Abstract
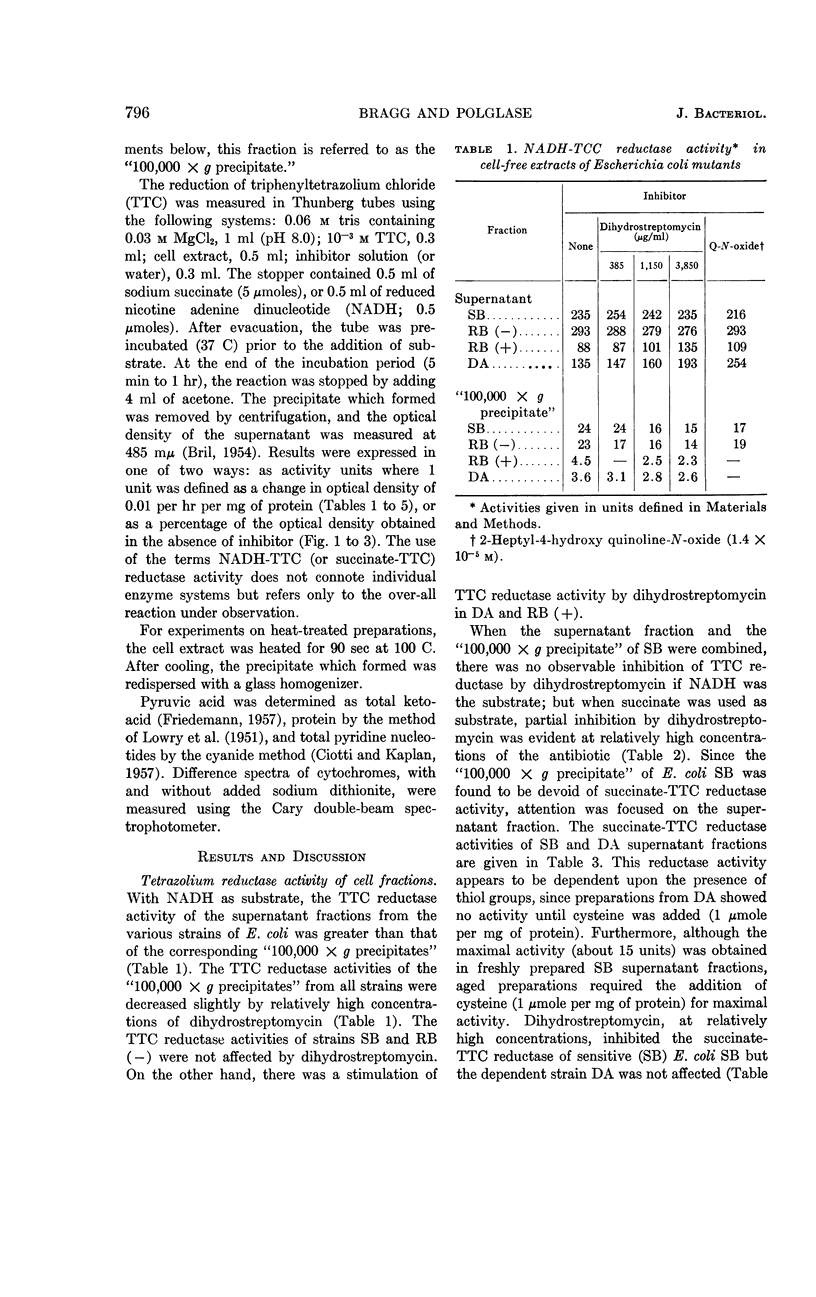
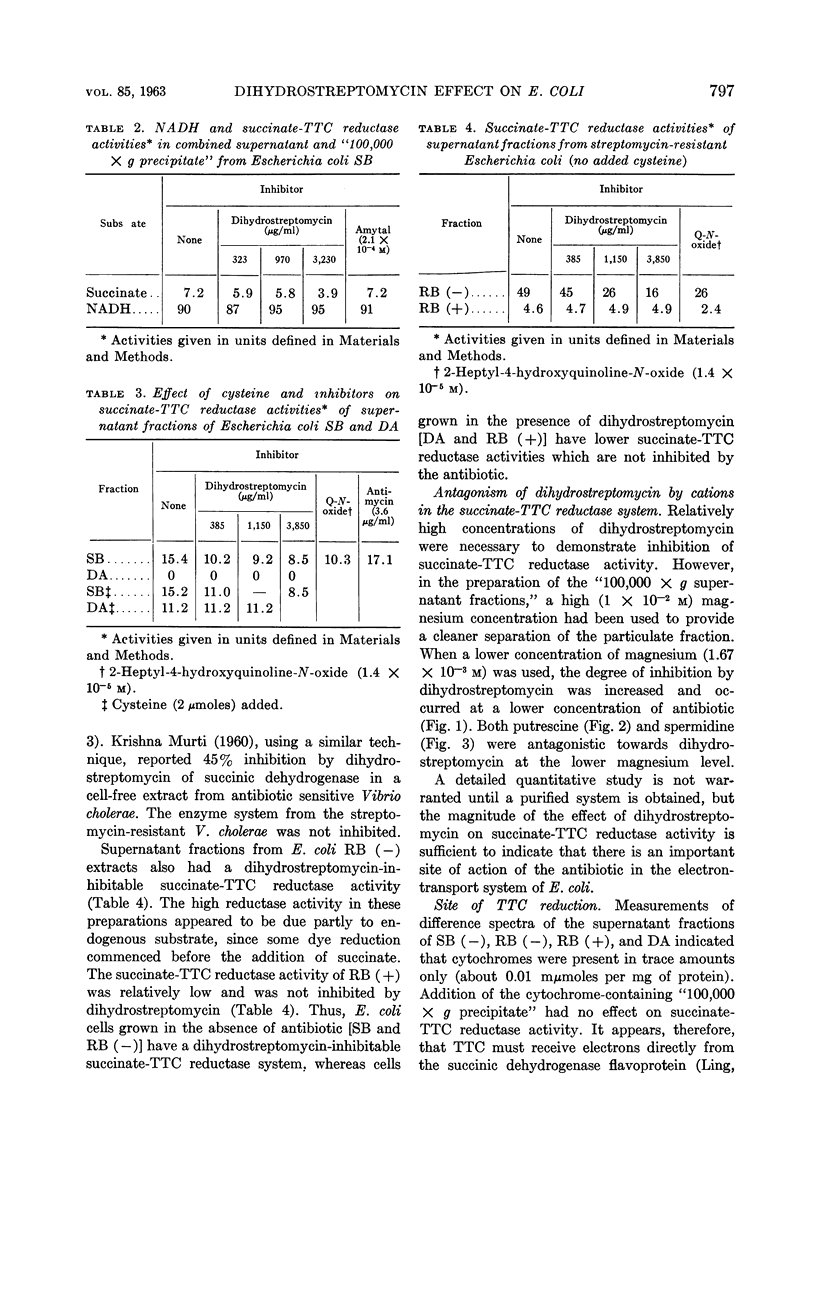
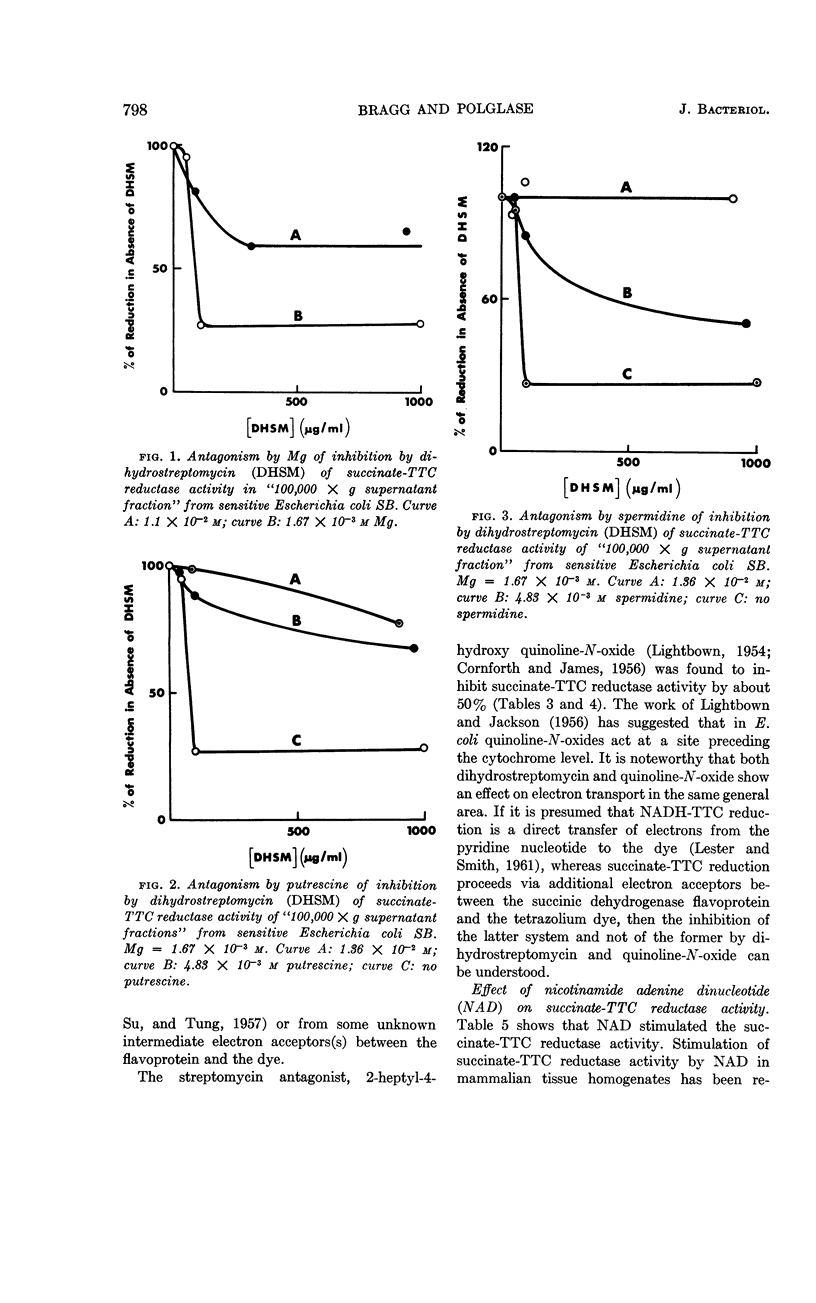
Bragg, P. D. (University of British Columbia, Vancouver, British Columbia, Canada) and W. J. Polglase. Effect of dihydrostreptomycin on tetrazolium dye reduction in Escherichia coli. J. Bacteriol. 85:795–800. 1963.—Sonic-disrupted extracts of Escherichia coli, grown without added antibiotic (sensitive and resistant), contained (in supernatant of fraction centrifuged at 100,000 × g) a dihydrostreptomycin-inhibitable, succinate-triphenyltetrazolium chloride (TTC) reductase activity. The succinate-TTC reductase activities of extracts of E. coli grown in the presence of dihydrostreptomycin (resistant and dependent) were relatively low and were not inhibited by the antibiotic. At a moderate magnesium concentration, the degree of inhibition by dihydrostreptomycin of succinate-TTC reductase activity was sufficiently marked to indicate an important site of action of the antibiotic. Magnesium, putrescine, and spermidine antagonized the action of dihydrostreptomycin in the succinate-TTC reductase system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAGG P. D., POLGLASE W. J. ACTION OF DIHYDROSTREPTOMYCIN AND ANTAGONISM BY CATIONS. J Bacteriol. 1963 Mar;85:590–594. doi: 10.1128/jb.85.3.590-594.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. Extracellular metabolites of streptomycin mutants of Escherichia coli. J Bacteriol. 1962 Aug;84:370–374. doi: 10.1128/jb.84.2.370-374.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIL C. Enzymic micro-determination of succinate and fumarate in tissue homogenates. Biochim Biophys Acta. 1954 Oct;15(2):258–262. doi: 10.1016/0006-3002(54)90067-0. [DOI] [PubMed] [Google Scholar]
- CORNFORTH J. W., JAMES A. T. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem J. 1956 May;63(1):124–130. doi: 10.1042/bj0630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WHITE J. R. Inhibition of polypeptide synthesis by streptomycin. Biochem Biophys Res Commun. 1962 May 11;7:385–389. doi: 10.1016/0006-291x(62)90320-0. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- KRISHNA MURTI C. R. Action of streptomycin on Vibrio cholerae. Biochem J. 1960 Aug;76:362–369. doi: 10.1042/bj0760362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTBOWN J. W. An antagonist of streptomycin and dihydrostreptomycin produced by Pseudomonas aeruginosa. J Gen Microbiol. 1954 Dec;11(3):477–492. doi: 10.1099/00221287-11-3-477. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING K. H., SU T. C., TUNG T. C. Mechanism of the reduction of triphenyltetrazolium chloride by succinoxidase. Arch Biochem Biophys. 1957 Sep;71(1):126–129. doi: 10.1016/0003-9861(57)90014-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGER J., BENEDICT M., ARTMAN M. A common site of action of polyamines and streptomycin. Biochim Biophys Acta. 1962 Jul 30;62:202–204. doi: 10.1016/0006-3002(62)90519-x. [DOI] [PubMed] [Google Scholar]
- ODA T., GOLDMAN D. S. Terminal electron-transport mechanisms in the tubercle bacillus. A reduced diphosphopyridine nucleotide-nitro-blue tetrazolium diaphorase. Biochim Biophys Acta. 1962 Jun 4;59:604–613. doi: 10.1016/0006-3002(62)90640-6. [DOI] [PubMed] [Google Scholar]


