Abstract
The appropriate timing of flowering is pivotal for reproductive success in plants; thus, it is not surprising that flowering is regulated by complex genetic networks that are fine-tuned by endogenous signals and environmental cues. The Arabidopsis thaliana flowering-time gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) encodes a MADS box transcription factor and is one of the key floral activators integrating multiple floral inductive pathways, namely, long-day, vernalization, autonomous, and gibberellin-dependent pathways. To elucidate the downstream targets of SOC1, microarray analyses were performed. The analysis revealed that the soc1-2 knockout mutant has increased, and an SOC1 overexpression line has decreased, expression of cold response genes such as CBFs (for CRT/DRE binding factors) and COR (for cold regulated) genes, suggesting that SOC1 negatively regulates the expression of the cold response genes. By contrast, overexpression of cold-inducible CBFs caused late flowering through increased expression of FLOWERING LOCUS C (FLC), an upstream negative regulator of SOC1. Our results demonstrate the presence of a feedback loop between cold response and flowering-time regulation; this loop delays flowering through the increase of FLC when a cold spell is transient as in fall or early spring but suppresses the cold response when floral induction occurs through the repression of cold-inducible genes by SOC1.
INTRODUCTION
Flowering, a transition from vegetative to reproductive phase, is the most dramatic change in the plant's life cycle. To maximize reproductive success, plants have evolved an intricate mechanism determining flowering time in response to both environmental factors, such as light and temperature, and endogenous signals that reflect the plant's developmental state and age (Boss et al., 2004; Baurle and Dean, 2006; Oh and Lee, 2007). It is also known that flowering is regulated by various abiotic stresses, such as nutrient deficiency, heat, and cold (Kim et al., 2004; Balasubramanian et al., 2006; Baurle and Dean, 2006). Extensive genetic and physiological analyses of Arabidopsis thaliana have revealed that floral induction is regulated by at least four major genetic pathways, namely, long-day, autonomous, vernalization (a long period of cold for flowering), and gibberellin-dependent pathways. These four pathways commonly regulate so-called flowering pathway integrators FT, SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1), and LEAFY (LFY), and the exact flowering time is determined by the expression level of these integrators (Blazquez and Weigel, 2000; Lee et al., 2000; Onouchi et al., 2000; Samach et al., 2000; Moon et al., 2003; Moon et al., 2005). Such integrators are regulated antagonistically by two central upstream regulators: CONSTANS (CO), encoding a zinc finger protein, and FLOWERING LOCUS C (FLC), encoding a MADS box transcription factor (Michaels and Amasino, 1999; Lee et al., 2000; Samach et al., 2000). CO, mediating the long-day pathway, acts as a positive regulator, whereas FLC, mediating the autonomous/vernalization pathway, acts as a negative regulator of flowering (Lee et al., 2000; Samach et al., 2000).
In addition to these four major pathways, flowering is known to be fine-tuned by other mechanisms. For example, flowering time is adjusted by the ambient temperature such that cool temperature delays flowering, whereas warm temperature accelerates flowering (Blazquez et al., 2003; Balasubramanian et al., 2006). In Arabidopsis, ambient cool temperature is sensed through genes such as FCA, FVE, and SHORT VEGETATIVE PHASE (SVP): the mutants, fca, fve, and svp, exhibit insensitivity to ambient cool temperature for flowering (Blazquez et al., 2003; Halliday et al., 2003; Lee et al., 2007b). FCA and FVE are two autonomous pathway genes that have a function to repress FLC expression, and SVP is a floral repressor that makes a flowering repressor complex together with FLC (Li et al., 2008). This so-called thermosensory pathway eventually regulates FT expression (Blazquez et al., 2003; Lee et al., 2007b). Similarly, ambient warm temperature accelerates flowering through an increase of FT expression (Balasubramanian et al., 2006). However, such effect is suppressed by FLC and is modulated by FLOWERING LOCUS M (FLM), an FLC homolog (Balasubramanian et al., 2006).
Intermittent cold treatment, a short-term cold treatment during the day, also delays flowering, an effect that is mediated by FVE (Ausin et al., 2004; Kim et al., 2004). The fve mutant, showing late flowering due to increased expression of FLC, exhibits ectopic expression of cold-regulated (COR) genes without cold treatment. In addition, it shows increased freezing tolerance, and its flowering time is not delayed by intermittent cold, indicating that FVE is a genetic linker between flowering time and cold response (Kim et al., 2004).
Cold induces the expression of many genes encoding a diverse array of proteins that enhance the tolerance of plants to freezing temperature. Such COR genes share C-repeat/dehydration response elements (CRT/DRE) in their promoters, and CRT/DRE binding factors (CBFs) act as the key regulators of cold response pathway in Arabidopsis (Thomashow, 1999). It was reported that overexpression of CBF1, CBF2, and CBF3 causes late flowering and dwarf phenotypes as well as phenotypes associated with freezing tolerance, such as increases of Pro and sugar concentrations and transcriptional activation of COR genes (Gilmour et al., 2004). CBFs are positively regulated by ICE1 (for inducer of CBF expression 1), which encodes a MYC-like basic helix-loop-helix transcription factor (Chinnusamy et al., 2003), whereas they are negatively regulated by HOS1 (for high expression of osmotically responsive genes), which encodes a RING finger protein, probably acting as a E3 ubiquitin ligase (Lee et al., 2001).
Although the regulation of flowering pathway integrator SOC1 has been relatively well studied, the downstream factors of SOC1 remain largely unknown. To elucidate the downstream targets of the floral integrator SOC1 encoding a MADS box transcription factor, we performed microarray experiments using both an overexpression line and a null mutant. Here, we report that the floral activator SOC1 functions as a negative regulator of the cold response pathway through the direct repression of CBFs. By contrast, overexpression of CBFs increases the expression level of FLC. In conclusion, our results suggest that SOC1 and FLC are the key regulators of crosstalk between cold response and flowering time regulation. Such a feedback loop involving SOC1, cold response genes, and FLC may prevent premature flowering under cold conditions in fall or early spring and therefore may be evolutionarily advantageous.
RESULTS
SOC1 Negatively Regulates Cold-Inducible Genes
To monitor the global gene expression regulated by SOC1, we performed a microarray analysis using the Affymetrix ATH1 GeneChip as a preliminary screen. We used RNA extracted from an overexpression allele soc1-101D, a null allele soc1-2, and wild-type Columbia (Col) grown for 7 d under long days. Sampling was specifically done with 7-d-old seedlings because all genotypes including the early flowering soc1-101D are in the vegetative phase at this time, which could be determined by the absence of APETALA1 expression (Hempel et al., 1997). Interestingly, six out of the top-ranked 20 negatively regulated genes by SOC1 were the well-known cold-inducible (COR) genes.
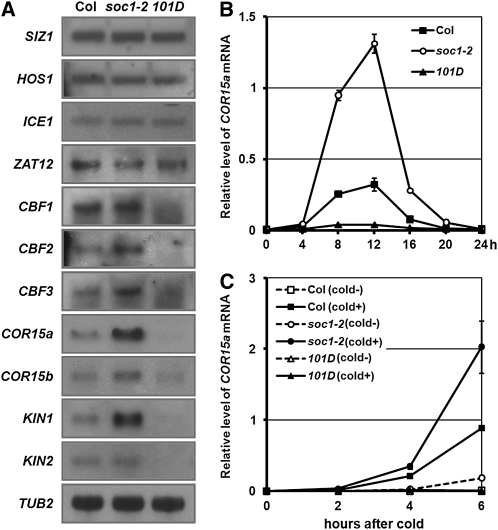
To confirm if a loss-of-function or a gain-of-function mutation in SOC1 affects the expression of cold-inducible genes, we analyzed the expression of a range of genes by RNA gel blot analysis in soc1-2 and soc1-101D (Figure 1A). As is shown, four cold-inducible genes, COR15a, COR15b, KIN1, and KIN2, exhibited increased expression in soc1-2 and decreased expression in soc1-101D under long days (16 h light/8 h dark) at 22°C. Because cold response genes are known to be under circadian control (Harmer et al., 2000; Fowler et al., 2005), we checked the daily rhythm of COR15a expression as a representative cold-inducible gene (Figure 1B). It showed a peak at 12 h after dawn and became minimal during the night period. During the circadian cycle, soc1-2 showed higher expression, whereas soc1-101D showed lower expression than the wild type, although the biggest difference was observed at the 12-h peak. This result suggests that the negative regulation of cold-inducible genes by SOC1 is not affected by the circadian rhythm, although the amplitude is changed. Next, we addressed whether SOC1 affects the induction kinetics of cold-inducible genes. For this, we treat with 4°C cold immediately after dawn because the daily temperature is usually the lowest at dawn in nature. As is shown in Figure 1C, soc1-2 exhibited much stronger, and soc1-101D showed much weaker induction of COR15a expression compared with the wild type. This result strongly suggests that SOC1 attenuates the induction of COR genes in response to cold.
Figure 1.
SOC1 Negatively Regulates Cold-Inducible Genes.
(A) Expressions of cold-responsive genes in wild-type (Col), soc1-2, and soc1-101D (101D) was detected by RNA gel blot analysis. TUB2 was used as a quantitative control. Plants grown at 22°C for 10 d under 16-h-light/8-h-dark long-day conditions were harvested at 8 h after dawn for RNA isolation.
(B) Daily rhythm of COR15a expression in wild-type, soc1-2, and soc1-101D grown under long days was detected by quantitative RT-PCR. The values and error bars represent mean value and sd, respectively, from three technical replicates. The 10-d-old seedlings grown 16-h-light/8-h-dark conditions were harvested every 4 h for RNA isolation. The zero time corresponds to right after dawn.
(C) Cold response of COR15a in wild-type, soc1-2, and soc1-101D. Expression level of COR15a was detected by quantitative RT-PCR. Plants were grown at 22°C for 10 d under long days and then transferred to 4°C (cold+) or maintained at 22°C (cold-) for 0, 2, 4, and 6 h in the light. The zero time corresponds to right after dawn.
SOC1 Directly Represses the Expression of CBF Genes
The majority of COR genes have cold- and dehydration-responsive DNA regulatory elements designated CRT/DRE in their promoter (Yamaguchi-Shinozaki and Shinozaki, 1994), and the expression of COR genes is mediated by CRT/DRE that is regulated by the CBF gene family (Stockinger et al., 1997). To determine whether the negative regulation of COR genes by SOC1 is mediated through CBFs, we compared the expression of CBF1, CBF2, and CBF3 in the wild type, soc1-2, and soc1-101D. The expression level of CBFs increased in soc1-2 and decreased in soc1-101D (Figure 1A). We also compared the daily rhythm of CBF3 expression in the three genotypes (Figure 2A). CBF3 expression exhibited a peak at 8 h after dawn, which is 4 h before the COR15a peak. Similar to COR15a, CBF3 was increased in soc1-2 and decreased in soc1-101D during the daily cycle. In addition, the expression of CBF3 in response to cold treatment was higher in soc1-2 but lower in soc1-101D especially at 2 h after cold treatment (Figure 2B). Thus, the increased expression of various COR genes in soc1-2 is most likely due to the enhanced expression of CBFs.
Figure 2.
SOC1 Directly Represses CBF Expression.
(A) Daily rhythm of CBF3 in wild-type (Col), soc1-2, and soc1-101D (101D) under long days. Expression level of CBF3 was detected by quantitative RT-PCR. The 10-d-old seedlings were harvested every 4 h for RNA isolation.
(B) Cold response of CBF3 in wild-type, soc1-2, and soc1-101D. Expression level of CBF3 was detected by quantitative RT-PCR. Plants grown at 22°C for 10 d under long days were transferred to 4°C for 0, 2, and 6 h in the light. The quantitative RT-PCR analysis was biologically repeated three times, and each time point consisted of three technical replicates in both (A) and (B). The error bars represent sd for three technical replicates.
(C) Four graphic bars represent the promoters of CBF1, CBF2, CBF3, and LFY. The arrowheads denote putative CArG box, and black lines (a-f, ProLFY-1, ProLFY-4) indicate the regions used for ChIP.
(D) ChIP assay with SOC1 antibody. Enrichment of CBFs promoters (a to f) was confirmed by ChIP-PCR. ProLFY-1 was used as a positive control, and ProLFY-4 was used as a negative control.
(E) Quantitative real-time PCR analysis using the same ChIP-PCR products in (D). Values are normalized against soc1-2 and are means of triplicate experiments with error bars representing sd. Negative controls, pTUB and CHS, are shown at right.
In the cold response pathway, ICE1 and HOS1 are positive and negative upstream regulators of the CBF family, respectively (Chinnusamy et al., 2007). To determine whether SOC1 regulates the transcriptional level of ICE1 and HOS1, the expression of ICE1 and HOS1 was also checked by RNA gel blot analysis (Figure 1A). In comparison to the wild type, the expressions of ICE1 and HOS1 were not changed in soc1-2 or soc1-101D. In addition, expression of ZAT12, a negative upstream regulator of CBF1 and CBF2 that is also induced by cold treatment (Rizhsky et al., 2004; Vogel et al., 2005), was not affected by soc1-101D or soc1-2 (Figure 1A). Thus, these results indicate that SOC1 suppresses the cold response pathway through the repression of CBF genes.
It is reported that SOC1, a MADS box transcription factor, binds to variant forms of the CArG box in the promoter of LFY (Lee et al., 2008; Liu et al., 2008). Promoter analysis revealed that all three CBF genes have two variant forms of CArG boxes at the distal and proximal regions (Figure 2C). Thus, we wondered if SOC1 binds to the promoters of CBF genes directly. The two regions of LFY promoter were used as negative and positive controls for chromatin immunoprecipitation (ChIP) based on a previous report (Lee et al., 2008). ProLFY-1, a distal region of LFY promoter, was highly enriched in soc1-101D compared with soc1-2, whereas ProLFY-4, a proximal region of LFY promoter, was not enriched in soc1-101D as reported (Figure 2D). Interestingly, the ChIP analysis revealed that all CArG-box regions in the CBF promoters are enriched by SOC1 overexpression (Figures 2D and 2E). Such results strongly suggest that SOC1 negatively regulates cold response through direct repression of the transcription of CBFs.
CBF Genes Activate FLC Expression
It has been reported that overexpression of CBF genes causes late flowering (Figure 3A; Liu et al., 2002; Gilmour et al., 2004), but it is not elucidated why. Because FLC is a central repressor of flowering in Arabidopsis, we checked if FLC expression is increased by overexpression of CBFs. Indeed, the FLC expression was increased more than twofold in 35S-CBF1, 35S-CBF2, and 35S-CBF3 (Figure 3B). We also checked the expression of SVP, a flowering repressor encoding another MADS box transcription factor (Hartmann et al., 2000) because SVP is known to mediate the ambient cool temperature delay of flowering and interacts with FLC to make a flowering repressor complex (Lee et al., 2007b; Li et al., 2008). In contrast with FLC, CBF overexpression did not affect on the expression of SVP (Figure 3B). FLM, a gene included in FLC clade genes, represses flowering and modulates flowering at warm temperature (Balasubramanian et al., 2006). The expression of FLM is not affected by CBF overexpression, similar to SVP (Figure 3B).
Figure 3.
CBFs Positively Regulate FLC Expression.
(A) Flowering time of wild-type (Wassilewskija) and CBF overexpression lines. Thirty plants were used to measure the flowering time, and the error bars represent sd.
(B) Expression levels of COR15a, FLC, SVP, and FLM were determined by quantitative RT-PCR.
(C) Suppression of FLC expression in CBF overexpression lines by vernalization. Expression level of FLC was detected by quantitative RT-PCR.
(D) Flowering time of CBF overexpression lines without or with 40 d of vernalization. Plants with −Vernalization were grown at 22°C for 9 d under long days, whereas plants with +Vernalization were grown at 22°C for 5 d under long days and then transferred to 4°C for 40 d.
To address if late flowering in 35S-CBF1, 35S-CBF2, and 35S-CBF3 is caused by increased expression of FLC, these lines were vernalized to suppress FLC expression. After 40 d of vernalization, the FLC expression was strongly suppressed (Figure 3C). Correlated with this, the late flowering phenotype of 35S-CBF1, 35S-CBF2, and 35S-CBF3 was also suppressed (Figure 3D), indicating that the late flowering phenotype of CBF overexpression line is caused by increase of FLC expression.
Intermittent Cold Delays Flowering through FLC Activation
It is reported that intermittent cold treatment delayed flowering time through upregulation of FLC (Kim et al., 2004). We further analyzed the regulation of flowering by intermittent cold: a treatment of 6 h cold (4°C) beginning at dawn every day. First, we checked the effect of intermittent cold on the daily rhythm of COR15a and CBF3 (Figures 4A and 4B). Without cold, COR15a expression peaked at 12 h after dawn and greatly decreased at dusk. However, with the intermittent cold treatment, higher peak expression of COR15a was observed at 6 h after dawn when the cold treatment was over and remained higher until 8 h after dawn. Subsequently, it was dramatically reduced at 12 h after dawn. CBF3 expression was also increased by cold treatment: the expression peaked at 6 h and then abruptly decreased to a minimal level at 8 h after dawn. Our results show that intermittent cold causes highly increased expression of COR and CBF genes in the morning.
Figure 4.
Effect of Intermittent Cold on Flowering.
(A) Comparison of COR15a expressions between plants grown with (Cold +) and without (Cold −) intermittent cold (4°C). Expression level of COR15a was detected by quantitative RT-PCR. Intermittent cold treatments were for 6 h from dawn every day. For RNA isolation, the 10-d-old seedlings were harvested at 0, 4, 6, 8, 12, 16, and 24 h after dawn.
(B) Expression level of CBF3 detected by quantitative RT-PCR. The quantitative RT-PCR analysis was biologically repeated three times, and each time point consisted of three technical replicates in both (A) and (B). The error bars represent sd for three technical replicates.
(C) The schematics of intermittent cold treatment. The white bars represent normal growth conditions, and the black bars represent intermittent cold treatment.
(D) Effect of intermittent cold treatment length on the flowering time.
(E) Effect of intermittent cold treatment length on the expression of FLC and SVP. Expression level of FLC and SVP was detected by quantitative RT-PCR. Col plants grown 20 d in each condition were harvested at 6 h after dawn for RNA isolation.
(F) The effect of intermittent cold on the flowering time of each mutant. The mutants of soc1-101D, soc1-2, and svp-41 in the left graph are in the Col background, whereas the flc-3 mutants in the right graph are in the Col:FRISF2 background. Plants were treated with (gray bars, Cold +) or without (white bars, Cold −) intermittent cold for 6 h from the dawn every day until they flowered.
Next, we addressed how many days of intermittent cold are required to delay flowering (Figures 4C to 4E). The result showed that 10 d of cold slightly delays flowering and 20 d of cold delays it further, indicating that the flowering is delayed in proportion to the days of the cold treatment. Consistent with this, FLC expression was increased according to the days of cold. By contrast, SVP expression was not changed by intermittent cold, which is correlated well with the fact that SVP expression is not affected in the 35S-CBF lines.
If the delay of flowering that is induced by intermittent cold is caused by the increase of FLC, it is expected that the flowering of flc, a null mutant, would not be delayed by intermittent cold. Indeed, flc showed the same flowering time with and without intermittent cold (Figure 4F). Interestingly, svp mutants also showed no response to intermittent cold, although SVP expression was not affected by cold treatment (Figure 4F). This may be because SVP produces a flowering repressor complex together with FLC as reported (Li et al., 2008). The soc1-101D mutants, in which strong suppression of CBFs is observed, also showed insensitivity to the intermittent cold (Figure 4F). Together, our results suggest that intermittent cold delays flowering through FLC activity, which is induced by CBFs.
Vernalization Overrides the Effect of Cold Stress on Delaying Flowering
Vernalization, an exposure to prolonged cold temperature, has the opposite effect on flowering compared with intermittent cold treatment. To understand the molecular basis of this difference, we examined the effects of vernalization and intermittent cold on COR15a and CBFs expression in Col:FRISF2, a line showing high expression of FLC and a dramatic acceleration of flowering by vernalization (Michaels and Amasino, 1999; Choi et al., 2005). As expected, intermittent cold treatment caused strong increase in CBF3 and COR15a and slight increase in FLC expression level, which causes a slight delay in flowering time (Figures 5A and 5B). By contrast, when the tissues were harvested immediately after 40 d of vernalization treatment, FLC expression was strongly suppressed in Col:FRISF2, although the plants showed strong induction of CBF1 and CBF3 and much stronger induction of COR15a (Figure 5A). Taken together, our results show that vernalization overrides the effect of cold stress on flowering and suggest that vernalization and cold stress affect FLC expression and flowering via distinct mechanisms.
Figure 5.
Comparison of Vernalization and Intermittent Cold.
(A) Expression levels of CBF1, CBF3, COR15a, and FLC in Col:FRISF2 grown with intermittent cold (4°C) for 6 h every day (Cold +) or with 40 d of vernalization (Ver +). The quantitative RT-PCR analysis was biologically repeated three times, and each time consisted of three technical replicates. The error bars represent sd from triplicate samples.
(B) Effect of vernalization and intermittent cold on the flowering time of Col:FRISF2. Thirty plants were used to measure the flowering time, and the error bars represent sd.
Effect of SOC1 Mutation on Freezing Tolerance
Because soc1-2 increases and soc1-101D decreases the induction of CBFs and COR genes, it was of interest to determine whether these mutants exhibit differences in freezing tolerance. To address this question, plants were exposed to −5°C for 6 h and transferred to room temperature to check the survival rate (Figure 6). As expected, more soc1-2 mutants survived than the wild type, whereas few soc1-101D mutants survived after the freezing treatment. Our result demonstrates that SOC1 regulates not only flowering but also freezing tolerance.
Figure 6.
Effect of soc1 Mutations on Freezing Tolerance.
(A) The freezing-tolerance of soc1-2 and soc1-101D (101D) plants compared with wild-type plants. Experiments were performed in triplicate, and percentage of the plants survived was calculated: n ≥ 30. Mean values and standard errors were plotted. The * and ** denote statistical significance with P < 0.05 and P < 0.01 (Student's t test), respectively.
(B) Sample plates showing plants subjected to freezing tolerance assays. Each plate contains 10 plants per line. The numbers on the plate to the right denote the number of plants that survived after freezing.
COR Gene Expression Is Regulated by Some Other Flowering-Time Genes
Because COR15a expression has known to be increased in fve mutants as well as in soc1-2 (Kim et al., 2004), we tested whether any other late-flowering mutants show a similar increase in COR15a expression (Figure 7). Increased levels of COR15a transcript were observed in gi, a long-day pathway mutant, and in fpa, an autonomous pathway mutant. By contrast, co and ft, two long-day pathway mutants, as well as fca and fld, two autonomous pathway mutants, did not show any difference in the expression of COR15a in comparison to that in the wild type. Interestingly, the ld mutant consistently showed reduced expression of COR15a (Figures 7A and 7B). This result indicates that late flowering per se is not the cause of the ectopic expression of COR15a. It also suggests that the increased COR15a expression is not simply due to the decreased level of SOC1 in the late-flowering mutants because all the late-flowering mutants used in this study have reduced levels of SOC1 transcripts (Lee et al., 2000). For example, among the late-flowering mutants analyzed here, the fca mutant, which has the lowest expression of SOC1, did not show any difference, whereas gi and fpa, which have relatively higher SOC1 expression, showed increased COR15a expression (Figures 7A and 7B; Lee et al., 2000). These findings suggest that the suppression of cold-inducible genes occurs through SOC1-dependent and SOC1-independent pathways.
Figure 7.
Expression of COR15a Is Regulated by Other Flowering Time Genes.
(A) RNA gel blot analysis of COR15a in various late-flowering mutants in Col background. TUB2 probe was used as a loading control.
(B) RNA gel blot analysis of COR15a in fpa-1, fve-1, and gi-1 mutants in Landsberg erecta background.
(C) RNA gel blot analysis of CBF1 in Col, soc1-2, fve-3, and gi-2.
(D) RNA gel blot analysis of COR15a in double mutants with soc1-2.
(E) RNA gel blot analysis of COR15a in double mutants with soc1-101D. Total RNAs were presented as quantitative control for RNA gel blot analysis in (B) to (E).
To confirm the hypothesis of two independent pathways, we compared the level of COR15a in the single mutant soc1-2 and the double mutants soc1-2 fve-3 and soc1-2 gi-2 (Figure 7D). The mutants, soc1-2, fve-3, and gi-2, we used in this experiment are null (Fowler et al., 1999; Borner et al., 2000; Ausin et al., 2004). As expected, the double mutants showed higher expression of COR15a than soc1-2. We also compared the level of COR15a between soc1-101D and the double mutants soc1-101D fve-3 and soc1-101D gi-2 (Figure 7E). The double mutants showed a similar reduced level of COR15a as the soc1-101D single mutant, indicating that overexpression of SOC1 overcomes the derepression caused by the mutations in GI and FVE.
Similar to soc1-2, the gi and fve mutants showed an increase in CBF1 expression, although the increase in fve was relatively less (Figure 7C). Thus, GI and FVE are also likely to suppress the cold response pathway through the repression of CBF genes. Taken together, our results indicate the existence of SOC1-dependent and SOC1-independent pathways for regulating flowering in response to cold signals.
DISCUSSION
In this study, we identified downstream targets of SOC1, which is a key integrator of flowering pathways, by microarray analysis. Unexpectedly, many of genes that are negatively regulated by SOC1 were identified as cold-inducible genes. By contrast, the overexpression of cold response genes delays flowering through the activation of FLC, as does cold stress. This finding reveals the presence of a feedback loop between cold response and flowering, which is another fine-tuning mechanism for flowering time regulation. We propose to name this mechanism as an intermittent cold-sensing pathway for flowering.
Model of Intermittent Cold-Sensing Pathway for Flowering
A model of the intermittent cold-sensing pathway for flowering in Arabidopsis is presented in Figure 8. When the ambient temperature is cold during vegetative growth, the expression of CBFs is induced in response to cold (Thomashow, 1999). The increased expression of CBFs then causes the activation of FLC, which represses the two flowering pathway integrators FT and SOC1, thereby delaying the flowering. On the other hand, a decreased level of SOC1 causes derepression of cold-inducible genes. Such derepression appears to enable plants to respond to cold more strongly, as was seen in the soc1-2 mutant (Figures 1C and 2B). Consistent with this, the soc1-2 mutant showed enhanced resistance to freezing tolerance (Figure 6). Therefore, cold temperature during vegetative growth not only delays flowering but also makes the plants more sensitive to cold. However, when flowering occurs, usually in late spring, SOC1 expression increases (Lee et al., 2000), and increased SOC1 represses cold-inducible genes and thus suppresses the cold response. Such a suppression of cold response can be observed in soc1-101D, an overexpression mutant (Figures 1C and 2B). In addition to SOC1, other flowering time genes, such as FPA, FVE, and GI, are involved in the cold-sensing pathway, although the mechanism needs to be further analyzed (Figure 7).
Figure 8.
Model of Crosstalk between Cold Response and Flowering Time Regulation.
Arrows indicate promotion, and T bars indicate repression. In cold early spring or fall, the expression of CBF genes is activated by the cold signal, and the increased CBFs activate FLC expression, which eventually delays flowering time. By contrast, in warm late spring, floral induction occurs and the increased SOC1, GI, FVE, and FPA suppress the CBF-dependent cold response pathway.
Such a feedback loop between the cold response and flowering could be evolutionarily advantageous. When cold conditions prevail in fall, the intermittent cold-sensing pathway would delay flowering time, providing protection against premature flowering. In addition, for annual plants that start growing from early spring, such a mechanism would delay flowering until full-blown spring has come. By contrast, if flowering sets in, plants suppress the cold response because the expression of CBFs is not desirable for reproductive development, as seen in overexpression lines of CBFs, which show growth retardation (Gilmour et al., 2004).
Cold Response and Vernalization
Both vernalization and intermittent cold-sensing pathways recognize cold temperature; however, their effects on flowering are opposite: whereas vernalization accelerates flowering, intermittent cold sensing delays it. Interestingly, the target of both vernalization and intermittent cold sensing is FLC. It is well known that vernalization suppresses the expression of FLC through histone modification (Sung and Amasino, 2004a). Here, we provide evidence demonstrating that FLC is also a target of the intermittent cold-sensing pathway. First, intermittent cold stress increases the transcript level of FLC and delays the flowering. Second, the flc null mutant does not exhibit delayed flowering in response to cold stress. Third, overexpression of CBFs delays flowering through the activation of FLC. Finally, vernalization, which suppresses the expression of FLC, offsets the effect of CBFs overexpression (Figures 3C and 3D). It is noteworthy that Liu et al. (2002) did not find that the CBF1 overexpression line increases FLC expression in the Col:FRISF2 background. However, this difference may be because Col:FRISF2 line has such a high basal level of FLC expression.
Although vernalization has an opposite effect on FLC, its effect on CBFs and COR genes is the same as that of cold stress; that is, vernalization causes a strong induction of CBF1, CBF3, and COR15a (Figure 5A). Thus, cold stress and vernalization cannot be distinguished at the CBF and COR15a expression level. On the other hand, this distinction can be made at the VIN3 gene expression level because VIN3 is induced not by a short period of cold but by a long period of cold that is sufficient to trigger vernalization (Sung and Amasino, 2004b). Vernalization-induced VIN3 expression initiates inactivation of FLC by histone modification of FLC chromatin. Thereafter, VRN1, VRN2, and LHP1 permanently inactivate FLC chromatin structure via heterochromatin formation (Bastow et al., 2004; Sung and Amasino, 2004b; Mylne et al., 2006; Sung et al., 2006). Thus, vernalization suppresses FLC expression epigenetically despite the presence of a positive regulator, such as the FRI complex (Kim et al., 2006). Currently, it is not known how CBFs regulate FLC expression, but it is very likely that they cannot resolve the heterochromatic state of FLC caused by vernalization. There are two CRT/DRE cold response elements at the proximal region of the FLC promoter; thus, it would be worthwhile to determine whether CBFs bind the FLC promoter in vivo.
SOC1-Dependent and SOC1-Independent Mechanisms for the Intermittent Cold-Sensing Pathway
SOC1 is not the only genetic factor that affects the intermittent cold-sensing pathway with respect to flowering. This and a previous study showed that three other flowering genes, namely, FPA, FVE, and GI, act in this pathway (Kim et al., 2004). In the current model of flowering time regulation, all three genes regulate SOC1 through the long-day pathway or the autonomous pathway (Parcy, 2005). Thus, SOC1 appeared to integrate the cold-sensing signal from the upstream factors FPA, FVE, GI, and FLC. However, our results indicate a more complex pathway. First, other flowering time mutants with strongly reduced expression of SOC1, such as fca and ld, did not exhibit increased expression of cold-inducible genes (Figure 7A). Second, CO and FT do not participate in the intermittent cold-sensing pathway, although GI regulates SOC1 through the activation of CO and FT (Figure 7A). Third, the double mutants soc1 gi and soc1 fve showed an additive effect with regard to the increase in COR15a expression (Figure 7D). This strongly indicates that the cold-sensing pathway is distinct from other well-defined genetic pathways for flowering. It also suggests that FPA, FVE, and GI act on the intermittent cold-sensing pathway independent of SOC1. One caveat is that the decreased level of SOC1 in the fca, fld, ld, co, and ft mutants does not cause increased COR15a expression, although the soc1 null mutation does. The low level of SOC1 remaining in such mutants probably is sufficient to repress COR15a since it has been reported that ∼30 to 70% of the wild-type SOC1 level is detected in these mutants (Lee et al., 2000).
FVE and GI are classified in different flowering pathways (Parcy, 2005; Oh and Lee, 2007). Consistently, no differences in GI expression between fve-1 mutants and wild-type plants were detected, indicating they do not affect the transcription of the other (Fowler et al., 1999). Thus, it is likely that these two genes affect the intermittent cold-sensing pathway via separate mechanisms. However, both fve and gi showed epistatic interaction with fpa in a double mutant analysis (Koornneef et al., 1998; Veley and Michaels, 2008). Thus, there is still an open possibility that these three genes participate in the same intermittent cold-sensing pathway for flowering, which is independent of SOC1. It is noteworthy that GI was identified as a gene that is highly induced in response to cold from a microarray analysis (Fowler and Thomashow, 2002). In addition, the gi mutant shows increased resistance against paraquat-induced oxidative stress (Kurepa et al., 1998). Therefore, GI may have a function in stress responses as well as in flowering.
Recently, it was reported that low red/far-red light ratio at a low ambient temperature (16°C) induces the expression of COR15a and COR15b through CBF activity (Franklin and Whitelam, 2007). In such low ambient temperature, phytochrome B (phyB) and phyD suppress the expression of COR genes; therefore, the mutations in phyB and phyD or low red/far-red increase the expression of COR genes. Although the mechanism is not well understood, such results with ours here can explain why phyB mutant at 16°C flowers later than the wild type, while it flowers earlier than the wild type at normal temperature, 22°C (Halliday et al., 2003). The increased activity of CBF in the phyB mutant at 16°C is likely to delay flowering. However, phyB at 16°C did not show an increase of FLC (Halliday et al., 2003), which is inconsistent with our results. Thus, it adds another layer of complexity in the crosstalk between the regulation of COR genes and flowering time control.
Crosstalk with the Thermosensory Pathway for Flowering
The key components of intermittent cold-sensing pathway are SOC1, CBFs, and FLC (Figure 8). SOC1 directly binds to the CBF promoters, which have modified CArG boxes, for the transcriptional repression (Figure 2). CBFs positively regulate FLC expression, then FLC represses flowering pathway integrators to delay flowering. In this report, we showed that SOC1 acts as transcriptional repressor of CBF genes. It is well known that SOC1 acts as transcriptional activator for the expression of LFY (Lee et al., 2008; Liu et al., 2008). However, it is reported that SOC1 also acts as transcriptional repressor by directly binding the SEP3 promoter (Liu et al., 2009). Therefore, it is likely that SOC1 can act as both an activator and a repressor depending on the cofactors.
Additional components in this pathway are FPA, FVE, and GI that negatively regulate CBF expression. In the thermosensory pathway, it is suggested that FT and SVP are major players, and FCA and FVE are involved in this pathway (Blazquez et al., 2003; Lee et al., 2007b). In addition, it is proposed that FLC is not involved in this pathway because the flc null mutant shows delayed flowering time in response to low temperature, although FLC expression is increased in this condition (Blazquez et al., 2003; Lee et al., 2007b). Thus, it appears that the two pathways are independent. However, our results here show that the two pathways are intertwined. First, the two pathways share the same component, FVE, one of the autonomous pathway genes that regulate FLC expression. Second, SVP is also involved in the intermittent cold-sensing pathway in genetic terms. Although the expression of SVP is not affected by either intermittent cold or CBFs, the svp mutant shows insensitivity to intermittent cold for flowering as does the flc mutant (Figures 3B, 4E, and 4F). Because SVP makes a flowering repressor complex with FLC (Li et al., 2008), it indicates that SVP-FLC complex is involved in intermittent cold-sensing pathway. Therefore, there is a crosstalk between the thermosensory pathway and the intermittent cold-sensing pathway, although they are partially independent as well. In conclusion, we elucidated a fine-tuning mechanism of flowering in response to cold, which must confer adaptability to an ever-changing environment.
METHODS
Plant Materials and Growth Conditions
Sterilized seeds were incubated on 0.85% plant agar (Duchefa) containing 1% sucrose and half-strength Murashige and Skoog (Duchefa) medium for 3 d at 4°C to break seed dormancy. The normal condition in long days was followed as previously described (Lee et al., 2008). To test the induction kinetics by cold, plants were grown at 22°C for 10 d under long days and then transferred to 4°C (cold+) or maintained at 22°C (cold-) for 0, 2, 4, or 6 h in the light. The zero time corresponds to right after dawn. For intermittent cold treatment, plants were placed at 4°C for 6 h from right after dawn every day. For vernalization treatment, 5-d-old seedlings were incubated at 4°C for 40 d under short-day conditions. The soc1-2 and soc1-101D mutants were described previously as agl20 and agl20-101D, respectively (Lee et al., 2000). The mutants of svp-41, ft-1, co-1, gi-2, ld-1, fve-3, fca-9, and fld-1 are in the Col background, and fpa-1, fve-1, and gi-1, are in the Landsberg erecta background (Koornneef et al., 1991; Lee et al., 1994; Putterill et al., 1995; Fowler et al., 1999; Kardailsky et al., 1999; Kobayashi et al., 1999; Page et al., 1999; Hartmann et al., 2000; He et al., 2003; Ausin et al., 2004; Kim et al., 2004). The flc-3 mutants are in the Col:FRISF2 background (Lee et al., 2000). At least 16 plants were used to measure the flowering time. The flowering time was measured as the number of rosette leaves produced when flowering occurs. The overexpression lines of CBF1, CBF2, and CBF3 in the Wassilewskija background were previously described (Gilmour et al., 2004).
Analysis of Gene Expression
Total RNA was isolated from plant tissues by the RNeasy plant mini kit (Qiagen) according to the manufacturer's instructions. For RNA gel blot analysis, 20 μg of RNA was separated on 1.5% denaturing formaldehyde agarose gels and transferred to Hybond N+ nylon membranes (Amersham Biosciences). All RNA probes were prepared from plasmid vectors containing the cDNA fragments of each gene amplified by RT-PCR with primers as follows: SIZ1, 5′-GCAGGAACTTGTCGACCGG-3′ and 5′-GCTTGCACCATCATTTGGGATAG-3′; HOS1, 5′-ATGGATACGAGAGAAATCAACGG-3′ and 5′-ATACAGACATTGGTGATATAATG-3′; ICE1, 5′-ATGGGTCTTGACGGAAACAATGG-3′ and 5′-ACAGAACTCAAATCCCTGTTCCC-3′; ZAT12, 5′-ATGGTTGCGATATCGGAGATC-3′ and 5′-TCAATAAACTGTTCTTCCAAGCTC-3′; CBF1, 5′-ATGAACTCATTTTCAGCTTT-3′ and 5′-TTAGTAACTCCAAAGCGACA-3′; CBF2, 5′-CTTCTACTTACTCTACTCTCATAAAC-3′ and 5′-ATTTGCATTTGACAACAACTTTTACC-3′; CBF3, 5′-GACGACGGATCATGGCTTC-3′ and 5′-TAATAACTCCATAACGATACGTCG-3′; COR15a, 5′-ATGCTCTCGAGGCTTCAGATTTCGTGACGG-3′ and 5′-ATGCTGGTACCTGAAGAGAGAGGATATGG-3′; COR15b, 5′-ATGGCGATGTCTTTATCAGGAG-3′ and 5′-TCAGGACTTTGTGGCATTCTTAG-3′; KIN1, 5′-AAGCCCACATCTCTTCTCATC-3′ and 5′-TTATTTGAATATAAGTTTGGCTCGTC-3′; KIN2, 5′-CATAATTGATTCTCGTACTCATCG-3′ and 5′-GGTAAAACAAAGTTCTTAGAACTTAAAC-3′; TUB2, 5′-CTCAAGAGGTTCTCAGCAGTA-3′ and 5′-TCACCTTCTTCATCCGCAGTT-3′. Each fragment was inserted at single 3′-T overhangs of pGEM-T Easy Vector (Promega) between T7 and SP6 RNA polymerase promoters. These plasmids were linearized with NcoI restriction enzyme that leaves a 5′ overhang. One microgram of the purified, linearized plasmid was used as template of in vitro transcription by SP6 RNA polymerase. RNA probes were made by the digoxigenin RNA labeling kit (Roche) according to the manufacturer's instructions. Prehybridization, hybridization, wash, and detection were performed as described in the digoxigenin application manual (Roche).
For cDNA production, 4 μg of total RNA was reverse transcribed with oligo(dT)18 primer (Fermentas) in a 20-μL reaction mixture using RevertAid M-MuLV reverse transcriptase (Fermentas). After heat inactivation, total volume of the reaction mixture was diluted in 580 μL of sterilized water, and 4 μL was used for the real-time quantitative RT-PCR. All quantitative RT-PCR analyses were performed by iQ5 multicolor real-time PCR detection system (Bio-Rad) using iQ SYBR Green supermix (Bio-Rad). We adopted the guidelines for the experimental design and statistical analysis of quantitative RT-PCR data (Rieu and Powers, 2009). The PCR condition was as follows: 40 cycles of PCR (95°C for 30 s, 60°C for 30 s, and 72°C for 20 s) after the initial denaturation step of 5 min at 95°C. Data was collected at 72°C in each cycle, and the expression levels of genes were calculated by iQ5 optical system software version 2.0 using TUB2 as the reference gene. The quantitative RT-PCR analysis was biologically repeated three times, and each time consisted of three technical replicates. The primers used for quantitative RT-PCR are as follows: COR15a, 5′-CTTACCTAATCAGTTAATTTCAAGCA-3′ and 5′-TTAAACATGAAGAGAGAGGATATGG-3′; CBF1, 5′-CTTGAAAAAGAAATCTACCTG-3′ and 5′-AGTACGTAGTTACTAGAGTTCTC-3′; CBF3, 5′-CGACGTATCGTTATGGAGTTATTA-3′ and 5′-CTAAAAATAATAATAAAATAAAAAGTATCGTAC-3′; FLC, 5′-GAGAATAATCATCATGTGGGAGC-3′ and 5′-CAACCGCCGATTTAAGGTGG-3′; SVP, 5′-CCGGAAAACTGTTCGAGTTC-3′ and 5′-TGACTGCAAGTTATGCCTCTCT-3′; FLM, 5′-TGAAGAACCAAATGTCGATAATGT-3′ and 5′-ATCAGTTCTGCCTTCCTAGC-3′; and TUB2, 5′-ATCGATTCCGTTCTCGATGT-3′ and 5′-ATCCAGTTCCTCCTCCCAAC-3′.
ChIP Assay
ChIP with SOC1 antibody was performed by following the method described previously (Lee et al., 2007a, 2008). Briefly, 600 mg of soc1-2 and soc1-101D seedlings grown under long days for 8 d was used for ChIP. After cross-linking with 1% formaldehyde, extracted cells were lysed and the DNA is broken into pieces of 0.3 to 1.0 kb length by sonication. Then, immunoprecipitation using anti-SOC1 serum, raised in rabbits by repeated injection of SOC1IKC-GST fusion proteins, was performed. The purified protein-DNA complexes were heated to reverse cross-linking, allowing the DNA to be separated from the proteins. One-twentieth of the purified DNA was used for PCR analysis, and 1/100 was used for real-time quantitative PCR. Fifteen microliters of the ChIP products resuspended in 400 μL of TE was used for PCR, and 4 μL of them was used for real-time quantitative PCR. In PCR analysis, sonicated input DNA (0.5%) was used as a quantitative control. In quantitative PCR analysis, expression levels were normalized against the expression in soc1-2. The primers for the CBFs promoter regions containing CArG box are as follows: a, 5′-CAGGACAGGACTAAGCGAAG-3′ and 5′-GCGAGAGGTAACGAGAGAGA-3′; b, 5′-CGTACGGACGTTCGTTTTTGAA-3′ and 5′-CCTCAATTATCTTCTTATCTCGC-3′; c, 5′-GAATATGCTAGAGTAATTTCCTAAGA-3′ and 5′-CCCTGCCACTTGTTAATTCTC-3′; d, 5′-GCCAAGGATTAGACCGATATAG-3′ and 5′-CATTCCTTGTCGATATATTTCTCC-3′; e, 5′-GAATTGGGAGAGTAGATATTTGTG-3′ and 5′-AAAATGTTACATTTGATCATTCACCC-3′; f, 5′-AGATCAATTAGAAGCATGCAGTTG-3′ and 5′-GAGGGCGTTGAGATTGTGATC-3′; ProLFY-1, 5′-CCGGATCCATCCATTTTTCGCAAAGG-3′ and 5′-CCGGATCCATCTGTTCTAAAGCCTCC-3′; ProLFY-4, 5′-CCGGATCCCCCATATGTCCAATCCCA-3′ and 5′-CCGGATCCATCTATCTGCGTTTTAGG-; ProTUB, 5′-ACAAACACAGAGAGGAGTGAGCA-3′ and 5′-ACGCATCTTCGGTTGGATGAGTGA-3′; and CHS, 5′-CCACCATTCCAATCTTGGTAAGTA-3′ and 5′-AGAAGCACCAGCCATCACCAT-3′.
Freezing-Tolerance Assays
Eleven-day-old plants were placed at −5°C for 6 h, and then they were incubated at 23°C for 2 d for recovery. The percentage of plants that survived after this freezing and recovery was calculated. Experiments were performed in triplicate, and each experiment was accomplished in a plate (diameter 150 × 20 mm) containing 10 plants per each control or mutant.
Microarray Analysis
Total RNA was prepared using Trizol reagent (Sigma-Aldrich). Double-stranded cDNA was synthesized using 10 μg of total RNA mixed with T7-(dT)24 primer using SuperScript Choice System (Invitrogen). Next, the cDNA was used to synthesize biotinylated cRNA using the Enzo BioArray High Yield RNA transcript labeling kit (Affymetrix). Twenty micrograms of cRNA was fragmented in a fragmentation buffer (40 mM Tris acetate, pH 8.1, 100 mM KOAc, and 30 mM MgOAc in DEPC water) at 94°C for 35 min before undergoing chip hybridization. We used Arabidopsis ATH1 Genome Array (Affymetrix). Hybridization, washing, and scanning steps were performed at the Affymetrix Service Center (Seoulin Bioscience Institute). Affymetrix GCOS software was used for scanning and basic analysis. More detailed analysis was performed using Affymetrix DMT software. The microarray data have been submitted to the Gene Expression Omnibus of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo/). Series accession number is GSE3279, which contains the whole experimental samples. Each sample accession number is GSM73643, GSM73646, GSM73647, GSM73648, GSM73649, GSM73650, and GSM73651.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: SOC1 (AT2G45660), FLC (AT5G10140), CBF1 (AT4G25490), CBF2 (AT4G25470), CBF3 (AT4G25480), KIN1 (AT5G15960), KIN2 (AT5G15970), COR15a (AT2G42540), COR15b (AT2G42530), ICE1 (AT3G26744), HOS1 (AT2G39810), SIZ1 (AT5G60410), ZAT12 (AT5G59820), SVP (AT2G22540), FLM (AT1G77080), FVE (AT2G19520), FPA (AT2G43410), GI (AT1G22770), FCA (AT4G16280), LD (AT4G02560), FLD (AT3G10390), FT (AT1G65480), LFY (AT5G61850), CO (AT5G15840), AP1 (AT1G69120), VIN3 (AT5G57380), VRN1 (AT3G18990), VRN2 (AT4G1684), LHP1 (AT5G17690), FRI (AT4G00650), phyB (AT2G18790), phyD (AT4G16250), and TUB2 (AT5G62690).
Acknowledgments
We thank M.F. Thomashow for providing the COR15a-overexpressing T8 line and 35S-CBF1, 35S-CBF2, and 35S-CBF3 seeds. This work was supported partially by the Korea Ministry of Science and Technology under the National Research Laboratory Program (2006-01952), a grant from Global Research Laboratory Program (2006-03870), a grant from Seoul R&BD Program, and a grant (Code 20070301034011) from the BioGreen 21 program, Rural Development Administration. E.S. and H.P. were supported by the Brain Korea 21 program.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ilha Lee (ilhalee@snu.ac.kr).
References
- Ausin, I., Alonso-Blanco, C., Jarillo, J.A., Ruiz-Garcia, L., and Martinez-Zapater, J.M. (2004). Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 36 162–166. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, S., Sureshkumar, S., Lempe, J., and Weigel, D. (2006). Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow, R., Mylne, J.S., Lister, C., Lippman, Z., Martienssen, R.A., and Dean, C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167. [DOI] [PubMed] [Google Scholar]
- Baurle, I., and Dean, C. (2006). The timing of developmental transitions in plants. Cell 125 655–664. [DOI] [PubMed] [Google Scholar]
- Blazquez, M.A., Ahn, J.H., and Weigel, D. (2003). A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33 168–171. [DOI] [PubMed] [Google Scholar]
- Blazquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404 889–892. [DOI] [PubMed] [Google Scholar]
- Borner, R., Kampmann, G., Chandler, J., Gleissner, R., Wisman, E., Apel, K., and Melzer, S. (2000). A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 24 591–599. [DOI] [PubMed] [Google Scholar]
- Boss, P.K., Bastow, R.M., Mylne, J.S., and Dean, C. (2004). Multiple pathways in the decision to flower: Enabling, promoting, and resetting. Plant Cell 16 S18–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy, V., Ohta, M., Kanrar, S., Lee, B.H., Hong, X.H., Agarwal, M., and Zhu, J.K. (2003). ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy, V., Zhu, J., and Zhu, J.K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12 444–451. [DOI] [PubMed] [Google Scholar]
- Choi, K., Kim, S., Kim, S.Y., Kim, M., Hyun, Y., Lee, H., Choe, S., Kim, S.G., Michaels, S., and Lee, I. (2005). SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 17 2647–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, S., Lee, K., Onouchi, H., Samach, A., Richardson, K., Coupland, G., and Putterill, J. (1999). GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 18 4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, S., and Thomashow, M.F. (2002). Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, S.G., Cook, D., and Thomashow, M.E. (2005). Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 137 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K.A., and Whitelam, G.C. (2007). Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat. Genet. 39 1410–1413. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., Fowler, S.G., and Thomashow, M.F. (2004). Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 54 767–781. [DOI] [PubMed] [Google Scholar]
- Halliday, K.J., Salter, M.G., Thingnaes, E., and Whitelam, G.C. (2003). Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J. 33 875–885. [DOI] [PubMed] [Google Scholar]
- Harmer, S.L., Hogenesch, L.B., Straume, M., Chang, H.S., Han, B., Zhu, T., Wang, X., Kreps, J.A., and Kay, S.A. (2000). Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290 2110–2113. [DOI] [PubMed] [Google Scholar]
- Hartmann, U., Hohmann, S., Nettesheim, K., Wisman, E., Saedler, H., and Huijser, P. (2000). Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 21 351–360. [DOI] [PubMed] [Google Scholar]
- He, Y.H., Michaels, S.D., and Amasino, R.M. (2003). Regulation of flowering time by histone acetylation in Arabidopsis. Science 302 1751–1754. [DOI] [PubMed] [Google Scholar]
- Hempel, F.D., Weigel, D., Mandel, M.A., Ditta, G., Zambryski, P.C., Feldman, L.J., and Yanofsky, M.F. (1997). Floral determination and expression of floral regulatory genes in Arabidopsis. Development 124 3845–3853. [DOI] [PubMed] [Google Scholar]
- Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J., and Weigel, D. (1999). Activation tagging of the floral inducer FT. Science 286 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., Hyun, Y., Park, J.Y., Park, M.J., Park, M.K., Kim, M.D., Lee, M.H., Moon, J., Lee, I., and Kim, J. (2004). A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat. Genet. 36 167–171. [DOI] [PubMed] [Google Scholar]
- Kim, S., Choi, K., Park, C., Hwang, H.J., and Lee, I. (2006). SUPPRESSOR OFFRIGIDA4, encoding a C2H2-type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 18 2985–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M., and Araki, T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286 1960–1962. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Alonso-Blanco, C., Blankestijn-de Vries, H., Hanhart, C.J., and Peeters, A.J.M. (1998). Genetic interactions among late-flowering mutants of Arabidopsis. Genetics 148 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Hanhart, C.J., and van der Veen, J.J. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229 57–66. [DOI] [PubMed] [Google Scholar]
- Kurepa, J., Smalle, J., Van Montagu, M., and Inze, D. (1998). Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J. 14 759–764. [DOI] [PubMed] [Google Scholar]
- Lee, H., Suh, S.S., Park, E., Cho, E., Ahn, J.H., Kim, S.G., Lee, J.S., Kwon, Y.M., and Lee, I. (2000). The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 14 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.J., Xiong, L.M., Gong, Z.Z., Ishitani, M., Stevenson, B., and Zhu, J.K. (2001). The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev. 15 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I., Aukerman, M.J., Gore, S.L., Lohman, K.N., Michaels, S.D., Weaver, L.M., John, M.C., Feldmann, K.A., and Amasino, R.M. (1994). Isolation of LUMINIDEPENDENS – A gene involved in the control of flowering time in Arabidopsis. Plant Cell 6 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., He, K., Stolc, V., Lee, H., Figueroa, P., Gao, Y., Tongprasit, W., Zhao, H.Y., Lee, I., and Deng, X. (2007. a). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19 731–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., Oh, M., Park, H., and Lee, I. (2008). SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 55 832–843. [DOI] [PubMed] [Google Scholar]
- Lee, J.H., Yoo, S.J., Park, S.H., Hwang, I., Lee, J.S., and Ahn, J.H. (2007. b). Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 21 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., Liu, C., Shen, L., Wu, Y., Chen, H., Robertson, M., Helliwell, C.A., Ito, T., Meyerowitz, E., and Yu, H. (2008). A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell 15 110–120. [DOI] [PubMed] [Google Scholar]
- Liu, C., Chen, H., Er, H.L., Soo, H.M., Kumar, P.P., Han, J.H., Liou, Y.C., and Yu, H. (2008). Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135 1481–1491. [DOI] [PubMed] [Google Scholar]
- Liu, C., Xi, W., Shen, L., Tan, C., and Yu, H. (2009). Regulation of floral patterning by flowering time genes. Dev. Cell 5 711–722. [DOI] [PubMed] [Google Scholar]
- Liu, J.Y., Gilmour, S.J., Thomashow, M.F., and van Nocker, S. (2002). Cold signalling associated with vernalization in Arabidopsis thaliana does not involve CBF1 or abscisic acid. Physiol. Plant. 114 125–134. [DOI] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, J., Lee, H., Kim, M., and Lee, I. (2005). Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 46 292–299. [DOI] [PubMed] [Google Scholar]
- Moon, J., Suh, S.S., Lee, H., Choi, K.R., Hong, C.B., Paek, N.C., Kim, S.G., and Lee, I. (2003). The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 35 613–623. [DOI] [PubMed] [Google Scholar]
- Mylne, J.S., Barrett, L., Tessadori, F., Mesnage, S., Johnson, L., Bernatavichute, Y.V., Jacobsen, S.E., Fransz, P., and Dean, C. (2006). LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc. Natl. Acad. Sci. USA 103 5012–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, M., and Lee, I. (2007). Historical perspective on breakthroughs in flowering field. J. Plant Biol. 50 249–256. [Google Scholar]
- Onouchi, H., Igeno, M.I., Perilleux, C., Graves, K., and Coupland, G. (2000). Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell 12 885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, T., Macknight, R., Yang, C.H., and Dean, C. (1999). Genetic interactions of the Arabidopsis flowering time gene FCA, with genes regulating floral initiation. Plant J. 17 231–239. [DOI] [PubMed] [Google Scholar]
- Parcy, F. (2005). Flowering: A time for integration. Int. J. Dev. Biol. 49 585–593. [DOI] [PubMed] [Google Scholar]
- Putterill, J., Robson, F., Lee, K., Simon, R., and Coupland, G. (1995). The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc-finger transcription factors. Cell 80 847–857. [DOI] [PubMed] [Google Scholar]
- Rieu, I., and Powers, S.J. (2009). Real-time quantitative RT-PCR: Design, calculations, and statistics. Plant Cell 21 1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky, L., Davletova, S., Liang, H.J., and Mittler, R. (2004). The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 279 11736–11743. [DOI] [PubMed] [Google Scholar]
- Samach, A., Onouchi, H., Gold, S.E., Ditta, G.S., Schwarz-Sommer, Z., Yanofsky, M.F., and Coupland, G. (2000). Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288 1613–1616. [DOI] [PubMed] [Google Scholar]
- Stockinger, E.J., Gilmour, S.J., and Thomashow, M.F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 94 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, S.B., and Amasino, R.M. (2004. a). Vernalization and epigenetics: How plants remember winter. Curr. Opin. Plant Biol. 7 4–10. [DOI] [PubMed] [Google Scholar]
- Sung, S.B., and Amasino, R.M. (2004. b). Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427 159–164. [DOI] [PubMed] [Google Scholar]
- Sung, S.B., He, Y.H., Eshoo, T.W., Tamada, Y., Johnson, L., Nakahigashi, K., Goto, K., Jacobsen, S.E., and Amasino, R.M. (2006). Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 38 706–710. [DOI] [PubMed] [Google Scholar]
- Thomashow, M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 571–599. [DOI] [PubMed] [Google Scholar]
- Veley, K.M., and Michaels, S.D. (2008). Functional redundancy and new roles for genes of the autonomous floral-promotion pathway. Plant Physiol. 147 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.T., Zarka, D.G., Van Buskirk, H.A., Fowler, S.G., and Thomashow, M.F. (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41 195–211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki, K., and Shinozaki, K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]