Abstract
Introduction:
This article outlines a theoretical framework for research concerning secondhand smoke exposure (SHSe) prevention as a means to curtail the tobacco industry.
Methods:
The Behavioral Ecological Model (BEM) assumes interlocking social contingencies of reinforcement (i.e., rewards or punishments) from the highest level of society (e.g., taxing cigarette sales) to physiological reactions to nicotine that influence smoking and SHSe. We review selected research concerning both policy and clinical efforts to restrict smoking and/or SHSe.
Results:
Research to date has focused on smoking cessation with modest to weak effects. The BEM and empirical evidence suggest that cultural contingencies of reinforcement should be emphasized to protect people from SHSe, especially vulnerable children, pregnant women, the ill, the elderly, and low-income adults who have not “elected” to smoke. Doing so will protect vulnerable populations from industry-produced SHSe and may yield more and longer-lasting cessation.
Conclusions:
Interventions that reduce SHSe may serve as a Trojan horse to counter the tobacco industry. Future studies should: (a) guide policies to restrict SHSe; (b) develop powerful community and clinical interventions to reduce SHSe; (c) test the degree to which policies and other contexts enhance the effects of clinical interventions (e.g., media programs disclosing the disingenuous marketing by the industry); and (d) investigate the effects of all health care providers’ ability to reduce SHSe and generate an antitobacco culture, by advising all clients to avoid starting to smoke, to protect their children from SHSe, and to quit smoking.
Behavior change is required to prevent morbidity. Behavior explains more than 50% of the variance in infectious diseases, degenerative diseases, and injuries (Cuff & Vanselow, 2004; McGinnis & Foege, 1993). An individual's health is a function of the behavior of many people on multiple levels, and a broad systems approach to understanding both the individual's and the population's behavior is critical to achieving health promotion for all. Investigators cannot ignore the behavior of politicians who enact legislative policies that influence public health research, the behavior of medical care providers and insurers, and the behavior of industries (e.g., pharmaceuticals, tobacco) that may profit from behavior that prevents disease or that harms the public. The confluence of these many agencies defines complex behavioral ecological subcultures that determine health-related behavior and morbidity outcomes. We have used a Behavioral Ecological Model (BEM), where social ecological systems are emphasized and integrated with individual factors (e.g., genetic and personal learning histories) to understand and engineer change in the populations’ behavior (Hovell, Wahlgren, & Adams, 2009; Hovell, Wahlgren, & Gehrman, 2002). In this context, we discuss the role of secondhand smoke exposure (SHSe) in the overarching process of tobacco control.
This article describes the BEM, how it applies to SHSe research, and how elevating SHSe as the key target within the overarching tobacco control science may be a means of preventing tobacco addiction in whole populations.
Need for a new model
The tobacco industry creates more smokers and disease than clinicians can prevent by clinical services alone. The focus on clinical care is understandable, as it helps seriously damaged members of society, but it only indirectly contributes to prevention. Alternatively, smoke-free policies and increased taxation hold promise for complete tobacco control, where no one uses tobacco products. Such policies are consistent with the BEM and illustrate a more comprehensive prevention model.
Popular theories offer “rational” or cognitive models of decision making that depend on understanding the health consequences of lifestyle practices (Bandura, 1989; Prochaska, DiClemente, & Norcross, 1992). However, these models are not complete, and they underemphasize principles of learning and the influence from physical and social environments. Jeffery (2004) and West (2005) have called for a return to principles of learning with emphasis on contingencies of reinforcement to influence lifestyle practices. Simplistically, contingencies of reinforcement reflect a specific behavior followed by a consequence (usually immediately) that influences the likelihood of future instances of the same behavior class. These behavior consequence chains ultimately become linked to antecedent events or contexts that prompt similar behavior. A contingency of reinforcement is the relationship between a behavior (e.g., smoking) and the preceding (e.g., offer of a cigarette) and following environmental events (e.g., relief from nicotine withdrawal symptoms and social approval from another smoker) that influence future instances of similar behavior (Skinner, 1969).
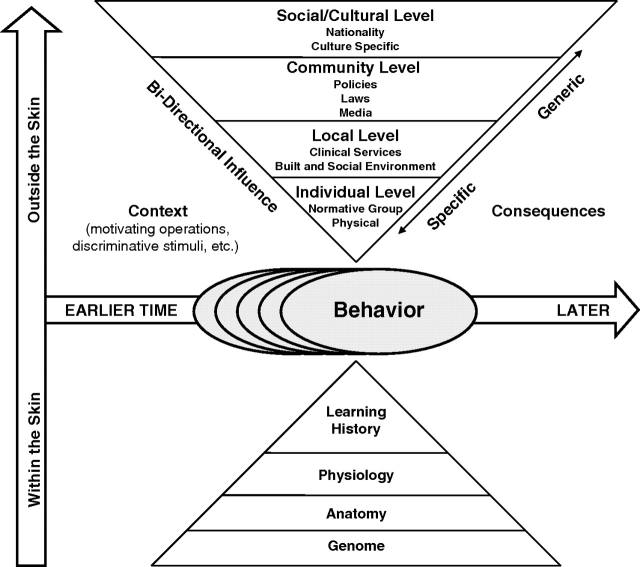
The BEM is based on principles of behavior (Baer & Wolf, 1987; Baer, Wolf, & Risley, 1968). However, the BEM extends these principles to the role of cultural factors in a complex web of influence that integrates concepts from biology, ecology, and Darwinian selection. Figure 1 depicts sources of influence on behavior, from within the body (lower triangle) and from the society (upper triangle). The model presumes that influences from genetics, and biological (e.g., experienced immune system) and behavioral-learning history (e.g., addiction to smoking), interact with influences from the local family, friends, and to societal ecology, all interacting with physical ecology. This perspective redirects public health and medical investigators to understand how contingencies define cultures, how cultures interact across levels of society, and how to change system factors to promote protective behavior community wide.
Figure 1.
Behavioral ecological model. Note. From “Adolescent Health: Understanding and Preventing Risk Behaviors,” by R. J. DiClemente, J. S. Santelli, and R. A. Crosby. Copyright 2009 by the John Wiley & Sons., San Francisco, CA. Reproduced with permission.
The BEM: Extension to the cultural level
Tobacco control requires more than altering nicotine addiction. Social processes can enhance the addicting properties of nicotine or compete with nicotine to prevent or treat addiction. This means that engineering social/cultural systems offers both prevention and treatment for tobacco addiction. Research on SHSe may inform such engineering.
Cultural practices and interlocking contingencies
Culture begins with interlocking behavior across individuals, within and across generations (Glenn, 1988, 2004; Malagodi & Jackson, 1989). “Interlocking behavior” occurs when a person's behavior, or its consequences, serves as prompt and reciprocal reinforcement for another's behavior (Houmanfar & Rodrigues, 2006). For example, accepting an offer to light a cigarette and later offering to return the favor promotes and reinforces smoking. These behaviors are embedded in a complex social context of attracting a sex partner and other possible reinforcers. These social reinforcers are achieved in a probabilistic (i.e., intermittent) but contingent manner, thereby sustaining smoking practices.
Nicotine addiction is one example of a biological contingency for smoking. The most proximal consequence for smokers is relief from nicotine withdrawal symptoms that build when they go without nicotine. These consequences interact or add to social consequences taking place proximal to smoking. These interactions represent interlocking physical contingencies (relief from nicotine withdrawal symptoms), social contingencies, and more delayed financial or sexual contingencies reflected by paying for one another's drinks or by establishing sexual relationships. These examples reflect a cluster of interacting contingencies that support smoking and recruitment of new smokers. These proximal contingencies are independent of health effects of smoking or SHSe. To emphasize health consequences in order to motivate smoking avoidance or cessation is to ignore inadvertently a myriad of more powerful and immediate contingencies.
Social contingencies
Social contexts are markers for unique contingencies of reinforcement. As an individual moves from one social context to another, his/her behavior changes to match the contingencies in the new context. For example, moving from one nation to another involves acculturation, where the contingencies from the new culture compete with those of the old (Kottak, 2006). Acculturation implies social contingencies and adoption of new social practices by the immigrant. However, the BEM guides the identification of explicit social contingencies operating that result in new normative behavior. Entering a bar where smoking is allowed increases the likelihood of reinforcement for smoking and for “tolerating” SHSe consistent with the “bar culture.”
In communities that prohibit smoking in pubs, such microenvironments no longer represent cultures defined by smoking, SHSe, and supportive contingencies. This change can have far-reaching effects (Pierce & León, 2008). It disrupts the interlocked behaviors of drinking, smoking, and SHSe; it disassociates social/sexual and other powerful reinforcers for smoking and tolerating SHSe. Theoretically, disruption of these interlocking contingencies may generalize to other microenvironments, to weaken the tobacco industry's influence on smokers more broadly.
Government policies also influence local social contingencies. Such is the case with bars that have restricted smoking. Most of these changes have been predicated on new national or local government policies that restrict SHSe in public buildings. Thus, policies can jump-start the prohibition of smoking (to prevent nonsmokers’ SHSe) and by doing so speed the new culture that disassociates smoking, SHSe, and drinking and their past common contingent reinforcers. The combination defines multiple levels of “contingencies” where policies alter bar owners’ practices that alter smoking, and that alters SHSe, and so forth. Changes in community norms in turn can produce feedback to influence new government policies. While more research is necessary to test these hierarchical relationships, there is already a growing body of research that has shown an association between implementation of smoke-free public policies and more favorable attitudes toward secondhand smoke (SHS) regulations (e.g., Fong et al., 2006; Hyland et al., 2009) and adoption of household smoking bans (e.g., Borland et al., 2006; Fong et al.; Norman et al., 2000).
Culture at the center of control of tobacco
Korean businessmen serve as a model subculture with respect to tobacco use and interlocking contingencies. Traditional Korean men smoke as part of a social contingency system that includes providing cigarettes and tobacco paraphernalia as gifts. Business success depends on smoking, drinking, and partying with supervisors and coworkers after work. However, when these men emigrate to California, their smoking prevalence drops from about 60% to about 30% (Song et al., 2004). We believe that this is due to the antitobacco culture in California countering the effects of former Korean cultural contingencies. Korean males emigrating from Korea to California might compromise their business standing if they continue to smoke. When Korean women move to California, their smoking prevalence goes from 7% in Korea to 17% in California as they approximate full acculturation (Song et al., 2004). This probably represents the effect of acculturation in California, with substantially more gender equity, interacting with the previous Korean contingencies that suppressed female smoking (Hofstetter et al., 2004). These results show that “gender” acts as a marker for unique social contingencies for males and females. This interaction demonstrates the power of subculture contingencies and the importance of engineering gender-specific contingencies to protect women or other “high risk” immigrants from the tobacco industry. One such example is a media program modeled after the “truth” campaign (Farrelly et al., 2002), to counter the industry's luring women to smoke with promises of equality and freedom.
The BEM and the tobacco industry
The tobacco industry knows how to change behavior
The tobacco industry has been changing cultures. Brandt (2007) summarized how the industry has employed concepts from the BEM (e.g., promoting tobacco use among women as a demonstration of gender equality) and made tobacco a central feature of Western culture, if not worldwide cultures. The industry lobbies for laws that protect it. The industry illustrates how to engineer social contingencies that define our tobacco culture.
Litigation is working but not without compromise
Even as the industry has come under attack through civil litigation, it has turned failures into protectionist policies. However, as recent large-scale litigation has produced “losses” for the industry, there is an impression that the larger culture can exact counter-control (Gostin, 2007). However, negotiated settlements have included clever stipulations. For example, awards to the Flight Attendant Medical Research Institute (FAMRI) were provided to conduct research to prevent or control diseases linked to SHSe. This spans basic, clinical, and epidemiological science, including large centers of excellence. The FAMRI will yield discoveries that contribute to disease prevention; some studies will improve clinical treatment of tobacco-related diseases, and some will inform effective smoking cessation treatments or prevention of SHSe. However, the settlement precluded research funding for new tobacco policies, including those that might inform means of population-wide protection from SHSe. Nevertheless, the FAMRI remains a model settlement, in contrast to the track record for the Master Settlement Agreement (MSA) between the tobacco industry and attorneys general of 46 states. The MSA is the largest dollar “award” from the industry (Wilson, 1999) but provides almost no support of tobacco control interventions such as media campaigns or tobacco prevention research. So when the industry “loses” a civil suit or fails to block new tobacco taxes, the industry promotes contingencies that deflect attention from tobacco control. As antismoking advocate Godshall (1999) has asserted, “[W]ith unprecedented future legal protection granted by the state [attorneys general] in exchange for money, it appears that the tobacco industry has emerged from the state lawsuits even more powerful.”
The Food and Drug Administration
With regulation of the industry (the Family Smoking Prevention and Tobacco Control Act), a new experiment in tobacco control may begin (Kennedy, Cornyn, Waxman, & Davis, 2007). Theoretically, such regulation would eliminate nicotine and toxic agents from cigarettes. However, it is unlikely that the Food and Drug Administration (FDA) will be allowed to eliminate the industry. Thus, the outcome of regulation, based on the BEM, will involve new contingencies that may limit the industry's life or prolong it.
Complex contingencies produce dependence
Since the World Health Organization tobacco treaty (Framework Convention Alliance, 2008), the processes now unfolding are a function of changes in the antitobacco subculture in the United States, western Europe, and other nations. This suggests future curtailments as nations develop antitobacco cultures, but the timing and degree remain uncertain. One reason for this is that the industry actively pursues systems that guarantee long-term existence. Its ability to lobby legislators is partially based on incredible wealth acquired by selling an addictive product. The industry has also systematically supported all levels of society, from funding local fire departments to funding science in our most prestigious universities (Chapman & Shatenstein, 2001). Its business success attracts investors, including pension funds. Thus, efforts to curtail the industry may hurt the retirement and health services of thousands of workers in the United States alone. Theoretically, such contingencies cause legislators to pause in their efforts to curtail a deadly industry. Research to confirm this is warranted.
The tobacco industry funds academic research in the context of academic freedom norms, offering the public possible “cures” for cancer, heart disease, and so forth. By targeting treatment first and smoking cessation second, the industry ensures minimal effect on its business. These industry contingencies support research and clinical care by the very professionals who should be leading efforts against a harmful industry. Potentially biased behavior of academics is supported by cultural contingencies of academic freedom. Recognizing these interacting contingencies is a step toward altering them. More research should focus on the direct and indirect effects of industry support for civil governments as well as academic investigators.
The right to smoke guarantees new addicts
Concepts of self-control and personal decision making prevail in most cultures. These are not consistent with systems theory or the BEM with regard to empirically defined bases for behavior, but such cultures define social contingencies that promote risk practices and suppress the communities’ effort to curtail them. Smokers assert that they have a “right” to smoke (Cardador, Hazan, & Glantz, 1995; Smith & Malone, 2007) and can decide whether to smoke or quit. Such cultural norms of self-control are contradicted by biological and behavioral sciences and retard tobacco control. Exaggerated concepts of self-determination are symbiotic with industry contingencies and are promoted by the industry (Cardador et al., 1995; Smith & Malone), producing a pathological social system. Thus, tobacco control might require reeducation of the public on the complex social systems that determine “personal decisions.”
Implications and a macro view
The picture we have provided is incomplete but sufficient to show that the United States and other cultures support the industry. This context is so pervasive and continuing to be built by the industry that it is critical to take a macro view of these systems and to launch a tobacco control research policy that includes study of policies that transition the industry to a health neutral or health promoting business. We suggest that one pathway toward this end might emphasize SHSe control.
Emphasis on SHSe
SHSe consequences
It is established that SHSe can harm nonsmokers. The adverse health effects include respiratory infections, otitis media, sudden infant death syndrome (SIDS), heart disease, and lung cancer (U.S. Department of Health and Human Services [USDHHS], 2006). Secondhand smoke exposure also increases the risk of a person's becoming an addicted smoker as a young adult (Becklake, Ghezzo, & Ernst, 2005). This SHSe may occur through prenatal or postnatal exposure to nicotine that could establish early sensitivity to nicotine and predispose the child to addiction. These theoretically plausible effects warrant confirmation by empirical studies. Exposure to secondhand smoke also might cause children to imitate smoking as a function of modeling and socially reinforcing contingencies for smoking, starting with parents, family members, close friends, and ultimately adolescent peers who smoke. For susceptible youth, symptoms of dependence appear within 2 days of first inhaling (Difranza et al., 2007). The industry exploits these modeling processes by advertisements that promote smoking by women and preteens and this perpetuates smoking into the next generation.
The damage done by SHSe is huge. From coronary heart disease alone, up to 75,000 deaths are attributable to SHSe (Lightwood, Coxson, Bibbins-Domingo, Williams, & Goldman, 2009). Secondhand smoke exposure costs billions of dollars in excess medical care for U.S. children and billions more in annual loss of life (Adams & Young, 1999; Aligne & Stoddard, 1997).
Population standards for SHSe
In 2000, 25% of U.S. children were exposed to SHS (Soliman, Pollack, & Warner, 2004). Healthy People 2010 objectives are to reduce child SHSe prevalence below 10% (USDHHS, 2000). However, the U.S. Surgeon General has declared that there is no safe level of SHSe (USDHHS, 2006). Ultimately, no one should be exposed.
Changing priorities to SHSe control
A recent study estimated that California's antismoking program saved the state $86 billion in health expenditures between 1989 and 2004 (Lightwood, Dinno, & Glantz, 2008). These estimates exclude the treatment costs for other diseases or indirect costs. This study also showed that declining SHSe rates can prevent disease, related deaths, and related costs. The results suggest that culture change can produce huge health effects and cost savings. Realizing billions of dollars in health benefits and financial savings warrants far more attention to SHSe prevention research and related policies to effect culture-wide change. Smokers are products of the industry and our cultures. Based on the BEM, antitobacco efforts should capitalize on existing societal contingencies to protect vulnerable members and members who have not “elected” to smoke but are exposed to SHS. One such norm is the protection of vulnerable members of society, such as infants, young children, the poor, and the ill. Another norm is that nonsmokers should not suffer the consequences of smokers. Imitating the techniques employed by the industry to address SHSe including half of the world's children (World Health Organization, 1999) would likely be powerful “medicine” against tobacco. However, these should be restricted to legal and ethical interventions. Consider SIDS: Some cases of SIDS probably reflect medically compromised infants who happen to obtain sufficient exposure to maternal smoking during pregnancy and to postnatal SHS (Brownson, Eriksen, Davis, & Warner, 1997; USDHHS, 2006). It seems critical to protect all infants from SHSe to avoid these unnecessary deaths. For others, lifelong exposure to low doses of SHS remains a public health problem of importance due to the increased risk of morbidity and premature mortality.
A greater investment in prohibiting SHSe is theoretically likely to curtail the industry. The United States and other nations should emphasize the elimination of SHSe in all microenvironments such as work settings, private homes, and outdoor settings where nonsmokers could be exposed, such as parks (Giles-Corti et al., 2001; Henriques, Newton, & Marshak, 2003). The new agenda represents a shift to prevention. To be comprehensive, modeling of smoking and SHSe should be avoided, including in artistic and recreational media (e.g., TV, movies, the Internet).
Public health harm-reduction proponents argue correctly that smokeless tobacco products are less harmful than smoke (and may eliminate SHSe). If emphasis on SHSe were to eliminate cigarette smoking and SHSe (possibly in favor of smokeless products), it would reduce the disease burden. However, the contribution from smokeless products and SHSe reduction requires empirical confirmation. We suggest that public health policies and tobacco control research that emphasizes SHSe prevention will move the industry to alter its products, possibly starting with smokeless tobacco. The harm reduction proponents would see such change as a major public health success. We agree. However, advertising and modeling smokeless products will communicate more use of smokeless products, leading to disease, even if in a smaller subgroup than is true of cigarettes. Thus, smokeless tobacco products are not free of harm. Should such a shift take place, we would argue that since there is no benefit derived by the tobacco user or the public from tobacco products, any harm justifies precluding the production and sale of even smokeless tobacco products.
The BEM provides concepts of additive and synergistic effects of community level contingencies. This includes the role of clinicians in treating existing tobacco-related diseases, promoting tobacco cessation, and even promoting SHSe prevention in private homes and cars for their patients. Increased services focused on tobacco control in general and SHSe reduction in particular will sensitize patients and whole families, possibly supporting larger scale community-wide interventions aimed at prevention of SHSe, tobacco initiation, and consequential addiction and disease. The combination will yield an antitobacco culture that might eliminate or change the industry. Such culture change might be sufficient to preclude tolerance of smokeless tobacco products. Research is needed to identify specific policies and social contingencies that greatly reduce smoking and SHSe.
Directions for clinical intervention to reduce SHSe
Secondhand smoke exposure is caused by smoking. In addition to smoking cessation interventions, we need programs that focus on SHSe to protect children and others, since most smokers do not quit and most who do quit return to smoking within 1 year (Fiore, Bailey, & Cohen, 2000; Fiore, Smith, Jorenby, & Baker, 1994; Ranney, Melvin, Lux, McClain, & Lohr, 2006; Rigotti, Munafo, & Stead, 2007).
Following the BEM, a community-wide systems approach should be constructed to protect all nonsmokers from SHSe. This will require designing interlocking contingencies that promote a culture that does not tolerate smoking in the presence of nonsmokers. Theoretically, such a system would involve community-wide “interventions,” including policies, media campaigns, educational services, and clinical services, to name a few.
The clinical service industries offer a potential means of moving the culture forward without having to build major new policies. All clinical services, not limited to smoking cessation, can include requests to stop smoking, referral to clinical cessation services, and advice to protect children and other nonsmokers by never smoking at home, in the car, or other settings. If such advice were routinely delivered, most of the population would obtain advice and recommendations to stop smoking and to protect all others from SHSe. Theoretically, the degree to which such advice becomes universal begins to define an antitobacco culture.
Smoking cessation services
Five minutes of brief smoking cessation advice by physicians could increase quit rates from about 5% at baseline to about 15% at follow-up (Folsom & Grimm, 1987; Janz et al., 1987; Russell, Wilson, Taylor, & Baker, 1979). The current U.S. policies promote the “five A's” (ask, advise, assess, assist, and arrange) and encouraging physicians to ask and advise their patients not to smoke. While more physicians are delivering brief advice, more research is needed to learn how to promote such advice from all health care providers. If all providers employed brief advice, the cumulative effects might be significant.
The most affordable interventions are likely to be brief advice, but they tend to be the least powerful. A subset of clinicians—for example, preventive medicine specialists—might offer more intensive interventions. This might include medications such as nicotine replacement therapy and Zyban for assisting with cessation. Some services are a good fit for this type of care. Families returning regularly for well-baby visits might be provided intensive clinical services by the pediatrician or assistants using tailored counseling, feedback, and incentives. Interventions could also provide financial and social contingencies to motivate cessation. Financial contingencies include vouchers—for example, $50/month to pregnant women for quitting smoking (Donatelle, Prows, Champeau, & Hudson, 2000), or $100 to employees for completing a smoking cessation program (Volpp et al., 2009). Social contingencies include social support provided by a nonsmoker who has a close relationship with the smoker (Donatelle et al.). More research is needed in these promising areas.
“Minimal interventions” for SHSe
Minimal interventions for SHSe have not yet been tested. In a randomized trial with more than 2,000 families, Wall, Severson, Andrews, Lichtenstein, and Zoref (1995) provided minimal counseling and video materials for maternal smokers and explained that quitting would protect their child from SHSe and its health consequences. Parents were shown a video at the first well-baby visit and were provided written materials and brief advice from the pediatrician at well-baby visits at 2 weeks and 2, 4, and 6 months. This resulted in 7-day abstinence of 2.7% in control families and 5.9% in experimental families at 6 months. However, the study focused on smoking cessation outcomes. No SHSe measures were obtained to determine whether changes in SHSe were obtained in homes, or whether parents smoked outside. Had this study included measures of SHSe, it might have demonstrated a direct effect on SHSe.
Future studies should determine the likely effect of brief and low-intensity interventions for SHSe that could be distributed across many clinical services. Such studies should involve direct instruction on how to establish home bans and how to keep children from contact with smokers outside the home. Following our work with tobacco prevention among preteens, we assume relatively small effects from such minimal interventions (Matt et al., 2008b; Wahlgren, Hovell, Meltzer, Hofstetter, & Zakarian, 1997). If so, these studies require large sample sizes. However, minimal interventions may be sufficient to promote change in an important but small percentage of the patient population. If so, population-wide effects could have profound clinical benefits.
Clinical interventions addressing SHSe
Clinical interventions for SHSe have emphasized counseling parents to smoke away from their children. Motivational interviewing techniques and similar counseling procedures provide a combination of health education about SHSe and its health consequences and practical means of avoiding smoking around children; and counselors who prompt and provide social reinforcement for parents’ report of change in exposure practices. The number of sessions have varied across studies from as few as 3 to as many as 14 over weeks. A limited number of sessions (e.g., <4) provides education and promotes verbal contracts to avoid smoking when the child is present (e.g., in the same room). Longer and more frequent sessions approximate shaping procedures by gradually encouraging the parent to reduce the child's exposure.
Several counseling trials have reported significant SHSe reductions, including studies of asthmatic children, Latinos, and low-income mothers (Emmons et al., 2001; Greenberg et al., 1994; Hovell et al., 1994, 2000, 2002; Wahlgren, Hovell, Slymen, Conway, Hofstetter, & Jones, 1997). Thus, individualized parent counseling may reduce children's SHSe for low-income and racially diverse families. Frequent contacts for home-based interventions appear most effective (Gehrman & Hovell, 2003). However, because most studies have been limited to parents avoiding smoking in the same room with a child, the parent may have met that standard, but SHSe reduction may have been insufficient to detect by air dosimeters or cotinine markers. Nicotine and cotinine markers may better reflect the degree to which all sources of exposure have been eliminated. No study has tried to eliminate all sources of exposure. Almost all studies have been efficacy trials; none has demonstrated effectiveness (Zakarian et al., 2004). Thus, advancing the clinical science requires testing more aggressive efficacy and effectiveness trials that target complete protection from SHSe.
Motivation versus guidance
In some of our studies, most of the reduction in SHSe followed after 3–5 sessions. This suggests that early responders make relatively easy changes that reduce their child's exposure. Such changes do not require parenting skills or much compromise in normal smoking patterns. To achieve more will require more powerful interventions not yet tested in counseling. For instance, a home “ban” on smoking might require teaching mothers skills to deal with husbands, grandparents, and other smokers who are not normally restricted from smoking. Future research should focus on the social skills needed to enforce complete tobacco bans in private homes and/or other means of protecting nonsmokers who establish home bans from smokers who disagree (Escoffery, Kegler, & Butler, 2009; Winickoff et al., 2009).
We have seen families in which the parent has skills to require a smoking ban but the child remains exposed. This is a problem of motivation and may not respond to counseling. In these cases, counseling should be bolstered by more powerful “incentives” or formal contingencies of reinforcement, such as payments for change in smoking behavior (Donatelle et al., 2000; Petry, Alessi, Marx, Austin, & Tardif, 2005).
Future clinical services for SHSe reduction
We have not yet identified the combination of procedures that reliably produces marked reductions in SHSe (Priest et al., 2008). One direction is to test combinations of existing clinical procedures. This might involve counseling, financial, and social contingencies for change. Evidence to date suggests that counseling can reduce exposure, but not always and not completely. To test more intensive interventions, clinical services should be more sophisticated by use of shaping and contingency contracting procedures and by immediate and ongoing feedback; cotinine assays should be available to providers; and both financial and social contingencies should be engineered as part of the intervention. With additional tests of intensive interventions for SHSe reduction and with new trials devoted to minimal interventions, it may be possible to identify both relatively intensive treatments for highly addicted smokers and minimal interventions that may be sufficient for most families to protect children from SHSe. Collectively, combined procedures, minimal interventions, and intensive interventions could yield interventions theoretically more powerful than any tested yet and might contribute to an antitobacco culture.
Improving community-wide interventions and changing cultures
Clinical services to influence culture
Clinical services are part of the process of influencing communities to change risk practices. Varying degrees of educational, clinical, and motivational interventions could be institutionalized in all types of services. This includes all medical specialties, incidentally providing brief advice not to start smoking, not to smoke around anyone else and never in one's home or car, and to quit, if a smoker. If this advice were provided for all nonemergent patient visits, it would reach a huge proportion of the population. Most parents and their children would obtain advice multiple times per year. Ignoring the potential direct effects, the cumulative effects of such advice might change community-wide norms from tobacco acceptance to intolerance of SHSe. Since this logic is dependent on both theory and limited empirical evidence that brief advice can change a small but important proportion of the population's behavior dramatically (e.g., quit rates when advised by physicians) and might result in more subtle changes in larger groups, wholesale adoption of brief advice may be premature. However, it could be implemented and tested in selected communities following quasi- and natural experimental models. Women, Infants, and Children programs might add tobacco control services as families return for nutritional services for one or more children over years. Extension to services outside the health care system (such as advice from one's barber or hairdresser) across social classes of the population could produce significant results. There is precedence for such research (Linnan, Emmons, & Abrams, 2002; Solomon et al., 2004) suggesting a prevention role for businesses in the community.
Engineering social contingencies
We have shown that measuring tobacco use and SHSe can be “reactive” and can decrease the “control group's” smoking level and its children's SHSe (Hovell et al., 1994; Wahlgren, Hovell, Meltzer, et al., 1997). Parents are sensitive when studies use cotinine biomarkers, nicotine wipes, air dosimeters, and vacuuming of dust samples from homes and cars (Matt et al., 2004, 2008a). Such reactivity complicates randomized trials and raises questions about use of minimal measurement to reduce reactivity. Most measures are not provided frequently or immediately following behavior change; this may reduce their reactive influence. Measurement itself might be designed as inexpensive interventions with relatively powerful effects. Principles of learning suggest that immediate and frequent feedback could be more influential. If clinicians provide parents with feedback at the office about their child's cotinine, it might prompt greater changes at home; if the child's cotinine decreased at subsequent visits, feedback and praise from the provider might reinforce decrease in SHSe to the child. If feedback were provided in the home immediately as the exposure occurs, it might prompt and reinforce changes in exposure even without the clinician's involvement. However, if the clinician also received home exposure readings via telemetry, he/she could provide additional prompts for change and/or offer social reinforcement for successfully keeping one's home smoke free for extended periods. The biomarker technology is available for clinic-based feedback but not immediate feedback. The technology for environmental markers to deliver immediate SHSe feedback to family members is available now but requires system development and effectiveness testing. Studies of feedback systems are exciting directions for SHSe research.
Addressing “thirdhand” exposure
Homes and cars become contaminated by cigarette smoke. Such contamination can be a source of thirdhand exposure when the child comes in contact with contaminated surfaces or breathes the air when such contamination “off-gasses” (Matt et al., 2004, 2008a). Children are more likely to engage in floor contact and hand-to-mouth behavior (e.g., putting contaminated objects in their mouths), and, compared with adults, their higher rate of respiration may result in greater inhalation of off-gassing from cigarette smoke contaminants. Thus, allowing children into homes contaminated by cigarettes, even if no one smokes when the child is present, may result in thirdhand exposure. Since most counseling studies have relied on cotinine as the “objective” outcome measure, and cotinine may be sensitive to contamination sources of exposure that were not the targets of change, this may contribute to failure to reduce cotinine or to find group-by-time interactions. Future trials should use environmental markers to verify parent reports for proximal effects of counseling, such as reduced air contamination. Eventually, counseling should promote complete bans of smoking in the home, car, and all microenvironments that children frequent. This will require more intensive counseling procedures than have been tested to date. Future research should also determine levels of SHSe attributable to contaminated homes and cars versus visible smoke and the time it takes to off-gas completely. Depending on the results, this information can be leveraged for stronger tobacco control policies against smoking in microenvironments that include children, such as banning smoking in housing where children live or might live in the near future (Winickoff et al., 2009).
Smoking policies, taxation, and media
Public smoking bans and workplace smoking bans may alter the social network in the community, making tobacco and SHSe culturally unacceptable. Policies that restrict smoking in public buildings have reduced SHSe, have been associated with lower rates of smoking, and have not caused a loss of business revenue (Brownson et al., 1997; USDHHS, 2006). Taxing tobacco sales has also suppressed tobacco purchases and contributed to lower level smoking if not cessation (Levy, Chaloupka, & Gitchell, 2004; USDHHS, 2006). Media campaigns offer face valid means of educating the public but may have limited influence for those already addicted. However, if media conveyed an antitobacco culture or a tough love approach, where nonsmokers were encouraged not to tolerate smokers while they are smoking, it might reduce smoking and SHSe rates (Bala, Strzeszynski, & Cahill, 2008; Evans et al., 2006; USDHHS, 2006). Media also can promote consumer activism by encouraging the public not to patronize businesses that directly or indirectly support tobacco. This could include boycotting movies that show smoking. Policies are difficult to pass through legislative systems and media advertising is expensive, with most “programs” designed to entertain and/or promote commercial products. Thus, it will require considerable empirical support and culture change to motivate legislators to aggressively establish policies that counter the industry and protect the public from SHSe. It will also require legislative policies to promote media programs to the same end. This will take time, and cumulative research concerning SHSe prevention may contribute to such media and policies.
Litigation and regulations
SHSe policy development is advancing for high-density housing and families undergoing divorce. Some communities are using regulations of the Environmental Protection Agency for protection from SHSe in apartments and condominiums (Bandura, 1989; Helburn, 2007; Hong, Barnes, & Glantz, 2007; Prochaska et al., 1992; U.S. Environmental Protection Agency, 2005). These usually involve formal restriction policies and even threats of fines or eviction if others are exposed to smoke. Divorce proceedings also have considered the child's SHSe in custody rulings (Rosenblatt, 2005). Again, these custody decisions threaten parents with aversive consequences, such as loss of custody. These policies are evolving in the absence of adequate consideration of policies that might promote positive means of restricting SHSe to children and nonsmoking adults. Addicted parents, even if divorcing, are a product of an industry and culture that promotes such addiction. They should not be treated as perpetrators of crime (Hovell & Daniel, 2005). Parents may require tobacco cessation services and intensive social skills training to establish and enforce complete home bans for tobacco use. They might warrant court contingencies for long-term monitoring of their home to ensure that it remains smoke free. This domain warrants more research that emphasizes “natural experiments” and experimental analyses.
Conclusions
Our research and review suggest that emphasis on treatment of disease or smoking cessation services cannot offset the industry's ability to produce new addicts. Similarly, smokeless tobacco products will likely promote new addicts via modeling and social contingencies. Thus, even if they reduce harm relative to smoke-producing cigarettes, they too may yield more harm than benefit. We believe that interventions can reduce SHSe, lower smoking initiation, and increase long-term cessation rates. Moreover, as more and more people get accustomed to a smoke-free environment, this strengthens an antitobacco culture.
Based on the BEM, we expect that emphasis on SHSe elimination in all micro- and macroenvironments, in both outdoor and indoor areas, if achieved, will eliminate places where smokers can smoke without criticism. As criticism increases, it may generalize to other sanctions. For example, used cars sell for about 9% less if contaminated by an owner who smoked compared with an uncontaminated car owned by a nonsmoker (Matt et al., 2008b). This suggests that the culture is shifting in subtle ways that generate financial costs for contaminating microenvironments. While the evidence is not yet available, we suspect similar and greater costs for contaminated private homes, apartment owners, and hotels. This is implicitly supported by the trend in hotels restricting all smoking in all rooms (Stoller, 2009; USDHHS, 2006). As smoking areas lessen, we expect smoking initiation to decrease and quitting to increase. Decreases in the prevalence of smokers will further decrease social acceptance of tobacco and make smoking less convenient. This might move many to question investments in the industry or acceptance of its support. With financial pressure, industry stockholders might be motivated to work with governments to transform their business from sale of an addictive product to sale of health-enhancing products.
These types of changes are predicated on cumulative and synergistic changes in contingencies within and across national cultures. It is guided by the BEM. To the extent that this model is valid, it serves as a basis for national and international research to inform the World Health Organization and national government agencies to design policies that could curtail SHSe and promote a change in the industry. One example is the first international health treaty—the Framework Convention for Tobacco Control—which has been signed by most member nations (Framework Convention Alliance, 2008).
Funding
This work was supported by intramural funding from the Center for Behavioral Epidemiology and Community Health (CBEACH), the Flight Attendant Medical Research Institute, and the Robert Wood Johnson Smoke-Free Families Program (grant No. 027946 SFP).
This research was supported by Grants R40 MC 00185 and R40 MC 02494 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, U.S. Department of Health and Human Services, and Grant R01 HL066307 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and Department of Health and Human Services.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We acknowledge the two anonymous reviewers for making helpful recommendations to improve the manuscript.
References
- Adams EK, Young TL. Costs of smoking: A focus on maternal, childhood, and other short-run costs. Medical Care Research and Review. 1999;56:3–29. [Google Scholar]
- Aligne CA, Stoddard JJ. Tobacco and children. An economic evaluation of the medical effects of parental smoking. Archives of Pediatrics and Adolescent Medicine. 1997;151:648–653. doi: 10.1001/archpedi.1997.02170440010002. [DOI] [PubMed] [Google Scholar]
- Baer DM, Wolf MM. Some still-current dimensions of applied behavior analysis. Journal of Applied Behavior Analysis. 1987;20:313–327. doi: 10.1901/jaba.1987.20-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer DM, Wolf MM, Risley TR. Some current dimensions of applied behavior analysis. Journal of Applied Behavior Analysis. 1968;1:91–97. doi: 10.1901/jaba.1968.1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala M, Strzeszynski L, Cahill K. Mass media interventions for smoking cessation in adults. Cochrane Database Systematic Reviews. 2008 doi: 10.1002/14651858.CD004704.pub2. CD004704. DOI: 10.1002/14651858.CD004704.pub2. [DOI] [PubMed] [Google Scholar]
- Bandura A. Human agency in social cognitive theory. American Psychologist. 1989;44:1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: A follow-up study of Montreal school children. Canadian Medical Association Journal. 2005;173:377–379. doi: 10.1503/cmaj.1041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Yong HH, Cummings KM, Hyland A, Anderson S, Fong GT. Determinants and consequences of smoke-free homes: Findings from the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15(Suppl. 3):iii42–iii50. doi: 10.1136/tc.2005.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt AM. The cigarette century the rise, fall, and deadly persistence of the product that defined America. New York: Basic Books; 2007. [Google Scholar]
- Brownson RC, Eriksen MP, Davis RM, Warner KE. Environmental tobacco smoke: Health effects and policies to reduce exposure. Annual Review of Public Health. 1997;18:163–185. doi: 10.1146/annurev.publhealth.18.1.163. [DOI] [PubMed] [Google Scholar]
- Cardador MT, Hazan AR, Glantz SA. Tobacco industry smokers’ rights publications: A content analysis. American Journal of Public Health. 1995;85:1212–1217. doi: 10.2105/ajph.85.9.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Shatenstein S. The ethics of the cash register: Taking tobacco research dollars. Tobacco Control. 2001;10:1–2. doi: 10.1136/tc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff PA, Vanselow NA. Improving medical education enhancing the behavioral and social science content of medical school curricula. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- Difranza JR, Savageau JA, Fletcher K, O'Loughlin J, Pbert L, Ockene JK, et al. Symptoms of tobacco dependence after brief intermittent use: The Development and Assessment of Nicotine Dependence in Youth-2 Study. Archives of Pediatrics and Adolescent Medicine. 2007;161:704–710. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant other supporter (SOS) program. Tobacco Control. 2000;9(Suppl. 3):III67–III69. doi: 10.1136/tc.9.suppl_3.iii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- Escoffery C, Kegler MC, Butler S. Formative research on creating smoke-free homes in rural communities. Health Education Research. 2009;24:76–86. doi: 10.1093/her/cym095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WD, Crankshaw E, Nimsch C, Morgan-Lopez A, Farrelly MC, Allen J. Media and secondhand smoke exposure: Results from a national survey. American Journal of Health Behavior. 2006;30:62–71. doi: 10.5555/ajhb.2006.30.1.62. [DOI] [PubMed] [Google Scholar]
- Farrelly MC, Healton CH, Davis KC, Messeri P, Hersey JC, Haviland ML. Getting to the truth: Evaluating national tobacco countermarketing campaigns. American Journal of Public Health. 2002;92:901–907. doi: 10.2105/ajph.92.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence: An introduction to the US Public Health Service Clinical Practice Guideline. Respiratory Care. 2000;45:1196–1199. [PubMed] [Google Scholar]
- Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. Journal of the American Medical Association. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Folsom AR, Grimm RH., Jr Stop smoking advice by physicians: A feasible approach? American Journal of Public Health. 1987;77:849–850. doi: 10.2105/ajph.77.7.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GT, Hyland A, Borland R, Hammond D, Hastings G, McNeill A, et al. Reductions in tobacco smoke pollution and increases in support for smoke-free public places following the implementation of comprehensive smoke-free workplace legislation in the Republic of Ireland: Findings from the ITC Ireland/UK Survey. Tobacco Control. 2006;15(Suppl. 3):iii51–iii58. doi: 10.1136/tc.2005.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Framework Convention Alliance. What is the framework convention on tobacco control? 2008. Retrieved August 12, 2008, from http://www.fctc.org. [Google Scholar]
- Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: A critical review. Nicotine & Tobacco Research. 2003;5:289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- Giles-Corti B, Clarkson JP, Donovan RJ, Frizzell SK, Carroll AM, Pikora T, et al. Creating smoke-free environments in recreational settings. Health Education and Behavior. 2001;28:341–351. doi: 10.1177/109019810102800308. [DOI] [PubMed] [Google Scholar]
- Glenn SS. Contingencies and metacontingencies:Toward a synthesis of behavior analysis and cultural materialism. Behavior Analyst. 1988;11:161–179. doi: 10.1007/BF03392470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SS. Individual behavior, culture, and social change. Behavior Analyst. 2004;27:133–151. doi: 10.1007/BF03393175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godshall WT. Giving 10% to gain eternity. Tobacco Control. 1999;8:437–439. doi: 10.1136/tc.8.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin LO. The “tobacco wars”: Global litigation strategies. Journal of the American Medical Association. 2007;298:2537–2539. doi: 10.1001/jama.298.21.2537. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Strecher VJ, Bauman KE, Boat BW, Fowler MG, Keyes LL, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. Journal of Behavioral Medicine. 1994;17:273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- Helburn A. A case for smokefree housing. Dorchester, MA: Asthma Regional Council of New England; 2007. Retrieved March 4, 2009, from http://www.asthmaregionalcouncil.org/SmokeFreePaperFINALcolor.pdf. [Google Scholar]
- Henriques CE, Newton DR, Marshak HH. Smoke-free parks: A 12-year-old made it happen. Journal of Community Health. 2003;28:131–137. doi: 10.1023/a:1022647430540. [DOI] [PubMed] [Google Scholar]
- Hofstetter CR, Hovell MF, Lee J, Zakarian J, Park H, Paik HY, et al. Tobacco use and acculturation among Californians of Korean descent: A behavioral epidemiological analysis. Nicotine & Tobacco Research. 2004;6:481–489. doi: 10.1080/14622200410001696646. [DOI] [PubMed] [Google Scholar]
- Hong M, Barnes RL, Glantz SA. Tobacco control in California 2003–2007: Missed opportunities. San Francisco: Center for Tobacco Control Research and Education Tobacco Control Policy Making: United States; 2007. [Google Scholar]
- Houmanfar R, Rodrigues NJ. The metacontingency and the behavioral contingency: Points of contact and departure. Behavior and Social Issues. 2006;15:13–30. [Google Scholar]
- Hovell M, Daniel J. Defining residential tobacco home policies: A behavioural and cultural perspective. Archives of Disease in Childhood. 2005;90:661–662. doi: 10.1136/adc.2004.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial. Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Wahlgren DR, Adams MA. The logical and empirical basis for the Behavioral Ecological Model. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging theories in health promotion practice and research: Strategies for enhancing public health. 2nd ed. San Francisco: Jossey-Bass; 2009. pp. 415–450. [Google Scholar]
- Hovell MF, Wahlgren DR, Gehrman CA. The behavioral ecological model: Integrating public health and behavioral science. In: DiClemente RJ, editor. Emerging theories in health promotion practice and research: Strategies for improving public health. San Francisco: Jossey-Bass; 2002. pp. 347–385. [Google Scholar]
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children's exposure to environmental tobacco smoke: Randomised controlled trial. British Medical Journal. 2000;321:337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Higbee C, Borland R, Travers M, Hastings G, Fong GT, et al. Attitudes and beliefs about secondhand smoke and smoke-free policies in four countries: Findings from the International Tobacco Control Four Country Survey. Nicotine & Tobacco Research. 2009;11:642–649. doi: 10.1093/ntr/ntp063. DOI: 10.1093/ntr/ntp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz NK, Becker MH, Kirscht JP, Eraker SA, Billi JE, Woolliscroft JO. Evaluation of a minimal-contact smoking cessation intervention in an outpatient setting. American Journal of Public Health. 1987;77:805–809. doi: 10.2105/ajph.77.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW. How can health behavior theory be made more useful for intervention research? International Journal of Behavioral Nutrition and Physical Activity. 2004;1:10. doi: 10.1186/1479-5868-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T, Cornyn J, Waxman H, Davis T. The Family Smoking Prevention and Tobacco Control Act. 2007 S. 625/H.R. 1108. [Google Scholar]
- Kottak CP. Anthropology: The exploration of human diversity. 11th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- Levy DT, Chaloupka F, Gitchell J. The effects of tobacco control policies on smoking rates: A tobacco control scorecard. Journal of Public Health Management and Practice. 2004;10:338–353. doi: 10.1097/00124784-200407000-00011. [DOI] [PubMed] [Google Scholar]
- Lightwood JM, Coxson PG, Bibbins-Domingo K, Williams LW, Goldman L. Coronary heart disease attributable to passive smoking: CHD policy model. American Journal of Preventive Medicine. 2009;36:13–20. doi: 10.1016/j.amepre.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood JM, Dinno A, Glantz SA. Effect of the California tobacco control program on personal health care expenditures. Public Library of Science Medicine. 2008;5:e178. doi: 10.1371/journal.pmed.0050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnan LA, Emmons KM, Abrams DB. Beauty and the beast: Results of the Rhode Island smokefree shop initiative. American Journal of Public Health. 2002;92:27–28. doi: 10.2105/ajph.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagodi EF, Jackson K. Behavior analysts and cultural analysis: Troubles and issues. Behavior Analyst. 1989;12:17–33. doi: 10.1007/BF03392474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Quintana PJ, Hovell MF, Bernert JT, Song S, Novianti N, et al. Households contaminated by environmental tobacco smoke: Sources of infant exposures. Tobacco Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Quintana PJ, Hovell MF, Chatfield D, Ma DS, Romero R, et al. Residual tobacco smoke pollution in used cars for sale: Air, dust, and surfaces. Nicotine & Tobacco Research. 2008a;10:1467–1475. doi: 10.1080/14622200802279898. [DOI] [PubMed] [Google Scholar]
- Matt GE, Romero R, Ma DS, Quintana PJ, Hovell MF, Donohue M, et al. Tobacco use and asking prices of used cars: Prevalence, costs, and new opportunities for changing smoking behavior. Tobacco Induced Diseases. 2008b;4:2. doi: 10.1186/1617-9625-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Actual causes of death in the United States. Journal of the American Medical Association. 1993;270:2207–2212. [PubMed] [Google Scholar]
- Norman GJ, Ribisl KM, Howard-Pitney B, Howard KA, Unger JB. The relationship between home smoking bans and exposure to state tobacco control efforts and smoking behaviors. American Journal of Health Promotion. 2000;15(2):81–88. doi: 10.4278/0890-1171-15.2.81. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Pierce JP, León M. Effectiveness of smoke-free policies. Lancet Oncology. 2008;9:614–615. doi: 10.1016/s1470-2045(08)70167-0. [DOI] [PubMed] [Google Scholar]
- Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, Webster P, Ferguson-Thorne G. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews. 2008;4 doi: 10.1002/14651858.CD001746.pub2. CD001746. DOI: 10.1002/14651858.CD001746.pub2. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. American Psychologist. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Ranney L, Melvin C, Lux L, McClain E, Lohr KN. Systematic review: Smoking cessation intervention strategies for adults and adults in special populations. Annals of Internal Medicine. 2006;145:845–856. doi: 10.7326/0003-4819-145-11-200612050-00142. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews, 2. 2007 doi: 10.1002/14651858.CD001837.pub2. CD001837. DOI: 10.1002/14651858.CD001837.pub2. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M. Should our judicial system and legislatures protect infants and children from second-hand tobacco smoke? Children's Legal Rights Journal. 2005;25:37–50. [Google Scholar]
- Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners’ advice against smoking. British Medical Journal. 1979;2:231–235. doi: 10.1136/bmj.2.6184.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. Contingencies of reinforcement: A theoretical analysis. New York: Appleton-Century-Crofts; 1969. [Google Scholar]
- Smith EA, Malone RE. ‘We will speak as the smoker’: The tobacco industry's smokers’ rights groups. European Journal of Public Health. 2007;17:306–313. doi: 10.1093/eurpub/ckl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman S, Pollack HA, Warner KE. Decrease in the prevalence of environmental tobacco smoke exposure in the home during the 1990s in families with children. American Journal of Public Health. 2004;94:314–320. doi: 10.2105/ajph.94.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon FM, Linnan LA, Wasilewski Y, Lee AM, Katz ML, Yang J. Observational study in ten beauty salons: Results informing development of the North Carolina BEAUTY and Health Project. Health Education and Behavior. 2004;31:790–807. doi: 10.1177/1090198104264176. [DOI] [PubMed] [Google Scholar]
- Song YJ, Hofstetter CR, Hovell MF, Paik HY, Park HR, Lee J, et al. Acculturation and health risk behaviors among Californians of Korean descent. Preventive Medicine. 2004;39:147–156. doi: 10.1016/j.ypmed.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Stoller G. More hotels go completely smoke-free. 2009. Retrieved March 4, 2009, from http://www.usatoday.com/travel/hotels/2008-11-17-smoke-free-hotels-no-smoking_N.htm. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2010. 2nd ed. Washington, DC: U.S. Government Printing Office; 2000. Understanding and Improving health. [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke. A report of the Surgeon General. Rockville, MD: Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- U.S. Environmental Protection Agency. 2005. Local programs promoting smoke free homes. http://www.epa.gov/smokefree/publications.html. [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Slymen DJ, Conway TL, Hofstetter CR, Jones JA. Predictors of tobacco use initiation in adolescents: A two-year prospective study and theoretical discussion. Tobacco Control. 1997;6:95–103. doi: 10.1136/tc.6.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: Impact on maternal smoking and relapse. Pediatrics. 1995;96:622–628. [PubMed] [Google Scholar]
- West R. Time for a change: Putting the transtheoretical (stages of change) model to rest. Addiction. 2005;100:1036–1039. doi: 10.1111/j.1360-0443.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- Wilson JJ. Summary of the Attorneys General Master Settlement Agreement. 1999 National Conference of State Legislatures. [Google Scholar]
- Winickoff JP, Friebely J, Tanski SE, Sherrod C, Matt GE, Hovell MF, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. 2009;123:e74–e79. doi: 10.1542/peds.2008-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Tobacco free initiative:International Consultation on Environmental Tobacco Smoke (ETS) and Child Health. Geneva, Switzerland: Author: 1999. [Google Scholar]
- Zakarian JM, Hovell MF, Sandweiss RD, Hofstetter CR, Matt GE, Bernert JT, et al. Behavioral counseling for reducing children's ETS exposure: Implementation in community clinics. Nicotine & Tobacco Research. 2004;6:1061–1074. doi: 10.1080/1462220412331324820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.