy's classification was not different among the other types. Pituitary adenoma frequently invade surrounding structures such as the cavernous sinus, sphenoid sinus, and even the brain (11). Invasive adenomas are not considered to be malignant; their biological behavior lies between non-infiltrative adenomas and pituitary carcinomas (27). The reported frequency of invasive pituitary adenomas varied greatly from 10% to 85%, a reflection of differenct criteria for the definition of invasiveness. Scheithauer et al. (27) reported a frequency of 35%. Tumor size did not correlate with the growth fraction in pituitary adenomas (28), whereas invasiveness correlated in most studies (2). Indeed, Gandour-Edwards et al. (29) did not observe any difference in the Ki-67 labeling indexes between 10 patients with pituitary adenomas involving the sphenoid sinus, and 10 patients with noninvasive tumors. By contrast, Thapar et al. (30) reported significantly higher Ki-67 labeling indexes of 3% or higher to distinguish noninvasive from invasive adenomas. Pituitary adenomas may be associated with a suprasellar extension, as well outlined by coronal and sagittal MR images. Tumor invasion leads to neurological complications and also compromises complete tumor excision and normalization of hormone levels (1). Residual tumor usually continues to grow, becoming evident as a recurrence with further invasion of surrounding structures requiring repeat surgery (12). Invasion of the cavernous sinus occurs in 6 to 10% of all pituitary adenomas (1). Kawamoto et al. (31) reported that adenomas with invasion of the cavernous sinus had a significantly greater volume than those without invasion, although they showed no significant difference in the cell proliferation indexes assessed. Landolt et al. (10) demonstrated a significantly higher Ki-67 labeling index in invasive pituitary adenomas (mean 1.15%) than in non-invasive adenomas (mean 0.06%). In our study, the Ki-67 labeling index in the recurrent group was higher than in the non-recurrent group. The recurrence rate of pituitary adenoma has been reported to be as high as 10% to 35% despite their slow growth and benign nature (32). Incomplete removal of the tumor was one of the most important causes of recurrence. Ekramullah et al. (13) and Shibuya et al. (21) reported that they observed higher Ki-67 labeling index values in recurrent adenomas.
In conclusion, the careful clinical, hormonal, and MR image follow-up is necessary to identify the predicted recurrence of the tumor as early as possible. The Ki-67 labeling index does not seem to provide independent information to identify tumor recurrences. Although studies on the Ki-67 labeling index from long-term follow-up series are limited, the index does appear to provide valuable prognostic information. The high Ki-67 labeling index might suggest the need for careful clinical and radiological follow-up, as a useful marker to determine the characteristics and recurrence of pituitary adenomas, and therefore on appropriate therapeutic strategy.
References
- 1.Pizaro CB, Oliveira MC, Coutinho LB, Ferreira NP. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res. 2004;37:235–243. doi: 10.1590/s0100-879x2004000200011. [DOI] [PubMed] [Google Scholar]
- 2.Losa M, Franzin A, Mangili F, Terreni MR, Barzaghi R, Veglia F, Mortini P, Giovanelli M. Proliferation index of non-functioning pituitary adenomas: correlations with clinical characteristics and long-term follow-up results. Neurosugery. 2000;47:1313–1319. [PubMed] [Google Scholar]
- 3.Sassolas G, Trouillas J, Treluyer C, Perrin G. Management of non-functioning pituitary adenomas. Acta Endocrinol (Copenh) 1993;129:21–26. [PubMed] [Google Scholar]
- 4.Petruson B, Jakobsson KE, Elfverson J, Bengtsson BA. Five-year follow-up of nonsecreting pituitary adenomas. Arch Otolaryngol Head Neck Surg. 1995;121:317–322. doi: 10.1001/archotol.1995.01890030051008. [DOI] [PubMed] [Google Scholar]
- 5.Shone GR, Richards SH, Hourihan MD, Hall R, Thomas JP, Scanlon MF. Non-secretary adenomas of the pituitary treated by transethmoidal sellotomy. J R Soc Med. 1991;84:140–143. doi: 10.1177/014107689108400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to transsphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991;68:860–866. doi: 10.1002/1097-0142(19910815)68:4<860::aid-cncr2820680431>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Carboni P, Jr, Detta A, Hitchcork ER, Postans R. Pituitary adenoma proliferative indices and risk of recurrence. Br J Neurosurg. 1992;6:33–40. doi: 10.3109/02688699209002899. [DOI] [PubMed] [Google Scholar]
- 8.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB1 and MIB3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 9.Gerdes J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluation in human malignancies. Seminar in Cancer Biology. 1990;1:199–206. [PubMed] [Google Scholar]
- 10.Landolt A, Shibata T, Kleihues P. Growth rate of human pituitary adenoma. J Neurosurg. 1987;67:803–806. doi: 10.3171/jns.1987.67.6.0803. [DOI] [PubMed] [Google Scholar]
- 11.Zhao D, Tomono Y, Nose T. Expression of P27, Kip 1 and Ki-67 in pituitary adenomas: An investigation of marker of adenoma invasiveness. Acta Neurochir (Wien) 1999;141:187–192. doi: 10.1007/s007010050285. [DOI] [PubMed] [Google Scholar]
- 12.Kitz K, Knosp E, Koos WT, Korn A. Proliferation of pituitary adenomas. Measurement by MAb Ki-67. Acta Neurochir (Wien) 1991;53(Suppl):60–64. doi: 10.1007/978-3-7091-9183-5_11. [DOI] [PubMed] [Google Scholar]
- 13.Ekramullah SM, Saitoh Y, Arita N, Ohnishi T, Hayakawa T. The correlation of Ki-67 staining indices with tumor doubling times in regrowing non-functioning pituitary adenomas. Acta Neurochir (Wien) 1996;138:1449–1455. doi: 10.1007/BF01411125. [DOI] [PubMed] [Google Scholar]
- 14.McCormick D, Chong H, Hobbs C, Datta C, Hall PA. Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB-1. Histopathology. 1993;22:355–360. doi: 10.1111/j.1365-2559.1993.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 15.Knosp E, Kitz K, Perneczky A. Proliferation activity in pituitary adenomas: measurement by monoclonal antibody Ki-67. Neurosurgery. 1989;25:927–930. [PubMed] [Google Scholar]
- 16.Parkins CS, Darling JL, Gill SS, Revesz T, Thomas DG. Cell proliferation in serial biopsies through malignant brain tumors: measurement using Ki-67 antibody labelling. Br J Neurosurg. 1991;5:289–298. doi: 10.3109/02688699109005189. [DOI] [PubMed] [Google Scholar]
- 17.Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Maira G. Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol. 1999;52:107–111. doi: 10.1136/jcp.52.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Chung SH, Kim HJ, Whang K, Han YP, Hong SK. Bcl-2 and Bax expression and Ki-67 proliferative index in astrocytic tumors: in relation to prognosis. J Korean Neurosurg Soc. 2004;35:465–471. [Google Scholar]
- 19.Yonezawa K, Tamaki N, Kokunai T. Clinical features and growth fractions of pituitary adenomas. Surg Neurol. 1997;48:494–500. doi: 10.1016/s0090-3019(97)00102-x. [DOI] [PubMed] [Google Scholar]
- 20.Jaffrain-Rea ML, Di Stefano D, Minniti G, Esposito V, Bultrini A, Ferretti E, Santoro A, Faticanti Scucchi L, Gulino A, Cantore G. A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: secreting versus non-secreting adenomas. Endocr Relat Cancer. 2002;9:103–113. doi: 10.1677/erc.0.0090103. [DOI] [PubMed] [Google Scholar]
- 21.Shibuya M, Saito F, Miwa T, Davis RL, Wilson CB, Hoshino T. Histochemical study of pituitary adenomas with Ki-67 and anti-DNA polymerase alpha monoclonal antibodies, bromodeoxyuridine labeling, and nucleolar organizer region counts. Acta Neuropathol (Berl) 1992;84:178–183. doi: 10.1007/BF00311392. [DOI] [PubMed] [Google Scholar]
- 22.Delgrane E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas; a clinical and proliferation marker study. J Clin Endoclinol Metab. 1997;82:2102–2107. doi: 10.1210/jcem.82.7.4088. [DOI] [PubMed] [Google Scholar]
- 23.Mindermann T, Wilson CB. Age-related and gender-related occurrence of pituitary adenomas. Clin Endocrinol. 1994;41:359–364. doi: 10.1111/j.1365-2265.1994.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S, Kovacs K, Horvath E, Aiba T. Morphological study of clinically nonsecreting pituitary adenomas in patients under 40 years of age. J Neurosurg. 1991;75:902–905. doi: 10.3171/jns.1991.75.6.0902. [DOI] [PubMed] [Google Scholar]
- 25.Hardy J. Transsphenoidal surgery of hypersecreting pituitary adenomas. In: Kohler PO, Ross GT, editors. Diagnosis and treatment of pituitary tumors. Excerpta Medica, America Elsevier; 1973. pp. 179–194. [Google Scholar]
- 26.Lath R, Chacko G, Chandy MJ. Determination of Ki-67 labeling index in pituitary adenomas using MIB-1 monoclonal antibody. Neurol India. 2001;49:144–147. [PubMed] [Google Scholar]
- 27.Scheithauer BW, Kovacs KT, Laws ER, Jr, Randall RV. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986;65:733–744. doi: 10.3171/jns.1986.65.6.0733. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Suh YL, Shin DI, Shin HJ, Kim JH. p53 expression and Ki-67 labeling index in brain tumor with special reference to tumor and histologic grade. Korean J Pathol. 1998;32:81–87. [Google Scholar]
- 29.Gandour-Edwards R, Kapadia SB, Janecka IP, Martinez AJ, Barnes L. Biologic markers of invasive pituitary adenomas involving the sphenoid sinus. Mod Pathol. 1995;8:160–164. [PubMed] [Google Scholar]
- 30.Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER., Jr Proliferation activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery. 1996;38:99–106. doi: 10.1097/00006123-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano T, Hirohata T. Analysis of the growth rate and cavernous sinus invasion of pituitary. Acta Neurochir. 1995;136:37–43. doi: 10.1007/BF01411433. [DOI] [PubMed] [Google Scholar]
- 32.Hus DW, Hakim F, Biller BM, de la Monte S, Zervas NT, Klibanski A, Hedley-Whyte ET. Significance of proliferating cell nuclear antigen index in predicting pituitary adenoma recurrence. J Neurosurg. 1993;78:753–761. doi: 10.3171/jns.1993.78.5.0753. [DOI] [PubMed] [Google Scholar]