Abstract
Objective To develop and assess the psychometric properties of a pictorial version of the Pediatric Asthma Quality of Life Questionnaire (PAQLQ). Methods A pictorial PAQLQ was administered to 101 children with mild to severe asthma between 5 and 7 years of age. A subgroup of 48 children followed longitudinally completed the established version of the PAQLQ. Results A confirmatory factor analysis with modifications supported the factor structure of the established PAQLQ. The pictorial measure exhibited internal consistency reliability and convergent, discriminant, and predictive validity. Conclusions Results suggest that the pictorial version of the PAQLQ has an underlying factor structure that is similar to that of the established PAQLQ. Future research with larger and diverse samples is needed to confirm the factor structure of the pictorial PAQLQ.
Keywords: asthma, children, psychometric validation, quality of life.
Pediatric asthma is a major health concern for children in the United States, as the prevalence of the disease has increased at a rate of 4.3% per year over the past two decades (Center for Disease Control [CDC], 2002). Furthermore, asthma is the third leading cause of hospitalization in children under 15 years of age and the cost of treating asthma in children has risen to over $3 billion a year (CDC, 2002). Due to the demanding nature of the disease, children with asthma are at greater risk for a decreased quality of life (QOL; Mrazek, Schuman, & Klinnert, 1998). QOL is broadly defined as an indication of how much a patient's illness interferes with daily life and how well the individual adapts to his or her illness across a broad range of domains (e.g., social, emotional, physical; Levi & Drotar, 1998).
In pediatric asthma, QOL measures provide physicians with additional information about the child's daily functioning that is not necessarily determined by spirometry readings or more objective measures of health status. For instance, Juniper suggested that children with asthma are not only distressed by their asthma symptoms (e.g., shortness of breath, chest tightness), but also by the limitations imposed by their disease. Moreover, children with asthma are likely to be frustrated by these limitations, may be angry that they have asthma, and likely fear having an asthma attack or an exacerbation of symptoms (Juniper, 1997). All of these aspects of having asthma are conceptualized as impacting a child's QOL (Juniper, 1997). Thus, measures of QOL serve to complement other health measures by providing a more comprehensive picture of how a child with asthma functions on a day-to-day basis.
QOL assessments are also used to measure the effectiveness of medical, environmental, and behavioral interventions to improve a child's daily functioning. For instance, in clinical trial research, QOL outcomes are used to evaluate changes in morbidity and the efficacy of pharmacological interventions (van Dellen et al., 2007). Thus, reliable measurement of QOL is essential to test the effectiveness of some randomized control trials aimed at improving functional morbidity of children with asthma. For health care providers, treatment decisions such as changing medications or altering dosage levels are based on QOL variations (Drotar, 2004; Naar-King, Ellis, & Frey, 2004).
Although there is a substantial amount of research utilizing QOL measures in older children and adolescents, the utilization of such measures among young children poses unique challenges related to emotional and cognitive development (Drotar, 2004). For instance, although several QOL measures, including the Pediatric Asthma Quality of Life Questionnaire (PAQLQ; Juniper et al., 1996a), have proven valid for children as young as 7 years of age, there is still some question as to whether younger children are developmentally capable of reporting on their own QOL. Children under 10 years of age are cognitively less able to make accurate judgments about events and emotions than older children (Drotar, 2004). As children are often able to understand pictorial representations (e.g., faces, pie charts, thermometers) more reliably than abstract, written answer formats (e.g., somewhat, quite often), pictorial versions of scales are often utilized with younger samples (Kamphaus & Frick, 2005). Pictorial scales are more likely to hold a young child's attention, be more readily understood, and lead to more reliable responses than a written format (Harter & Pike, 1984). Popular response formats of pictorial scales include the use of faces to depict increasing degrees of distress (e.g., pain intensity; LeBaron & Zeltzer, 1984) and pain or feeling thermometers that increase in their level of “fill” to represent greater feelings of distress (e.g., Jay, Ozolins, Elliott, & Caldwell, 1983). Such pictorial response formats serve to anchor the child's perceptions between two extremes that are developmentally appropriate for the child. Thus, the goal of this study was to develop a pictorial version of the widely used PAQLQ (Juniper et al., 1996a) for use with children between the ages of 5 and 7 years and to evaluate the reliability and initial validity of such a measure.
PAQLQ
Juniper and colleagues (1996a) developed the disease-specific PAQLQ to measure the QOL of children with asthma and to provide healthcare professionals with daily functioning information pertinent to treatment planning. Items on the PAQLQ are designed to capture information about a child's functioning in three domains: symptoms, activities, and emotions. Utilizing a sample of 52 children between the ages of 7 and 17 years, Juniper et al. (1996a) reported that the 23-item measure was responsive to within-subject changes in asthma symptoms, was validated with β-agonist use and morning peak-flow rates, and could detect meaningful changes in QOL across a 4-week period. Reliability was established through intraclass correlation coefficients ranging from .95 for overall QOL, to .84 for the activities subscale.
The purpose of our study was to develop a downward extension of the PAQLQ for use with young children and to assess the psychometric properties of such a measure.1 First, we expected the factor structure of the pictorial PAQLQ to be similar to that of the established PAQLQ. Second, given a stable factor structure, we aimed to demonstrate internal consistency coefficients of at least .60 for the total scale and subscales of the pictorial PAQLQ (Ware et al., 1980). Third, based on research suggesting disparities in health outcomes among children with asthma (Chen, Bloomberg, Fisher, & Strunk, 2003; Ortega & Calderon, 2000), we expected the pictorial PAQLQ to exhibit group differences with child race/ethnicity. Specifically, we hypothesized that Caucasian children would report higher levels of QOL than minority children. Furthermore, we expected QOL scores to differ based on recruitment site. Conversely, we did not expect group differences in QOL based on gender. Fourth, consistent with previous reports that a relationship between caregiver and child QOL may exist (Annett, Bender, DuHamel, & Lapidus, 2003), we sought to establish preliminary convergent validity with caregiver QOL scores on the parallel parent version of the PAQLQ (Juniper et al., 1996b). As QOL is conceptualized as being influenced by physical factors related to asthma severity (Guyatt, Juniper, Griffith, Feeny, & Ferrie, 1997), we also expected preliminary convergent validity of the pictorial PAQLQ with pulmonary functioning. Fifth, we aimed to establish preliminary discriminant validity by demonstrating a lack of relationship between QOL and measures of verbal ability. Finally, we expected the pictorial measure to demonstrate preliminary predictive validity with scores on the established version of the PAQLQ collected when a subset of children was 8 years of age.
Methods
Participants
Participants included 101 children (64% boys) between the ages of 5 and 7 years (mean age of 5.93 years) with mild to severe asthma (46% mild, 22% mild persistent, 29% moderate persistent, 3% severe)2 and their primary caregivers (mean age of 33.48 years). Data were collected as part of a larger study aimed at assessing family routines and medication adherence among families with a child with persistent asthma. Ninety-six percent of families identified the primary caregiver as the child's mother, 51% of caregivers were married, and 91% had at least a high school degree or equivalent. Fifty-six percent of children were Caucasian, 25% were African American, 3% were Hispanic, 1% were Native American, and 15% were of mixed race.
Participants were recruited through a pediatric pulmonary clinic (31%), a general pediatric practice clinic at a teaching hospital (37%), and private pediatric practices (32%) in the surrounding area including urban and rural regions. The distribution of participant race/ethnicity was representative of our sampling base. Inclusion criteria included a primary diagnosis of asthma, the prescribed use of a daily controller medication, and a child between 5 and 7 years of age. Exclusion criteria included exercise-induced asthma, the presence of other chronic health conditions that required a daily medication (e.g., diabetes), being in foster care (due to informed consent restrictions), and not being able to read English. Two-hundred fifty families were identified as potential participants and 49% (122 families) agreed to participate in the study. Of the families that were not enrolled, 52% declined to participate after receiving a brochure or completing an interest card, 22% were not reachable by telephone, and 26% were ineligible. Finally, of the families that were enrolled, 17% did not attend the lab visit.
Procedure
In this IRB-approved (institutional review board) study, families were first contacted via telephone and invited to attend a laboratory visit where questionnaires and interviews about family life and asthma were completed. At this visit, caregivers completed a background information questionnaire and a measure of QOL, among other measures. Children were interviewed separately and completed a lung function test, the pictorial measure of QOL, and a measure of verbal ability in addition to other measures not used in these analyses. Informed consent from caregivers and assent from children was obtained at the laboratory visit. All families received $50 for their participation.
Of these 101 families, 48 families were part of a subgroup of children followed longitudinally until the child with asthma was 8 years of age. The families comprising this longitudinal subgroup were the first families with a 5–7-year-old child that agreed to participate in the study. This subgroup was followed longitudinally as part of the broader study to determine variability among family routines. Thus, the sampling strategy for the larger study did not include following all 101 families over time. The composition of this longitudinal subgroup was distributed equally across recruiting sites. After completing the initial laboratory visit when the child was between the ages of 5 and 7, these families were invited to return to the laboratory for a final visit when the child was 8 years of age. Caregivers again completed a background questionnaire and measure of QOL among other measures not used in analyses. Children completed the established version of QOL, as well as other measures. Families were again compensated $50 for their participation.
Materials
PAQLQ (Established Version)
At a second time point, children who were 8 years of age completed the PAQLQ (Juniper et al., 1996a), a 23-item asthma-specific measure of QOL that assesses physical (10 questions), emotional (eight questions), and social (five questions) impairment due to asthma over the past week. Children rate their responses on a 7-point scale that ranges from “all of the time/extremely bothered” to “none of the time/not at all bothered” with higher scores representing no impairment. Subscale scores are calculated for each category of impairment and a total QOL score is calculated from the mean of all responses. This measure has been validated with clinical asthma-control outcomes (e.g., β-agonist use and morning peak-flow rates; Juniper et al., 1996a). Cronbach's α of .80 for the symptoms subscale and Cronbach's α of .87 for the emotions subscale were reported for the subgroup of children who completed this measure.
PAQLQ (Pictorial Version)
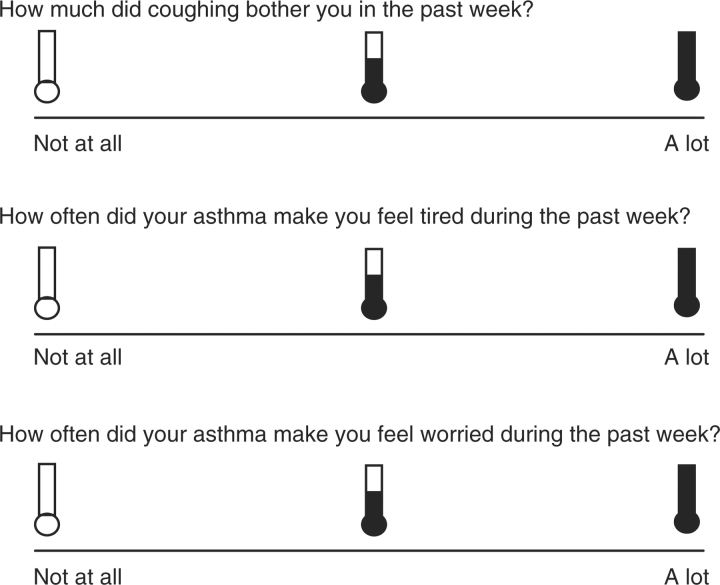
We developed a developmentally appropriate version of Juniper et al.'s (1996a) PAQLQ for use with 5–7-year-olds. We first converted the 23-item version to a pictorial version using pictures of three thermometers to represent “none”, “some”, or “all of the time” for the response format. Such a format provides a visual anchoring of three extremes by presenting empty and filled thermometers, as well as an intermediary thermometer that is halfway filled. We chose to utilize the thermometer response format based on its prior use with a similar age group among studies assessing children's reactions to trauma (Praver, DiGiuseppe, Pelcovitz, Mandel, & Gaines, 2000; Richters & Martinez, 1990) and internalizing and externalizing symptoms (Anan & Barnett, 1999). Furthermore, this response format allows the child to point to a thermometer to indicate how they are thinking or feeling, providing a nonthreatening way for children to communicate their emotions (Praver et al., 2000).
In the pictorial PAQLQ, the thermometer that is empty corresponds to “none of the time/not at all bothered” on the established PAQLQ and the full thermometer corresponds to “all of the time/extremely bothered”. Children are asked to rate their response to each item anywhere on a line below the three thermometers. Each item response is then scored such that a mark below the empty thermometer is converted to a score of 7, a mark below the intermediary thermometer is converted to a score of 4, and a mark below the filled thermometer is converted to a score of 1. A scoring template is also used to consistently score responses that fall between thermometers. After listening to instructions on how to complete the measure, children are asked to practice using the scale to answer questions such as “How much do you like ice cream?” and “How much do you like carrots?” The measure is then administered to the child by the interviewer. As with the established PAQLQ, subscale scores are calculated from the mean of responses for each subscale and total QOL is calculated from the mean of all responses (see Figure 1 for sample items).
Figure 1.
Sample Items From the Pictorial PAQLQ.
In the initial pilot phase, we administered the 23-item pictorial version to five children with asthma between 5 and 7 years of age. We found that young children were unable to understand the words “frustrated”(two items) and “uncomfortable” (one item) and also had difficulty identifying activities from the past week (e.g., running, laughing, playing at recess) that had bothered their asthma (four items). For instance, items containing the words “frustrated” and “uncomfortable” did not follow the same pattern as the other responses. Our team discussed the children's responses to these items and questionnaire administrators (advanced students in clinical psychology) remarked that children often seem confused by these items and hesitated in their responses. To follow up, we sent the items to researchers in another laboratory who agreed that these items would likely present difficulties for younger children. Following our pilot work, we consulted with a pulmonologist and respiratory therapist at our site and an expert psychosocial researcher at another site to confirm our findings regarding these seven items. After consultation, we eliminated these seven items from the measure, resulting in a 16-item questionnaire (see Table I for a list of eliminated items).
Table I.
Items from the established PAQLQ that were eliminated in constructing the pictorial PAQLQ
| Activity subscale | Emotions subscale |
|---|---|
| Items 1–3: How much have you been bothered by your asthma in (activity 1, 2, or 3) during the past week? (activities are chosen by the child from a list of activities.) | Item 5: How often did your asthma make you feel frustrated during the past week? |
| Item 22: Think about all of the activities that you did during the past week. How much were you bothered by your asthma doing these activities? | Item 15: How often did you feel frustrated because you couldn’t keep up with others during the past week? |
| Item 17: How often did you feel uncomfortable because of your asthma during the past week? |
Note. PAQLQ = Pediatric Asthma Quality of Life Questionnaire.
Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ)
Caregivers completed the 13-item PACQLQ (Juniper et al., 1996b) which measures the impact of the child's asthma on the caregiver's daily activities (four questions) and the fear and worry associated with having a child with asthma (nine questions). Caregivers respond to a seven-item scale with item responses ranging from “all of the time” to “none of the time” or “very, very worried/concerned” to “not worried/concerned.” Higher scores represent no impairment. A total score is derived from the mean of all responses. Cronbach's α of .93 for overall caregiver QOL was computed for this sample. The PACQLQ has been validated with other health-related measures, including the Global Rating of Change Questionnaires (Juniper, Guyatt, Willan, & Griffith, 1994).
Wechsler Intelligence Scale for Children-III (WISC-III)
Children completed the vocabulary subtest of the WISC-III (Wechsler, 1991), which assesses the intellectual abilities of children aged 6–17 years. We utilized scores on the vocabulary subtest of the verbal IQ index to determine child verbal ability for our 6- and 7-year-old participants.
Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-Revised)
Children who were 5 years of age completed the vocabulary subtest of the WPPSI-Revised (Wechsler, 1989). This measure assesses the intellectual abilities of children between the ages of 2 and 7 years. We utilized scores on the vocabulary subtest of the verbal IQ portion of the measure to determine verbal ability in our participants who were 5 years of age.
Pulmonary Functioning
A trained respiratory therapist administered a spirometry test to measure lung functioning during the laboratory visit. Three spirometry measurements were obtained from each child, with the largest forced expiratory volume in one second (FEV1) used in analyses. Lung functioning was first measured while the child was at rest. Following an administration of albuterol and a period of 10 min, the spirometry measurements were repeated. A board-certified pulmonologist classified level of asthma severity based on National Heart, Lung, and Blood Institute guidelines (NHLBI, 2007).
Hollingshead Index of Social Status
As part of the background questionnaire, caregivers completed the Hollingshead measure (Hollingshead, 1975). This measure determines socioeconomic status (SES) from the education level and occupation of each parent. Scores on the Hollingshead measure ranged from 12 to 66 (M = 39.20, SD = 15.12), suggesting that all SES levels were represented in our sample.
Statistical Analyses
We first performed a confirmatory factor analysis (CFA) using AMOS version 16.0 (Analysis of Moment Structures 16.0; SPSS, Inc., Chicago, IL) to determine whether the pictorial PAQLQ shared a factor structure similar to that of the established PAQLQ. CFA is a technique used to confirm an underlying, theory-driven model of the data (Tabachnick & Fidell, 2007). As our pictorial measure was based on the established PAQLQ, we utilized a confirmatory over an exploratory factor analysis (EFA) to determine whether the pictorial PAQLQ mirrored the theory-driven structure of the established PAQLQ. As the activities subscale of the pictorial PAQLQ contained only one item (“How often did you feel you couldn’t keep up with others because of your asthma?”), we removed this item and the associated latent variable, activities. Thus, the 15-item PAQLQ was utilized for all subsequent analyses.
The goodness of fit of the hypothesized and observed model was judged to be adequate if the following criteria were met: Tucker–Lewis Index (TLI) >.90, Comparative Fit Index (CFI) >.90, and Root Mean Square error of approximation (RSMEA) <.08 (Tabachnick & Fidell, 2007). If the CFA did not reveal an adequate fit of the model based on these criteria, we planned to use modification indices suggested by AMOS to guide possible path additions to the model and improve the model goodness of fit (Kline, 1998). We reasoned that as our CFA was guided by the theoretical structure of the PAQLQ, we would only include modification indices that were guided by this structure and allowed items to load on their hypothesized latent variables (e.g., Pai et al., 2007). Furthermore, to maintain a parsimonious model, we would add the fewest number of path covariances between error terms that would lead to a better fitting model, beginning with path covariances that had the largest modification indices (Tabachnick & Fidell, 2007).
All subsequent analyses were performed using SPSS version 16.0 software (Statistical Product and Service Solutions 16.0; SPSS Inc., Chicago, IL). Bivariate correlations were performed between factor scores that emerged from the CFA. Internal consistency coefficients (Cronbach's α) were calculated for the total scale and subscales. A t-test was utilized to assess for group differences in subscale scores on the pictorial PAQLQ based on child gender or child race/ethnicity (categorized as minority status or Caucasian). As our sample had a low percentage of Hispanic, Native American, and mixed-race families, we included children in these categories as minority along with African American families. An analysis of variance (ANOVA) was used to determine PAQLQ differences by recruitment site. We also utilized chi-square analyses to assess for differences in race/ethnicity based on recruitment site. An ANOVA was used to assess for differences in SES based on recruitment site.
Convergent validity was assessed by correlating the QOL subscale scores with a measure of lung functioning and caregiver scores on the PACQLQ. A median split was performed on PAQLQ and PACQLQ scores. We then used a Pearson chi-square analysis to determine whether parent and child QOL corresponded by level (i.e., both scores were above or below the median QOL value). Correlational analyses were used to assess for differences in QOL subscale scores based on verbal ability. To assess predictive validity, a partial correlational analysis controlling for child age at the initial visit was performed between subscale scores on the pictorial PAQLQ and the established PAQLQ. As the time between the completion of the pictorial PAQLQ (when the child was 5–7 years of age) and completion of the established PAQLQ (when the child was 8 years of age) could vary by as much as 3 years, we controlled for child age at the initial visit to eliminate this time interval as a potential confound.
Results
Confirmatory Factor Analysis
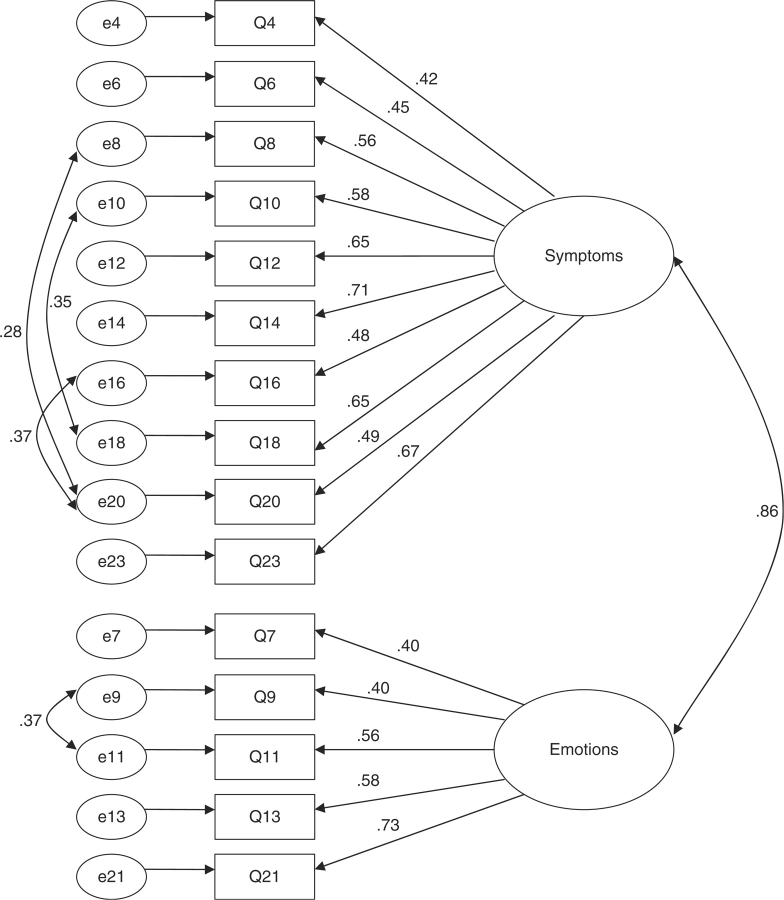
Results of the CFA revealed a significant chi-square of 151.2 (df = 89, p <.01), suggesting a lack of fit between the hypothesized and observed models. Further, the resultant fit indices failed to reach the minimum criteria for an adequate fitting model (TLI = .826, CFI = .852, and RSMEA = .084). Guided by the modification indices, paths between error variances for items 8 and 20, items 10 and 18, items 16 and 20, and items 9 and 11 were added (see Table II for questionnaire items). The addition of these paths improved the fit of the model, most likely because the paired items inquired about similar content. For instance, item 8 (“How much did asthma attacks bother you?”) and item 20 (“How often have trouble sleeping at night?”) are related in that difficulty sleeping is often a risk factor for symptom exacerbation, such as asthma attacks (Strunk, Sternberg, Bacharie, & Szefler, 2002). The final model showed adequate fit to the data, χ2(df = 85) = 107.07, p =.053, CFI =.948, TLI =.935, RSMEA =.051. This model is presented in Figure 2. A bivariate correlation of .57 (p <.01) was found between Factors 1 and 2. The mean of Factor 1, the symptoms subscale, was 4.68 (SD = 1.54) and the mean of Factor 2, the emotions subscale, was 4.92 (SD = 1.69). The mean total QOL score was 4.77 (SD = 1.42).
Table II.
CFA Items by Factor, Item Means, and Standard Deviations for the Pictorial PAQLQ
| Factor, established PAQLQ item number, and item | M | SD |
|---|---|---|
| Symptoms | ||
| 4. Coughing bothers you | 3.94 | 2.42 |
| 6. Asthma makes you feel tired | 4.41 | 2.54 |
| 8. Asthma attacks bother you | 4.86 | 2.34 |
| 10. Wheezing bothers you | 4.88 | 2.47 |
| 12. Tightness in chest bothers you | 5.10 | 2.45 |
| 14. Shortness of breath bothers you | 5.06 | 2.33 |
| 16. Asthma wakes you up during the night | 4.44 | 2.51 |
| 18. Feel out of breath | 4.58 | 2.46 |
| 20. Trouble sleeping at night | 4.56 | 2.55 |
| 23. Difficulty taking deep breath | 4.63 | 2.56 |
| Emotions | ||
| 7. Asthma makes you feel worried | 4.73 | 2.53 |
| 9. Asthma makes you feel angry | 4.90 | 2.53 |
| 11. Asthma makes you feel cranky | 5.03 | 2.35 |
| 13. Feel left out because of asthma | 4.91 | 2.45 |
| 21. Feel frightened by asthma attack | 5.01 | 2.54 |
| Item removed | ||
| 19. Feel couldn’t keep up with others | 4.50 | 2.61 |
Note. Confirmatory factor analysis = CFA; Pediatric Asthma Quality of Life Questionnaire = PAQLQ.
Figure 2.
CFA Model of the Pictorial PAQLQ With Error Covariance Path Modifications.
Subscale Reliabilities
Internal consistency coefficients were calculated for the two factors revealed from the CFA. Cronbach's α of .83 was calculated for Factor 1 and Cronbach's α of .71 was calculated for Factor 2. For the total QOL score, Cronbach's α was .86.
Group Differences
Scores on the symptoms subscale differed by child race: t(97) = −2.45, p <.05. Minority children reported significantly poorer scores on the symptoms subscale (M = 4.27, SD = 1.49) than Caucasian participants (M = 5.02, SD = 1.51). Similarly, scores on the emotions subscale differed by child race: t(99) = −2.69, p <.01. Minority children reported worse scores on the subscale (M = 4.42, SD = 1.68) than Caucasian children (M = 5.30, SD = 1.61). Scores on the symptoms subscale also differed by recruitment site, F(2, 96) = 4.73, p <.05, as did scores on the emotions subscale, F(2, 98) = 3.28, p <.05. Children recruited from the teaching hospital reported significantly worse QOL scores than children recruited from the pulmonary clinic or private pediatric clinics. Conversely, scores on the symptoms subscale did not differ by child gender t(97) = −1.26, p = .21, nor did scores on the emotions subscale, t(99) = –1.14, p =.26.
With respect to differences in our demographic variables based on recruitment site, we found that recruitment site was related to child race/ethnicity χ2(2, N = 98) = 36.39, p <.01. Specifically, we found that families recruited from the teaching hospital were more likely to be of minority status. Further, we found that SES differed by recruitment site, F(2, 96) = 24.44, p <.01. Families recruited from the teaching hospital had significantly lower SES scores (M = 29.34, SD = 11.52) than families recruited from the pulmonary clinic (M = 47.90, SD = 13.20) or private pediatric clinics (M = 44.18, SD = 12.86).
Convergent Validity
Symptoms subscale scores of the pictorial PAQLQ were significantly correlated with total scores on the PACQLQ (M = 5.34, SD = 1.49; r = .23, p < .05). Scores on the emotions subscale of the pictorial PAQLQ were also significantly correlated with total PACQLQ scores (r = .23, p < .05). A chi-square analysis revealed agreement among level of total QOL scores, χ2(1, N = 100) = 6.76, p < .01, with parent and child reporting correspondence in scores (i.e., both above or below the median QOL score) 63% of the time.
Scores on the symptoms subscale were also significantly related to FEV1 scores (M = 1.48, SD = .51; r = .22, p < .05). A statistical trend between scores on the emotions subscale and FEV1 scores was found (r = .19, p = .06).
Discriminant Validity
Verbal ability for 5-year-old children who completed the WPPSI (M = 9.57, SD = 3.13) was not significantly correlated with scores on the symptoms subscale (r = .04, p = .82) or scores on the emotions subscale (r = .05, p = .77). Scores on the emotions subscale were not significantly correlated with verbal ability for 6- or 7-year-old children who completed the WISC measure (M = 11.0, SD = 4.06; r = .20, p = .14). However, scores on the symptoms subscale were significantly related to verbal ability on the WISC (r = .29, p < .05).
Predictive Validity
For those children followed longitudinally, scores on the symptoms subscale of the pictorial QOL measure demonstrated predictive validity with the symptoms subscale of the established PAQLQ measure (M = 5.56, SD = 1.25; r = .51, p < .01) after controlling for child age at the initial visit. Further, scores on the emotions subscale of the pictorial PAQLQ demonstrated predictive validity with the emotions subscale of the established PAQLQ (M = 5.65, SD = 1.35; r = .41, p < .01) after controlling for child age at the initial visit.
Discussion
The aim of this study was to develop and provide initial validation of a pictorial version of the PAQLQ (Juniper et al., 1996a) for use with children between the ages of 5 and 7 years. For the most part, our findings suggest we have developed a downward extension of the established PAQLQ that demonstrates adequate psychometric properties and convergent, discriminant, and predictive validity. We suggest that with more extensive validation of the measure's factor structure, the pictorial PAQLQ could be incorporated into use by researchers and health care professionals. Prior to any modifications, our initial CFA did not adequately describe the factor structure of the picture version of the PAQLQ as evidenced by inadequate fit indices. However, with the modifications suggested by the modification indices, our CFA model did support the factor structure of the pictorial PAQLQ. The selection of these modification indices was driven by the theoretical underpinnings of child QOL. Thus, we suggest that the pictorial PAQLQ does follow a factor structure that is similar to that of the established PAQLQ.
Furthermore, the emotions and symptoms subscales of the pictorial PAQLQ were found to be distinct and consistent with the established PAQLQ (Juniper et al., 1996a). However, as we eliminated most of the items from the activities subscale in developing the pictorial PAQLQ (and subsequently eliminated the sole item from the activities subscale from our CFA analysis), our CFA results did not yield an activities subscale. We suggest that this two-factor structure perhaps makes better sense developmentally for young children than the three-factor structure of the established PAQLQ (emotions, symptoms, activities). For instance, we noted in our early pilot phase that younger children had difficulty recalling specific activities that were affected by their asthma. However, these children were able to recall feelings related to fatigue and exhaustion. Thus, the QOL of young children with asthma may be influenced differently than the QOL of older children.
Our internal consistency coefficients for total score and subscale scores suggest that the pictorial PAQLQ is a reliable measure. We found that the emotions subscale had the lowest internal consistency coefficient (α =.71) of the subscales and total scale. This might suggest that young children have difficulty understanding questions related to their emotional state (Drotar, 2004). For instance, in our pilot work, we found that young children had difficulty understanding items containing the words “uncomfortable” and “frustrated.” Items with these terms in them were subsequently eliminated in our initial scale development. Although the emotions subscale of the pictorial PAQLQ scale had adequate internal consistency reliability, future refinement of the pictorial scale might suggest testing other emotional items or rewriting such items to increase subscale reliability.
Our findings with respect to group differences have implications for future research on the QOL of young children with asthma. For instance, our findings suggest that minority children may experience a poorer QOL than Caucasian children. As children from minority families are more likely to utilize emergency departments instead of routine physician visits, it might be that a lack of effective management behaviors leads to increased asthma morbidity among this subgroup of children (Chen et al., 2003). We further found that families recruited through a teaching hospital were more likely to be of minority status and lower SES levels. Children from these families were also more likely to report a poorer QOL on the pictorial PAQLQ. Minority status may actually be a marker for other risk factors, such as economic resources, access to health care, and parental education (McLoyd, 2004). In fact, a recent review suggests that family income may be a correlate of child QOL in pediatric asthma (Olson, Lara, & Frintner, 2004). Thus, it is likely that our differences in QOL based on recruitment site were due to the presence of more diverse patients at the teaching hospital. Future studies should carefully consider what factors might account for a poorer QOL among children from diverse families. We also suggest that future research continues to focus on including more families from minority racial and ethnic groups. As we had a low number of Hispanic and Native American families participating in our study, we were unable to determine specifically how the QOL of young children in these minority groups might differ from Caucasian children, as well as other minority children.
Our findings also suggest that agreement between parent and child QOL exists among a younger group of children. The relationship between parent and child QOL has not been extensively studied, with a previous study finding that agreement between the QOL of parents and the QOL of children increases as the child matures (Annett et al., 2003). Future studies should seek to replicate our findings using the pictorial PAQLQ measure to determine the relative concordance between parent and child QOL. Doing so would also expand the knowledgebase about family-level QOL (Levi & Drotar, 1998).
With respect to lung functioning, we found that the symptoms subscale of the pictorial PAQLQ exhibited convergent validity with child FEV1 scores. We found a statistical trend between the emotions subscale and child FEV1 scores. Although several studies have reported that a relationship exists between asthma severity and child QOL (Guyatt et al., 1997; Okelo et al., 2004), others report little to no association (Erickson et al., 2002; Vila et al., 2003). It might be that asthma severity affects the QOL of younger children more so than that of older children with asthma due to the unstable nature of the disease in its early stages (NHLBI, 2007). However, future research using the pictorial PAQLQ among younger children with more severe asthma is warranted to clearly identify this association. Moreover, it may be that lung functioning has a greater influence on the symptom domain of QOL than the emotional functioning domain in young children with asthma. Future studies utilizing the pictorial PAQLQ should further investigate the effect of asthma severity on child QOL.
Our findings with respect to discriminant validity with a measure of verbal ability were also inconsistent. We found that the pictorial PAQLQ subscales were not related to verbal ability for 5-year-old participants, but that scores on the symptoms subscale were related to verbal ability for 6- and 7-year-olds. As few studies to date have considered the relationship between QOL in pediatric asthma and verbal ability, we suggest that more research is needed to determine whether verbal ability is a correlate of child QOL. Such an inconsistent finding speaks to the complexity of determining the correlates of QOL among young children with asthma (Everhart & Fiese, in press).
Thus, future work and refinement of the pictorial PAQLQ should focus on the two-factor structure (symptoms, emotions) that resulted from our CFA with modifications. Researchers should utilize the mean of scores on items 6, 8, 10, 12, 14, 16, 18, 20, 23 to generate the symptoms subscale score and the mean of scores on items 7, 9, 11, 13, and 21 to calculate the emotions subscale score (see Table II for specific questionnaire items).
As it stands now, the pictorial version of the PAQLQ shows promise as a tenable measure for health care providers and researchers to incorporate in their assessments of young children with asthma. The pictorial PAQLQ could be utilized by health care providers and researchers in their decisions about treatment planning in young children with asthma. Child report of QOL also avoids some of the pitfalls of parent report that can be influenced by expectations and emotional states (Drotar, 2004).
Limitations
Due to the relatively small size of our sample, we were unable to cross-validate the factor structure of the pictorial PAQLQ with a random subset of our sample. A larger sample would allow for a more extensive look at the factor structure of the pictorial version. A general guideline for performing a CFA is to utilize a sample size that is at least 5–10 times the number of observed indicators (Kline, 1998). Our study is also limited in its ability to generalize to other groups of children with asthma. For instance, although the full spectrum of asthma severity levels was represented in our sample, only 3% of our sample was classified as having severe asthma. Thus, our findings regarding the pictorial PAQLQ may not generalize to a sample of children with more severe asthma. Furthermore, as children were required to be prescribed a daily controller medication at study enrollment, our findings may not generalize to samples of children who are not prescribed a daily controller medication or those with exercise-induced asthma.
Conclusion
Findings from this study suggest that the pictorial PAQLQ shows promise as an important measure for use with children between 5 and 7 years of age. With modifications, the pictorial QOL measure did reveal a factor solution that was similar to that of the established PAQLQ. Integrating the pictorial PAQLQ into assessments of QOL would allow for researchers and health care providers to receive feedback on child functioning directly from the patient. This important information could then be used in the treatment planning and decision-making process of providers who treat young children with asthma. However, future research is needed to confirm the proposed factor structure of the pictorial PAQLQ.
Funding
National Institute of Mental Health (R01 MH51771, partial to B.H.F.).
Conflict of interest: None declared.
Footnotes
1Please contact the corresponding author for a copy of the pictorial PAQLQ.
2Data on lung functioning were available for 98 of the 101 children. Children with these data missing did not differ on any demographic variables from children with these data present (e.g., gender, age, and race).
References
- Anan RM, Barnett D. Perceived social support mediates between prior attachment and subsequent adjustment: A study of urban African-American children. Developmental Psychology. 1999;35:1210–1222. doi: 10.1037//0012-1649.35.5.1210. [DOI] [PubMed] [Google Scholar]
- Annett RD, Bender BG, DuHamel TR, Lapidus J. Factors influencing parent reports on quality of life for children with asthma. Journal of Asthma. 2003;40:577–587. doi: 10.1081/jas-120019030. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Asthma's impact on children and adolescents. 2002. Retrieved June 15, 2007, from http://www.cdc.gov/asthma/children.htm.
- Chen E, Bloomberg GR, Fisher JEB, Strunk RC. Predictors of repeat hospitalizations in children with asthma: The role of psychosocial and socioenvironmental factors. Health Psychology. 2003;22:12–18. doi: 10.1037//0278-6133.22.1.12. [DOI] [PubMed] [Google Scholar]
- Drotar D. Validating measures of pediatric health status, functional status, and health-related quality of life: Key methodological challenges and strategies. Ambulatory Pediatrics. 2004;4:358–364. doi: 10.1367/A03-101R.1. [DOI] [PubMed] [Google Scholar]
- Erickson SR, Munzenberger PJ, Plante MJ, Kirking DM, Hurwitz ME, Vinuya RZ. Influence of sociodemographics on the health-related quality of life of pediatric patients with asthma and their caregivers. Journal of Asthma. 2002;39:107–117. doi: 10.1081/jas-120002192. [DOI] [PubMed] [Google Scholar]
- Everhart RS, Fiese BH. Patient Education and Counseling. 2008. Asthma severity and child quality of life in pediatric asthma: A systematic review. doi:10.1016/j.pec.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Juniper EF, Griffith LE, Feeny DH, Ferrie PJ. Children and adult perceptions of childhood asthma. Pediatrics. 1997;99:165–168. doi: 10.1542/peds.99.2.165. [DOI] [PubMed] [Google Scholar]
- Harter S, Pike R. The pictorial scale of perceived competence and social acceptance for young children. Child Development. 1984;55:1969–1982. [PubMed] [Google Scholar]
- Hollingshead AB. A four-factor classification of social status. Unpublished manuscript submitted to Yale University: New Haven, CT; 1975. [Google Scholar]
- Jay SM, Ozolins M, Elliott CH, Caldwell S. Assessment of children's distress during painful medical procedures. Health Psychology. 1983;2:133–147. [Google Scholar]
- Juniper EF. How important is quality of life in pediatric asthma? Pediatric Pulmonology. 1997;15:17–21. [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Quality of Life Research. 1996a;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Quality of Life Research. 1996b;5:27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. Journal of Clinical Epidemiology. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Kamphaus RW, Frick P. Clinical assessment of children's personality and behavior. 2nd. New York: Springer; 2005. [Google Scholar]
- Kaugars AS, Klinnert MD, Bender BG. Family influences on pediatric asthma. Journal of Pediatric Psychology. 2004;29:475–491. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 1998. [Google Scholar]
- LeBaron S, Zeltzer L. Assessment of acute pain and anxiety in children and adolescents by self-reports, observer reports, and a behavior checklist. Journal of Consulting and Clinical Psychology. 1984;52:729–738. doi: 10.1037//0022-006x.52.5.729. [DOI] [PubMed] [Google Scholar]
- Levi R, Drotar D. Critical issues and needs in health-related quality of life assessment in children and adolescents with chronic health conditions. In: Drotar D, editor. Measuring health-related quality of life in children and adolescents: Implications for research and practice. Mahwah, NJ: Lawrence Erlbaum; 1998. pp. 3–24. [Google Scholar]
- McLoyd VC. Linking race and ethnicity to culture: Steps along the road from inference to hypothesis testing. Human Development. 2004;47:185–191. [Google Scholar]
- Mrazek DA, Schuman WB, Klinnert MD. Early asthma onset: Risk of emotional and behavioral difficulties. Journal of Child Psychology and Psychiatry. 1998;39:247–254. [PubMed] [Google Scholar]
- Naar-King S, Ellis DA, Frey MA. Assessing children's well-being: A handbook of measures. Mahwah, NJ: Lawrence Erlbaum; 2004. [Google Scholar]
- National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- Okelo SO, Wu AW, Krishnan JA, Rand CS, Skinner EA, Diette GB. Emotional quality-of-life and outcomes in adolescents with asthma. Journal of Pediatrics. 2004;145:523–529. doi: 10.1016/j.jpeds.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Olson LM, Lara M, Frintner MP. Measuring health status and quality of life for US children: Relationships to race, ethnicity, and income status. Ambulatory Pediatrics. 2004;4:377–386. doi: 10.1367/A03-156.1. [DOI] [PubMed] [Google Scholar]
- Ortega AN, Calderon JG. Pediatric asthma among minority populations. Current Opinion in Pediatrics. 2000;12:579–583. doi: 10.1097/00008480-200012000-00012. [DOI] [PubMed] [Google Scholar]
- Pai ALH, Mullins LL, Drotar D, Burant C, Wagner J, Chaney JM. Exploratory and confirmatory factor analysis of the child uncertainty in illness scale among children with chronic illness. Journal of Pediatric Psychology. 2007;32:288–296. doi: 10.1093/jpepsy/jsl021. [DOI] [PubMed] [Google Scholar]
- Praver F, DiGiuseppe RA, Pelcovitz D, Mandel FS, Gaines R. A preliminary study of a cartoon measure for children's reactions to chronic trauma. Child Maltreatment: Journal of the American Professional Society on the Abuse of Children. 2000;5:273–285. doi: 10.1177/1077559500005003007. [DOI] [PubMed] [Google Scholar]
- Richters JE, Martinez P. Levonn: A cartoon-based structured interview for assessing children's distress symptoms. Washington, DC: National Institute of Mental Health; 1990. [Google Scholar]
- Strunk RC, Sternberg AL, Bacharie LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. Journal of Allergy and Clinical Immunology. 2002;110:395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th. Boston: Allyn & Bacon; 2007. [Google Scholar]
- van Dellen QM, Stronks K, Bindels PJ, Ory FG, Bruil J, van Aalderen WM. Health-related quality of life in children with asthma from difference ethnic origins. Journal of Asthma. 2007;44:125–131. doi: 10.1080/02770900601182459. [DOI] [PubMed] [Google Scholar]
- Vila G, Hayder R, Bertrand C, Falissard B, de Blic J, Mouren-Simeoni MC, et al. Psychopathology and quality of life for adolescents with asthma and their parents. Psychosomatics. 2003;44:319–328. doi: 10.1176/appi.psy.44.4.319. [DOI] [PubMed] [Google Scholar]
- Ware JE, Brook RH, Ross Davies A, Williams KN, Stewart AL, Rogers WH, et al. Conceptualization and measurement of health for adults in the Health Insurance Study. Vol. 1: Model of health and methodology. Santa Monica, CA: RAND Corporation; 1980. [Google Scholar]
- Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence-Revised. San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for children. 3rd. New York: Psychological Corporation; 1991. [Google Scholar]