Abstract
In filamentous fungi, Sfp-type 4′-phosphopantetheinyl transferases (PPTases) activate enzymes involved in primary (α-aminoadipate reductase [AAR]) and secondary (polyketide synthases and nonribosomal peptide synthetases) metabolism. We cloned the PPTase gene PPT1 of the maize anthracnose fungus Colletotrichum graminicola and generated PPTase-deficient mutants (Δppt1). Δppt1 strains were auxotrophic for Lys, unable to synthesize siderophores, hypersensitive to reactive oxygen species, and unable to synthesize polyketides (PKs). A differential analysis of secondary metabolites produced by wild-type and Δppt1 strains led to the identification of six novel PKs. Infection-related morphogenesis was affected in Δppt1 strains. Rarely formed appressoria of Δppt1 strains were nonmelanized and ruptured on intact plant. The hyphae of Δppt1 strains colonized wounded maize (Zea mays) leaves but failed to generate necrotic anthracnose disease symptoms and were defective in asexual sporulation. To analyze the pleiotropic pathogenicity phenotype, we generated AAR-deficient mutants (Δaar1) and employed a melanin-deficient mutant (M1.502). Results indicated that PPT1 activates enzymes required at defined stages of infection. Melanization is required for cell wall rigidity and appressorium function, and Lys supplied by the AAR1 pathway is essential for necrotrophic development. As PPTase-deficient mutants of Magnaporthe oryzea were also nonpathogenic, we conclude that PPTases represent a novel fungal pathogenicity factor.
INTRODUCTION
Secondary metabolism and virulence are closely linked traits in fungi attacking plants and mammals. Many of the structurally diverse secondary metabolites contributing to pathogenicity are polyketides (PKs) or nonribosomal peptides (NRPs), and this diversity is reflected by the large number of genes encoding polyketide synthases (PKSs) or nonribosomal peptide synthetases (NRPSs). For example, in Cochliobolus heterostrophus, the causal agent of Southern Leaf Blight of maize (Zea mays), 25 PKS- and 11 NRPS-coding genes have been identified (Kroken et al., 2003; Lee et al., 2005). In several Colletotrichum species and in the rice blast fungus Magnaporthe oryzae, PK-derived melanin is required for generation of turgor pressure in infection cells called appressoria and, thus, for pathogenicity (Howard et al., 1991; De Jong et al., 1997; Bechinger et al., 1999; Deising et al., 2000). Also, in fungi attacking humans (e.g., Aspergillus fumigatus and Cryptococcus neoformans), melanin contributes to virulence, presumably by quenching reactive oxygen species and protecting hyphae against human monocytes (Langfelder et al., 2003). In addition to melanin, toxins synthesized by PKSs determine fungal virulence on plants. The carbon chain of the host-selective T-toxin of C. heterostrophus is probably assembled by the action of two PKSs (Baker et al., 2006). A closely related pathogen, the Northern Leaf Blight fungus, Cochliobolus carbonum, produces the host-selective HC toxin, a cyclic tetrapeptide synthesized by a NRPS (Panaccione et al., 1992; Walton, 2006). Genes encoding other NRPS proteins (e.g., NPS6 of C. heterostrophus and orthologs in different economically relevant plant pathogenic Ascomycota, such as Cochliobolus miyabeanus, Gibberella zeae, Alternaria brassicicola, and M. oryzae) are involved in siderophore synthesis and iron acquisition, tolerance to H2O2, and virulence (Oide et al., 2006; Hof et al., 2007). Siderophores are also pathogenicity factors in fungi infecting man (Schrettl et al., 2007). In addition to PKSs and NRPSs, bioinformatics approaches have recently revealed the existence of hybrid PKS-NRPS genes in several fungi. Interestingly, in the rice blast fungus M. oryzae, one of the PKS-NRPS genes, ACE1, acts as an avirulence gene, and the metabolite likely formed by the ACE1 enzyme elicits defense responses in rice cultivars carrying the Pi33 resistance gene (Fudal et al., 2007). Thus, a large body of literature indicates that PKs, NRPs, and PK-NRP hybrid molecules govern the interaction between pathogenic fungi and their hosts.
Despite the enormous chemical diversity in the secondary metabolites they synthesize, PKSs, NRPSs, and hybrid PKS-NRPSs share a common point of regulation. With the exception of type III PKSs, all of these enzymes require activation by 4′-phosphopantetheinylation at conserved Ser residues by an enzyme called 4′-phosphopantetheinyl transferase (PPTase). In yeast, three phosphopantetheinylated proteins (i.e., the cytoplasmic fatty acid synthase complex, a mitochondrial acyl carrier protein, and an α-aminoadipate reductase [AAR] involved in Lys biosynthesis) are known (Ehmann et al., 1999; Fichtlscherer et al., 2000). Different 4′-phosphopantetheinyl transferase (4′-PPTase) mutants are specifically affected in only one of these activities, with the other two remaining functional, indicating that specific 4′-PPTases exist in primary metabolism in Saccharomyces cerevisiae (Fichtlscherer et al., 2000). In the filamentous ascomycete Aspergillus nidulans, a single 4′-PPTase appears to regulate enzymes involved in both primary and secondary metabolism. A sound body of literature demonstrates that mutants deficient in the 4′-PPTase-encoding gene npgA/cfwA are Lys auxotrophs unable to synthesize several PK, including the conidiospore pigments and the mycotoxin sterigmatocystin, and NRPs such as penicillin and siderophores (Han and Han, 1993; Guzmán-de-Peña et al., 1998; Keszenman-Pereyra et al., 2003; Oberegger et al., 2003; Marquez-Fernandez et al., 2007). Like A. nidulans, other filamentous ascomycetes, such as A. fumigatus, M. orzyae, G. zeae, and Neurospora crassa, have single-copy cfwA/npgA homologs (Marquez-Fernandez et al., 2007).
The contribution of PKS-, NRPS-, and PKS-NRPS-derived secondary metabolites to fungal virulence has been demonstrated in several pathogens (Rasmussen and Hanau, 1989; Henson et al., 1999; Oide et al., 2006; Greenshields et al., 2007; Steiner and Oerke, 2007; Collemare et al., 2008), indirectly arguing that PPTases may play a central role in the infection process. However, no data directly demonstrate that PPTases are required for pathogenicity on plants, and the role of individual enzymes that require 4′-phosphopantetheinylation has not been analyzed in different stages of the infection process.
Mootz et al. (2002) deleted the PPTase-encoding lys5 gene of S. cerevisiae and demonstrated that Lys prototrophy was restored by complementation with genes from Bacillus sp encoding Sfp- and Gsp-type PPTases. The Schizosaccharomyces pombe lys7 and the A. nidulans npgA gene also complemented the lys5 deletion and have thereby been functionally characterized as PPTase genes. Mootz et al. (2002) suggested that this functional complementation system could allow for the simple cloning of 4′-PPTase genes from cDNA libraries of diverse origin. Taking advantage of this strategy, we cloned the Sfp-type PPTase gene PPT1 of the maize pathogen Colletotrichum graminicola, the causal agent of leaf anthracnose and stalk rot (Bergstrom and Nicholson, 1999). Targeted mutagenesis revealed that PPT1 is required for Lys prototrophy, acquisition of iron, tolerance to reactive oxygen intermediates, formation and melanization of infection cells, invasion of intact maize leaves, switch to necrotrophic development, formation of anthracnose leaf symptoms, and asexual sporulation. Infection assays performed with melanin-deficient UV mutants (Rasmussen and Hanau, 1989) and with AAR-deficient mutants produced by targeted gene deletion allowed us to dissect the pleiotropic effects of this enzyme and to define the infection stages at which enzymes activated by PPT1 are required for pathogenicity. Furthermore, deletion of the PPT1 homolog in M. oryzae confirmed the role of PPT1 in pathogenicity in another pathosystem. Thus, PPT1 is a novel fungal pathogenicity gene and may represent a novel multifunctional antifungal target.
RESULTS
Complementation of Yeast Mutant Δlys5 and 4′-Phosphopantetheinylation of a PKS Fragment Demonstrate the PPTase Function of PPT1 of C. graminicola
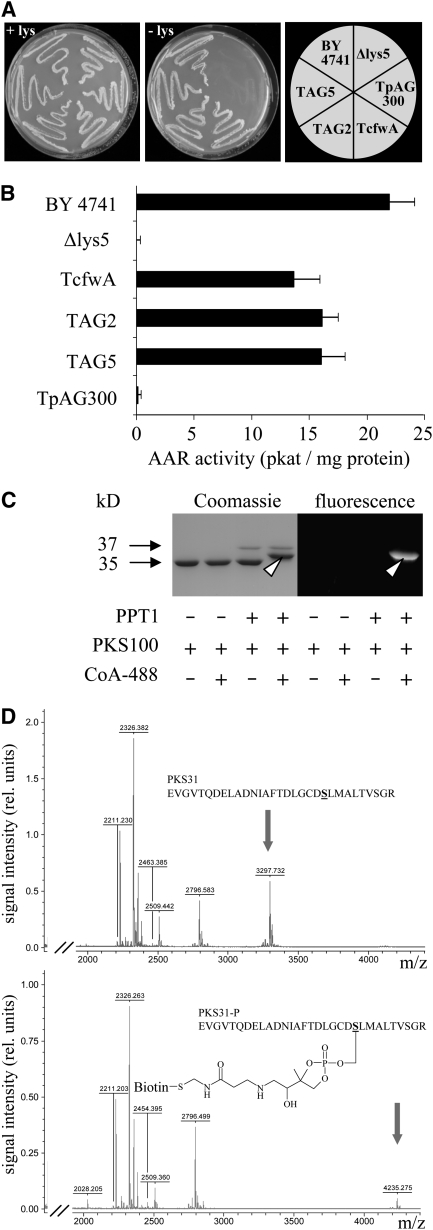
To clone the C. graminicola PPTase gene by complementation of the PPTase-deficient yeast mutant strain BO4421 (Δlys5), which is auxotrophic for Lys, a cDNA library was constructed from melanized saprophytic mycelium. The binary vector pAG300 allowed expression of C. graminicola cDNAs in yeast under control of the constitutive yeast ADH1 promoter. As a positive control, the yeast Δlys5 strain was complemented with the A. nidulans PPTase gene cfwA (Figure 1A, TcfwA). Of 4.5 million yeast transformants harboring C. graminicola cDNA, eight were prototrophic for Lys (TAG1 to TAG8) (Figure 1A, TAG2 and TAG5), indicating the presence of a functional PPTase. In contrast with the Lys prototrophic reference strain BY4741 and transformants TAG2, TAG5, and TcfwA, the Δlys5 strain BO4421 and strain BO4421 transformed with the empty vector (TpAG300) were auxotrophic for Lys (Figure 1A).
Figure 1.
Cloning of the C. graminicola PPTase Gene by Complementation of the S. cerevisiae Δlys5 Mutant, Confirmation of AAR Activity, and 4′-Phosphopantetheinylation of a PKS Fragment.
(A) The reference yeast strain BY4741 and Δlys5 transformants TcfwA, TAG2, and TAG5 expressing PPTase cDNAs from A. nidulans and C. graminicola, respectively, were able to grow on medium lacking Lys (-lys). By contrast, Δlys5 or the Δlys5 transformant containing the empty expression vector (TpAG300) were auxotroph for Lys. All yeast strains grew on medium amended with Lys (+lys).
(B) AAR activity was detectable in cell-free extracts from strains BY4741, TcfwA, TAG2, and TAG5 but not in extracts from the Δlys5 strain and TpAG300. Three independent experiments were performed; bars represent sd.
(C) 4′-phosphopantetheinylation of a 100–amino acid C. graminicola PKS1 fragment (PKS100) fused to GST, as indicated by a band shift in Coomassie blue–stained gels and by fluorescence (arrowheads), only occurred when affinity-purified PPT1 and PKS100 and fluorochrome ATTO488-conjugated CoA were present in the reaction mixture.
(D) Affinity-purified PKS100 was incubated with PPT1 in the absence (top) or presence (bottom) of biotin-conjugated CoA. After tryptic digest, MALDI-TOF MS analysis of a 31–amino acid peptide (PKS31) containing the conserved DSL phosphopantetheinylation site showed a mass shift only when biotin-conjugated CoA was present in the reaction mixture. Arrows indicate peaks corresponding to PKS31 (calculated m/z = 3299.645, measured m/z = 3297.732, error of 0.058%) and the phosphopantetheinylated PKS31-P (calculated m/z = 4237.705, measured m/z = 4235.275, error of 0.057%).
In liquid culture, the S. cerevisiae wild-type strain BY4741 (see Supplemental Figure 1 online, black line) and the transformants TcfwA (see Supplemental Figure 1 online, green line), TAG2, and TAG5 (see Supplemental Figure 1 online, blue lines) showed similar growth rates. Doubling times were 71 min for BY4741, 70 min for TcfwA and TAG5, and 67 min for TAG2. However, initiation of exponential growth of BY4741 lagged ∼120 min behind transformants TAG2 and TAG5 and ∼70 min behind TcfwA. In the absence of Lys, the Δlys5 strain BO4421 and BO4421 transformed with the empty vector (TpAG300) did not grow (see Supplemental Figure 1 online, gray lines).
The sequences of the cDNAs expressed in all transformants prototrophic for Lys were identical and showed significant similarity with those of other fungal PPTase genes in BLAST analyses, as indicated by e-values (M. oryzae 2e−91, G. zeae 5e−75, N. crassa 1e−64, and A. nidulans 4e−34). Based on functional complementation of the Δlys5 mutants and sequence homology, we designated PPT1 to the C. graminicola PPTase gene. This gene consists of 1113 bp and contains an intron of 178 bp, starting at position 85 from the beginning of the start codon. The intron is flanked by characteristic 3′GT and 5′AG splicing signals and contains a typical branching point motif (CTGAC). The deduced protein (PPT1) consists of 311 amino acids and has a calculated relative molecular mass (Mr) of 34.854. PPT1 of C. graminicola is 54 and 50% identical to the PPTases of M. oryzae and G. zeae, respectively, and 32% identical to the A. nidulans protein CfwA. Remarkably, PPT1 of C. graminicola is only 20% identical to S. cerevisiae Lys5p.
As activation of AAR strictly depends on 4′-phosphopantetheinylation, AAR activity can be taken as indirect evidence for PPTase activity. AAR activity was detected in cell-free extracts of wild-type strain BY4741, and transformants TcfwA, TAG2, and TAG5, but not in extracts of the Δlys5 mutant BO4421 and transformant TpAG300 (Figure 1B). Furthermore, 4′-phosphopantetheinylation of a C. graminicola PKS fragment containing a conserved Ser (amino acids 1618 to 1717 of PKS1, see below) demonstrated PPTase activity of PPT1 directly (Figure 1C). 4′-Phosphopantetheinylation of the GST-PKS100 fragment only occurred if the reaction mixture contained both affinity-purified recombinant proteins, for example, the 37.1-kD (His)6-PPT1 [consisting of the 34.9-kD PPTase and a (His)6-tag accounting for 2.2 kD] and the 37.4-kD GST-PKS100 (consisting of the 100–amino acid 10.9-kD PKS100 fragment and the 26.5-kD glutathione S-transferase [GST] tag), as well as CoA conjugated to fluorophore ATTO 488. This was indicated by an electrophoretic mobility shift in SDS-PAGE (Figure 1C, Coomassie-stained gel, arrowhead). As expected, only the shifted band representing the 4′-phosphopantetheinylated GST-PKS100 fragment fluoresced upon irradiation at 473 nm, due to the fluorophore being linked to the 4′-phosphopantetheinyl residue (Figure 1C, fluorescence image, arrowhead).
Matrix-assisted laser-desorption ionization time of flight mass spectrometry (MALDI-TOF MS) analysis was used to further demonstrate the covalent link between the 4′-phosphopantetheinyl residue and the GST-PKS100 target protein. The 4′-phosphopantetheinylated and the non-4′-phosphopantetheinylated fragments were tryptically digested, and shifts in the molecular mass of the resulting fragments were analyzed. The affinity-purified GST-PKS100 fragment was tryptically digested, and a 31–amino acid fragment contained a conserved Ser at position 22 (Figure 1D, PKS31, S underlined) and the DSL consensus signal for 4′-phosphopantetheinylation (Mofid et al., 2002). Indeed, the mass-to-charge (m/z) values of the non-4′-phosphopantetheinylated and the 4′-phosphopantetheinylated 31–amino acid fragments were 3297.732 and 4235.275, respectively (Figure 1D, arrows). The difference exactly corresponds to the mass of the biotinylated 4′-phosphopantetheinyl residue.
Thus, yeast complementation and biochemical data show that PPT1 of C. graminicola is capable of 4′-phosphopantetheinylating and activating proteins containing a conserved Ser. These data are in agreement with reports from other Sfp-type PPTases, including A. nidulans CfwA, which is required for activation of AAR, PKSs, and NRPSs (Neville et al., 2005; Yin et al., 2005; Marquez-Fernandez et al., 2007).
PPT1 Mutants of C. graminicola Are Auxotrophic for Lys, Nonmelanized, Unable to Synthesize PKS- and NRPS-Dependent Secondary Metabolites, Unable to Grow under Iron-Limiting Conditions, and Hypersensitive to Reactive Oxygen Species
To functionally characterize the PPT1 gene of C. graminicola, targeted mutagenesis was performed (see Supplemental Figure 2 online). In the knockout vector, the 182-bp Eco81I fragment of the gene was replaced by a 2.6-kb fragment carrying the Escherichia coli hygromycin phosphotransferase gene (hph) (see Supplemental Figure 2A online). As indicated by DNA gel blot analyses performed with PstI-digested genomic DNA, the 1597-bp wild-type band had been replaced by the 2279-bp fragment in all independent knockout strains (Δppt1; KO). Transformants with ectopically integrated KO vector (ect.) showed the 2279-bp band in addition to the 1597-bp wild-type band (see Supplemental Figure 2B online). To confirm gene inactivation at the transcript level, RT-PCR experiments were performed with primers specific for PPT1 transcripts, using total RNA from the wild-type and three independent KO strains. Primers corresponding to transcripts of the constitutively expressed C. graminicola CHSII gene (Werner et al., 2007) were used as a control (see Supplemental Figure 2C online). As expected, the 380-bp CHSII fragment was amplified from RNA from wild-type and KO strains, but the 936-bp C. graminicola PPT1 fragment only occurred when RNA from the wild-type strain was used as a template (see Supplemental Figure 2C online). These data confirm the successful targeted mutagenesis of PPT1.
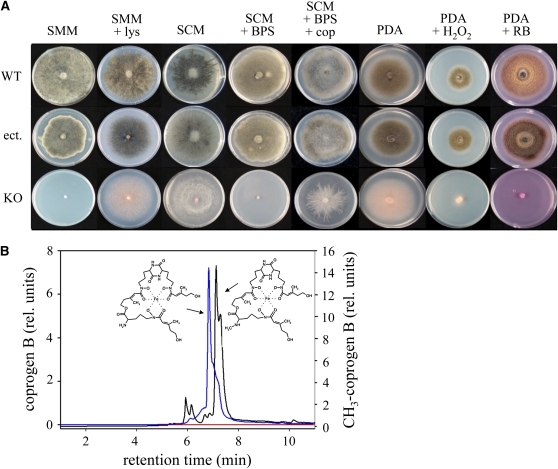
Hyphal pigmentation and growth assays on Lys- and iron-limited synthetic minimal medium indicated the requirement of PPT1 for activation of AAR, PKSs, and NRPSs (Figure 2). The wild-type strain and the transformants with ectopic integration of the KO vector (ect.), but not the KO strain, were able to grow on Lys-deficient synthetic minimal medium (Figure 2A, SMM). Although slightly reduced growth rates sometimes occurred in ectopic transformants, these results fully support the yeast complementation assays (Figure 1A; see Supplemental Figure 1A online). As expected, supplementing the medium with Lys rescued the growth defect of Δppt1 strains but was insufficient to restore melanization (Figure 2A, SMM + lys). Likewise, on rich media such as synthetic complete medium (Figure 2A, SCM) or potato dextrose agar (Figure 2A, PDA), Δppt1 strains were able to grow but failed to melanize. On potato dextrose agar, Δppt1 strains appeared grayish due to the color of the medium. These data suggest that PPT1 is required for activating both AAR and PKS.
Figure 2.
Growth Assays and Formation of Siderophores.
(A) In comparison with the wild-type strain and a strain carrying the ectopically integrated knockout vector (ect.), the KO strain is unable to synthesize melanin or to grow under iron-depleting conditions and is hypersensitive to reactive oxygen species. SMM, synthetic minimal medium; SCM, synthetic complete medium; BPS, bathophenanthroline-disulfonate; cop, desferri-coprogen; PDA, potato dextrose agar; RB, rose bengal.
(B) Analysis of siderophores formed by wild-type and Δppt1 strains. HPLC profiles of culture filtrates indicate that the wild-type strain secretes coprogen B (blue line) and 2-N-methylcoprogen B (black line) into the culture medium. Δppt1 strains do not secrete any siderophores (red line).
Siderophores are NRPs that mediate iron uptake and growth under iron-limiting conditions (Antelo et al., 2006; Haas et al., 2008). Addition of the iron chelator bathophenanthroline-disulfonate to synthetic complete medium caused complete growth inhibition of the Δppt1 strains, but the wild-type strain and ectopic transformants were able to grow (Figure 2A, SCM + BPS). Growth of the KO strains was restored by adding the siderophores desferri-ferrichrome (see Supplemental Figure 3 online) or desferri-coprogen (cop) (Figure 2A, SCM + BPS + cop). Indeed, HPLC-MS analyses of culture filtrates of the wild-type strain showed that C. graminicola secreted the siderophores coprogen B (Figure 2B, blue line) and 2-N-methylcoprogen B (Figure 2B, black line) under iron-limiting conditions; culture filtrates of Δppt1 strains did not contain any siderophore (Figure 2B, red line). Furthermore, only the wild-type strain but not the mutants contained the intracellular siderophore ferricrocin (see Supplemental Figure 4 online). These results are in agreement with reports of growth defects of siderophore-deficient mutants of G. zeae and C. heterostrophus under iron-limiting conditions (Oide et al., 2006; Greenshields et al., 2007). Chelation of iron is required to prevent an increase in the levels of reactive oxygen species (Haas et al., 2008). As expected, growth of Δppt1 strains was severely inhibited on media containing H2O2 or rose bengal, which produces singlet oxygen radicals when illuminated (Figure 2A, PDA + H2O2 and PDA + RB).
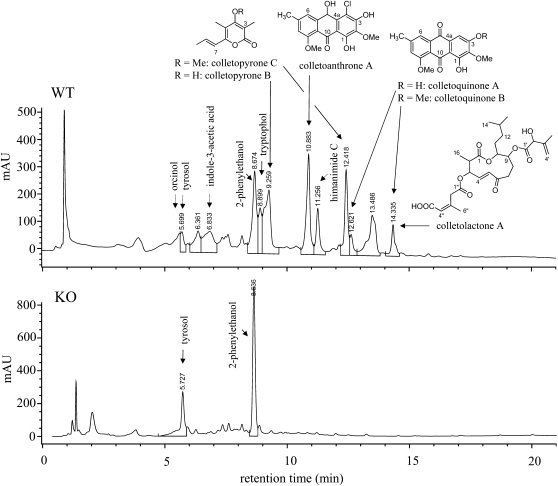
Differential metabolite analyses in culture filtrates revealed that several secondary metabolites were formed by the wild type but not by the Δppt1 strains (Figure 3). In addition to the extracellular NRPs coprogen B and 2-N-methylcoprogen B (Figure 2B), the intracellular iron storage NRP ferricrocin was detected in hyphal extracts (see Supplemental Figure 4 online). Furthermore, we identified six novel fungal metabolites using HPLC-MS and nuclear magnetic resonance (NMR) spectrometry, including two pyrones (colletopyrone B and C), two anthraquinones (colletoquinone A and B), a 10-hydroxyanthrone (colletoanthrone A), and an 11-membered macrolactone (colletolactone A) (Figure 3; see Supplemental Figure 5 Supplemental Tables 1 to 4 online). In addition, the compounds orcinol, tryptophol, indole-3-acetic acid, himanimide C, and 2-phenylethanol have been found in culture filtrates of the wild-type strain (Supplemental Figure 5 online). Interestingly, tyrosol, which has been described as a PK in the fungus Beauveria bassiana (Fuganti et al., 1996), was present in culture filtrates of both wild-type and Δppt1 strains, suggesting that tyrosol is synthesized via the shikimate pathway.
Figure 3.
HPLC Profiles and Secondary Metabolites Identified in Culture Filtrates of Wild-Type and Δppt1 (KO) Strains.
Chemical structures of orcinol, tryptophol, colletopyrone B and C, colletoanthrone A, colletoquinone A and B, and colletolactone A were identified by NMR. Tyrosol, indole-3-acetate, phenylethanol, and himanimide C were identified by employing reference data (HPLC retention time, UV, and mass spectra) from the IBWF compound library. The detection wavelength was 210 nm.
Toxicity assays were performed by applying 5-μL droplets containing all of the purified compounds individually at a concentration of 1 μg/μL onto wounded and nonwounded maize leaves. One, two, and three days after application of the secondary metabolites, leaves were macroscopically investigated for the occurrence of necroses and microscopically for the occurrence of cell wall or whole-cell fluorescence under UV excitation. None of the compounds produced in liquid cultures caused cell death or autofluorescence of whole cells or cell walls under these conditions, suggesting that they are not phytotoxic.
Taken together, these data show that the Sfp-type PPTase PPT1 plays a central role in Lys biosynthesis and synthesis of secondary metabolites required for pigmentation, acquisition of iron, and tolerance to oxidative stress in C. graminicola.
PPT1 of C. graminicola Is Required for Asexual Sporulation, Germ Tube Morphology, and Differentiation of Functional Appressoria
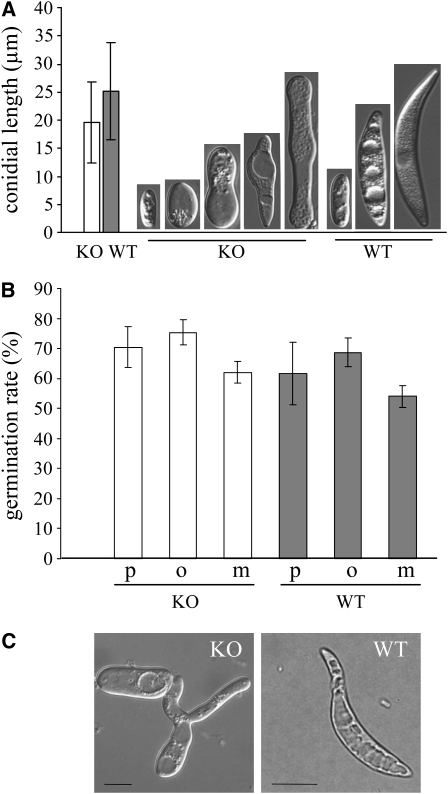
Marquez-Fernandez et al. (2007) demonstrated that severe asexual sporulation defects occurred in PPTase-deficient cfwA mutants of A. nidulans. To analyze the effect of PPT1 of C. graminicola on the size and morphology of oval and falcate conidia, wild-type and Δppt1 strains were allowed to sporulate in liquid complete medium. Conidia of the Δppt1 strains were smaller than wild-type conidia and showed drastic morphological defects (i.e., small conidia had round to spherical shapes and larger spores had central protrusions or round poles; Figure 4A). Interestingly, germination rates of mutant and wild-type conidia were similar on different hydrophobic substrata (i.e., polyester sheets, onion [Allium cepa cv Grano] epidermis, and maize surface; Figure 4B). However, germ tubes of Δppt1 strains frequently branched (Figure 4C).
Figure 4.
Formation of Asexual Spores.
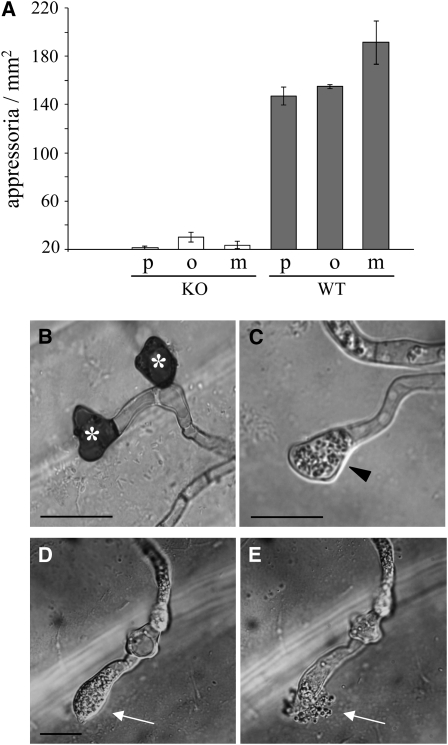
(A) Size and shape of conidia formed by wild-type and Δppt1 (KO) strains in liquid medium. Bars represent ± sd (n = 300). Conidial lengths of wild-type and KO strains differed significantly from each other (P < 0.05).
(B) Comparison of germination rates of conidia of KO strains (white columns) and the wild type (gray columns) on different hydrophobic substrata. p, polyester sheets; o, onion epidermis; m, maize surface. Bars represent ± sd (n = 600, P > 0.05).
(C) Germlings of a KO strain and the wild type. Note the branching of the germ tube of the KO strain. Bars = 10 μm.
Differentiation of melanized functional infection cells, such as appressoria or hyphopodia, is essential for pathogenicity of C. graminicola (Rasmussen and Hanau, 1989; Deising et al., 2000; Werner et al., 2007). To investigate the role of PPT1 in appressorium differentiation and melanization, wild-type and Δppt1 conidia were inoculated onto artificial and natural hydrophobic substrata (i.e., polyester, onion epidermal layers, and maize leaf surfaces; Figure 5). While the wild-type strain germinated and efficiently formed appressoria on all hydrophobic surfaces, Δppt1 strains showed severely reduced rates of appressorium formation (Figure 5A), although they had no defects in germination rates (Figure 4B). As expected, wild-type appressoria were strongly melanized (Figure 5B, asterisk), but infection cells of Δppt1 strains lacked melanization (Figure 5C, arrowhead). To determine appressorium function, penetration rates on maize leaves were quantified. While penetration rates of wild-type appressoria were 86% ± 6% in laboratory experiments with maize leaf segments, invasion of epidermal cells by those rarely formed nonmelanized appressoria of Δppt1 strains was not observed. Surprisingly, microscopy analyses revealed that most nonmelanized appressoria of Δppt1 strains ruptured on the leaf surface, as indicated by the spontaneous release of cytoplasm (cf. Figures 5D and 5E, arrows). By contrast, we never observed bursting of wild-type appressoria. These data suggest that a PKS activated by PPT1 is involved in appressorial melanization and cell wall stability.
Figure 5.
Δppt1 (KO) Strains Are Unable to Differentiate Functional Appressoria.
(A) In contrast with the wild-type strain (gray columns), KO strains (white columns) formed significantly lower numbers of appressoria on hydrophobic substrata (P < 0.05). p, polyester sheets; o, onion epidermis; m, maize surface. Three independent experiments were performed (total number of fungal structures counted = 1842). Bars represent ± sd.
(B) Melanized wild-type appressoria (asterisks) formed on onion surface.
(C) Appressoria of KO strain (arrowhead) lacked melanization.
(D) and (E) Bursting appressorium and cytoplasm release from a KO strain (arrows, compare [D] and [E]). Bars = 10 μm.
PPT1 Is Expressed at All Stages of Pathogenesis
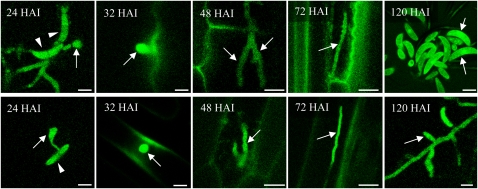
The role of PPT1 in both primary and secondary metabolism suggests that this gene would be expressed at all stages of pathogenesis. As infection structure differentiation and leaf infection do not occur perfectly synchronously, RT-PCR experiments do not provide information on PPT1 expression in individual infection structures. Therefore, we analyzed expression of this gene during the infection process on leaves of the host plant, maize, and on epidermal layers of the alternate host, onion, using a C. graminicola transformant carrying a PPT1:enhanced green fluorescent protein (eGFP) fusion under the control of the native PPT1 promoter (Figure 6). Preliminary experiments had shown that virulence was not affected in this transformant. In both host backgrounds, infection structure formation was comparable. Conidia (arrowheads) had germinated and differentiated appressoria (arrow) by 24 h after inoculation (HAI), and biotrophic infection vesicles and thick primary hyphae had formed by 32 and 48 HAI, respectively (arrows). Thin secondary hyphae, indicative of necrotrophic development, were visible at 72 HAI (arrows), and newly formed conidia (arrows) were present at 120 HAI. GFP fluorescence was observed in all fungal structures formed (i.e., in conidia, germ tubes, and appressoria differentiated on the plant cuticle) and in biotrophic and necrotrophic hyphae formed in planta. These data indicate that PPT1 was expressed at all stages of pathogenesis.
Figure 6.
Expression of PPT1 during Pathogenic Development of C. graminicola.
Expression of the PPT1:eGFP fusion in infected maize (top row) and onion (bottom row). By 24 HAI, conidia (arrowheads) had formed appressoria (arrows) on the plant surface. At 32, 48, 72, and 120 HAI, infection vesicles, primary hyphae, secondary hyphae, and newly formed conidia had formed, respectively, as indicated by arrows. GFP fluorescence is visible in all infection structures formed. Bars = 10 μm.
PPT1 Is Indispensable for Pathogenicity and Symptom Formation but Not for Growth in Planta
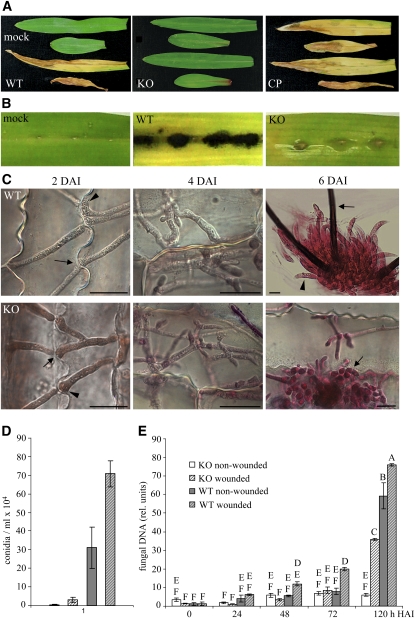
As PPT1 is essential for siderophore and melanin biosynthesis, both of which represent pathogenicity or virulence determinants in plant pathogenic fungi (Rasmussen and Hanau, 1989; Nosanchuk and Casadevall, 2003; Greenshields et al., 2007; Hof et al., 2007; Haas et al., 2008), we analyzed the role of this gene in pathogenicity on maize leaves (Figure 7). When inoculated onto nonwounded leaves, the wild-type strain, but not the Δppt1 strains, was able to cause leaf anthracnose disease symptoms (Figure 7A, WT and KO). Complementation of the Δppt1 strains with the PPT1 gene under control of the native promoter fully rescued growth defects on minimal medium lacking Lys (see Supplemental Figure 6 online), and complemented Δppt1 strains (CP) regained full virulence on intact maize leaves (Figure 7A, KO and CP).
Figure 7.
Δppt1 Strains Are Nonpathogenic and Have Defects in Asexual Sporulation but Can Colonize Maize Leaf Tissue.
(A) Plant infection assays showed that the wild type, but not a representative Δppt1 (KO) strain, caused anthracnose disease symptoms. Mock inoculation was performed with sterile 0.01% (v/v) Tween 20. In KO strains complemented with the PPT1 gene (CP), full virulence on intact leaves was restored.
(B) Macroscopy evaluation of symptom development at 6 DAI on wounded leaves inoculated with the wild type and a KO strain; mock-inoculated leaves served as control.
(C) Micrographs of wounded leaves inoculated with the wild type and a representative KO strain showing fungal in planta development over 6 DAI. Black arrows indicate hyphae penetrating anticlinal plant cell walls at 2 DAI, and the arrowhead indicates a hyphal appressorium-like structure at the site of penetration; at 4 DAI, epidermal cells were colonized by wild type and the Δppt1 strain; at 6 DAI, acervuli with conidia (arrowhead) and setae (arrow) were formed by the wild-type strain, whereas development of the KO strain was terminated when initials of acervuli (arrow) had formed. Samples were stained with acid fuchsin. Bars = 10 μm.
(D) Relative DNA amounts of the GPD1 gene from wild-type and KO strains in wounded and nonwounded leaves as determined by quantitative PCR at five time points. Letters indicate significance groups, and bars indicate ± sd (n = 3).
(E) Number of conidia produced by wild-type and KO strains on wounded and nonwounded leaves at 6 DAI. Patterns of columns are as in (D). Bars indicate ± sd (n = 80, P < 0.05).
Melanin-deficient mutants of C. graminicola obtained after UV irradiation were impaired in host cell penetration, but lesion formation was restored when wounded leaves were inoculated with conidia of these mutants (Rasmussen and Hanau, 1989). To analyze whether the nonpathogenicity of Δppt1 strains was only due to defects in melanization, we compared virulence of C. graminicola wild-type and Δppt1 strains on wounded leaf segments (Figure 7B). At 6 d after inoculation (DAI), the wild-type strain caused severe anthracnose symptoms indicative of necrotic tissue; however, only minor chloroses but no typical anthracnose disease symptoms occurred after inoculation with the Δppt1 strains (Figure 7B, WT and KO).
Interestingly, microscopy inspection of infection sites showed that the Δppt1 strains were able to colonize the host tissue. At 2 DAI, both wild-type and Δppt1 strains showed spreading growth and efficiently penetrated anticlinal plant cell walls (Figure 7C, arrows). In both wild-type and Δppt1 strains, hyphal swellings reminiscent of inconspicuous appressoria were formed prior to penetration, which often occurred through plasmodesmata (Figure 7C, arrowhead). Massive colonization of leaves by both strains was observed at 4 DAI, and at 6 DAI, the wild-type strain had formed acervuli with conidia (Figure 7C, arrow) and melanized setae (Figure 7C, arrowhead). Although Δppt1 strains were able to grow in the host tissue, they were arrested when initials of acervuli formed (Figure 7B, arrow) and exhibited major defects in conidiation. At 6 DAI, 3 × 105 and 7 × 105 conidia were recovered from nonwounded and wounded leaves infected by the wild-type strain, respectively; 0.3 × 104 and 3 × 104 were isolated from nonwounded and wounded Δppt1-infected leaves, respectively (Figure 7D).
To quantify pathogenic development of wild-type and Δppt1 strains in wounded and nonwounded host leaves, quantitative PCR was performed using primers specific for the glyceraldehyde-3-phosphate dehydrogenase gene of C. graminicola (Figure 7E). The wild-type strain developed hyphae in both wounded and nonwounded leaves, with minor differences in the amount of fungal DNA, taken as an indicator of fungal biomass. The switch to necrotrophic development occurred at ∼72 HAI, and massive growth of the wild-type strain was observed thereafter in both wounded and nonwounded leaves (cf. 72 and 120 HAI). By contrast, the Δppt1 strains formed hyphae only in wounded but not in intact leaves. Significant development of the Δppt1 strains was evident in wounded leaves, although the amount of DNA was only approximately half that of the wild-type strain on wounded but about two-thirds of the wild type on nonwounded leaves at 120 HAI (Figure 7E). Comparisons of fungal mass and symptom intensity (Figure 7B) suggest that it is unlikely that insufficient fungal mass would account for lack of necroses in leaves inoculated with the Δppt1 strains. Thus, inactivation of the PPT1 gene drastically diminished pathogenic development, but in planta growth of the Δppt1 strains was reminiscent of an endophytic lifestyle.
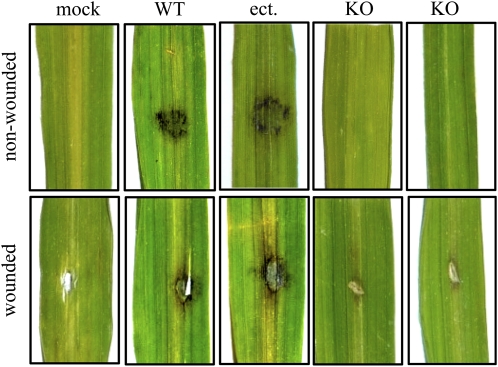
To address the question of whether PPTase is a pathogenicity factor also in other plant pathogenic fungi, the PPT1 gene was deleted in strain 70-15 of the rice blast fungus M. oryzae. The Magnaporthe genome (Dean et al., 2005) contains a single-copy PPTase gene. Replacement of PPT1 by a hygromycin resistance cassette was performed by transforming conidia of M. oryzae with a KO construct consisting of the hph cassette flanked by ∼1 kb of 5′- and 3′-flanks of the PPT1 gene (see Supplemental Figure 7 online). Successful deletion was confirmed by DNA gel blots (see Supplemental Figure 7 online). Two independent Δppt1 strains of M. oryzae (Figure 8, KO) and one strain carrying an ectopically integrated KO vector (Figure 8, ect.) were subsequently used in infection assays with intact and wounded leaves of the susceptible rice cultivar CO 39. As observed in infection assays with Δppt1 strains of C. graminicola on maize, none of the Δppt1 strains of M. oryzae were able to cause rice blast disease symptoms on wounded or on nonwounded leaves. By contrast, the wild-type strain and the strains carrying the ectopically integrated KO vector were able to cause disease symptoms in both wounded and nonwounded leaves (Figure 8).
Figure 8.
Δppt1 Strains of M. oryzae Are Nonpathogenic on Rice.
Plant infection assays showed that the wild-type strain and a strain with an ectopic integration of the KO construct (ect.) infected and caused symptoms on wounded and nonwounded leaves 7 DAI. The ΔMoppt1 (KO) strains were unable to cause disease symptoms on wounded or on nonwounded leaves. Representative photographs are shown. Three independent experiments were performed.
Thus, infection assays performed with PPTase-deficient mutants of C. graminicola (Figure 7) and M. oryzae (Figure 8) suggest that PPTase is a fungal pathogenicity factor.
Enzymes Activated by PPT1 Are Required at Specific Stages of Pathogenesis
To investigate in more detail the pleiotropic phenotype of the PPTase-deficient mutant of C. graminicola, we compared the Δppt1 strains with the melanin-deficient mutant M1.502 (Rasmussen and Hanau, 1989) and a Lys-auxotrophic mutant lacking the AAR gene (Δaar1) (Figure 9).
Figure 9.
Synthesis of Melanin and Lys Is Required for Pathogenicity.
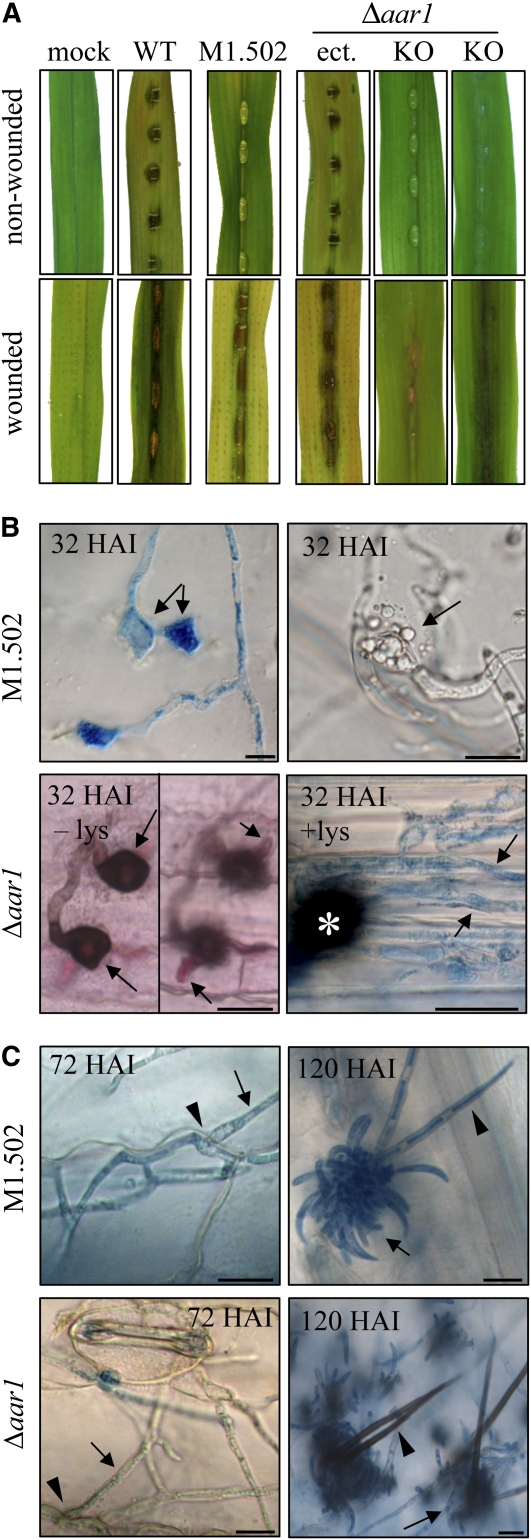
(A) Plant infection assays showed that the wild type, but not the melanin-deficient strain M1.502 or the Lys-deficient Δaar1 strain (KO), caused anthracnose disease symptoms on nonwounded maize leaves. Symptom development was restored on wounded leaves; a strain with an ectopic integration of the AAR1 KO construct and mock-inoculated leaves served as controls.
(B) Micrographs of intact leaves inoculated with M1.502 showed functionally aberrant appressoria on the intact plant surface, as indicated by lateral germination (32 HAI, left image, arrows) or ruptured appressoria (32 HAI, right image, arrow). Δaar1 strains formed melanized appressoria (32 HAI, – lys, left image, arrows) and primary infection hyphae (32 HAI, – lys, right image, arrows), which were unable to form secondary hyphae and to switch to necrotrophic development. Addition of Lys restored the ability to switch to necrotrophy, as indicated by the occurrence of thin intracellular secondary hyphae (32 HAI, + lys, arrows; asterisk marks appressorium). All samples, except Δaar1, 32 HAI, – lys, which was acid fuchsin-stained, were aniline blue stained. Bars = 10 μm.
(C) On wounded leaves, M1.502 and Δaar1 strains formed secondary hyphae (72 HAI, arrows) penetrating anticlinal cell walls (72 HAI, arrowhead). Asexual sporulation occurred in both strains (120 HAI, arrows). In contrast with melanized setae of strain Δaar1 (120 HAI, arrowhead), setae of strain M1.502 were nonmelanized (120 HAI, arrowhead). Samples were stained with aniline blue. Bars = 10 μm.
As described previously (Rasmussen and Hanau, 1989), the UV-induced mutant M1.502 was unable to cause disease symptoms when inoculated onto nonwounded maize leaves (Figure 9A), due to its inability to differentiate melanized appressoria (Figure 9B, M1.502). Even 32 HAI, the nonmelanized appressoria (note aniline blue–stained hyaline appressoria on the cuticle) had not penetrated the cuticle. Several of these nonmelanized appressoria had either germinated laterally (Figure 9B, 32 HAI, left micrograph, arrows) or ruptured (Figure 9B, 32 HAI, right micrograph, arrow). Cell wall rupturing had also been observed with the nonmelanized appressoria of Δppt1 strains (Figures 5D and 5E). Disruption of appressorial cell walls in nonmelanized strains due to misregulated turgor pressure is unlikely, as cytorrhizis experiments with increasing concentrations of polyethylene glycol 6000 showed that the turgor pressure of appressoria of strain M1.502 was comparable to that of the wild-type strain (see Supplemental Figure 8 online). General defects in cell wall integrity, as indicated by hyphal swellings, were not observed in saprophytically growing or in pathogenic hyphae developing in wounded leaves. Hyphae of strains Δppt1 and M1.502 exhibited normal cell walls even at apical regions and were able to penetrate anticlinal cell walls of plant cells (Figure 7C, 2 DAI, arrows; Figure 9C, 72 HAI, arrow) after inoculation onto wounded leaves. On wounded leaves, strain M1.502, but not the Δppt1 strains, was able to cause spreading anthracnose disease symptoms (Figures 7B and 9A). These data suggest that melanization catalyzed by a 4′-PPTase-dependent PKS contributes to the appressorial cell wall stability specifically required in infection cells generating high turgor pressure.
As expected, the nonmelanized strain M1.502 was able to form acervuli and conidia normally (Figure 9C, 120 HAI, arrow). Like appressoria, setae were nonmelanized in this strain (Figure 9B, 120 HAI, arrowhead).
To assess the role of AAR activity and Lys biosynthesis in pathogenesis, an AAR-deficient mutant of C. graminicola was created by deletion of the AAR-encoding gene AAR1. As described for deletion of the PPT1 gene of M. oryzae, a KO construct consisting of the hph cassette flanked by ∼1 kb of 5′- and 3′-flanks of the AAR1 gene (see Supplemental Figure 9 online) was transformed into protoplasts of the WT strain CgM2 of C. graminicola. Homologous integration of the KO fragment was confirmed by DNA gel blot analysis (see Supplemental Figure 9 online).
Infection assays were performed on wounded and nonwounded leaf segments with the wild-type strain, transformants carrying an ectopically integrated KO construct, and two independent Δaar1 strains (Figure 9). In contrast with the wild-type strain and the ectopic strain (Figure 9A, WT and ect.), the Δaar1 strains (Figure 9A, Δaar1, KO) were unable to cause disease symptoms on nonwounded leaves. Microscopy analyses showed that both independent Δaar1 strains used in these assays formed melanized appressoria on the cuticle and were able to penetrate intact maize leaves (Figure 8B, Δaar1, 32 HAI, left micrograph), although penetration rates were reduced (32.7% ± 2.1% and 26.0% ± 4.5% penetration in two independent transformants compared with 69.3% ± 4.5% in the wild-type strains at 32 HAI). Reduced penetration rates are likely not due to reduced turgor pressure in appressoria of Δaar1 strains, as indicated by cytorrhizis experiments (see Supplemental Figure 8 online).
Surprisingly, Δaar1 strains were only able to differentiate biotrophic infection structures (i.e., infection vesicles and young primary hyphae) (Figure 9B, Δaar1, 32 HAI, –lys, arrows) and failed to form necrotrophic secondary hyphae, possibly due to Lys starvation. Supporting this assumption, when 0.34 mM Lys was added to the infection droplet (Figure 9B, Δaar1, 32 HAI, + lys, arrows) or when conidia were inoculated onto wounded leaves, necrotrophic secondary hyphae formed (Figure 9B, Δaar1, 72 HAI, arrow) and caused anthracnose disease symptoms (Figure 9A, AAR1, KO wounded). Δaar1 strains were delayed in sporulation, but by 120 HAI, acervuli with melanized setae (Figure 9C, Δaar1, 120 HAI, arrowhead) and normal spores (Figure 9C, Δaar1, 120 HAI, arrow) had formed on infected leaves.
In summary, melanization is required at the stage of appressorium formation, and AAR activity is needed to enter necrotrophic development. The Sfp-type PPTase PPT1 of C. graminicola activates AAR, PKSs, and NRPSs and is required for conidiation, infection-related morphogenesis, appressorium function, and anthracnose disease development. Furthermore, our data show that PPTase activity is indispensable for pathogenicity in two different fungi and very likely in other filamentous pathogenic fungi.
DISCUSSION
In crop plants, fungi cause more economic damage than any other group of microorganisms, with annual losses estimated at more than $200 billion (Birren et al., 2002). Reduction of yield, however, represents only part of problems associated with fungal plant pathogens. Aspergillus and Fusarium species, and others, produce highly toxic secondary metabolites called mycotoxins, and uptake of contaminated food may result in increased incidence of cancer (Desjardins et al., 2000). Furthermore, several secondary metabolites (e.g., various toxins, melanin, and siderophores) represent pathogenicity factors in fungi attacking plants and mammals (Howard et al., 1991; Langfelder et al., 2003; Baker et al., 2006; Oide et al., 2006; Haas et al., 2008). Therefore, understanding fungal secondary metabolism at the molecular level may allow developing novel strategies to combat fungal diseases in both agriculture and medicine.
The use of drugs interfering with the formation of single secondary metabolites may have a limited application range, as many of these only play a role in some fungi. Melanin biosynthesis inhibitors, for example, are specifically used in rice blast control (Sawada et al., 2004), but other economically important plant pathogens, such as rusts and powdery mildews, are not affected as they are nonmelanized. By contrast, PPTases, which simultaneously activate AAR, type I and II PKSs, NRPSs, and NRPS/PKS hybrids, could represent novel targets for drugs and alternative antifungal strategies to efficiently combat a broad range of pathogens infecting either mammals or plants. In addition to blocking synthesis of PKs, NRPs, and NRP/PK hybrid molecules, interference with AAR activity may increase drug efficiency and specificity, as only in fungi does Lys biosynthesis occur via AAR. Indeed, deletion of the A. fumigatus Lys biosynthesis gene lysF encoding homoaconitase led to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis (Liebmann et al., 2004).
Infection assays with PPTase-deficient mutants of C. graminicola revealed that PPT1 is required for differentiation of functional melanized appressoria and penetration competence, anthracnose disease symptom formation, and asexual sporulation. Surprisingly, however, when inoculated onto wounded leaves Δppt1 mutants were able to invade and spread within the host tissue. These observations suggest that in Δppt1 strains, which are unable to activate NRPSs and AAR, additional components may guarantee supply with iron and Lys during in planta growth. For example, a high affinity iron uptake system (Eichhorn et al., 2006) may compensate for the lack of siderophores and uptake of free Lys, which is present at a concentration of ∼0.1 mM in maize cells (Chapman and Leech, 1979), may compensate for Lys auxotrophy. Alternatively, Lys may be provided by protein degradation by secreted proteases (Krijger et al., 2008). Interestingly, PPTase-deficient mutants are able to ramify within the host tissue but are unable to cause anthracnose disease symptoms, suggesting that toxins formed by PKSs, NRPSs, or PKS/NRPS hybrids might be required for a necrotrophic lifestyle.
To further investigate the different roles played by the enzymes and metabolic pathways activated by PPT1, a melanin-deficient mutant strain, M1.502 (Rasmussen and Hanau, 1989), and AAR-deficient mutants produced in this work were analyzed for defects in pathogenesis. Strain M1.502 formed nonmelanized appressoria and was nonpathogenic on an intact plant surface but produced normal anthracnose disease symptoms on wounded leaves, confirming previous reports on melanin-deficient mutants of this and other plant pathogenic fungi (Rasmussen and Hanau, 1989; Tsuji et al., 2000; Wilson and Talbot, 2009). Interestingly, our study showed that nonmelanized appressoria of strain M1.502 (frequently) ruptured on the plant surface, as did the rarely formed appressoria of Δppt1 strains, suggesting that melanization contributes to cell wall rigidity, which counteracts the enormous turgor pressure (5.35 MPa) generated in these infection cells (Bechinger et al., 1999). Although it is widely accepted that in the appressorial cell walls of the rice blast fungus M. oryzae melanin acts as a barrier to the efflux of osmolytes and is needed for turgor generation (Howard and Ferrari, 1989; Wilson and Talbot, 2009), studies with Gaeumannomyces graminis var graminis have shown that melanization is also associated with cell wall rigidity (Money et al., 1998). Other causes for appressorial rupture in Δppt1 strains and in strain M1.502 of C. graminicola (e.g., the occurrence of general cell wall or cytoskeleton defects) (Kopecká and Gabriel, 1995; Scheffer et al., 2005; Takeshita et al., 2006; Werner et al., 2007) are unlikely, as hyphae of these strains growing in vitro or in planta showed no apical or subapical swellings. In addition, cytorrhizis experiments revealed that no significant differences in appressorial turgor exist between the wild type and strain M1.502. Thus, melanization of appressoria is the first step requiring PPT1 activity in the infection process and is mediated by activating a PKS (Figure 10). In C. graminicola, the PKS required for melanization of appressoria likely is PKS1, as the gene encoding this enzyme is specifically activated during appressorium differentiation (Sugui and Deising, 2002).
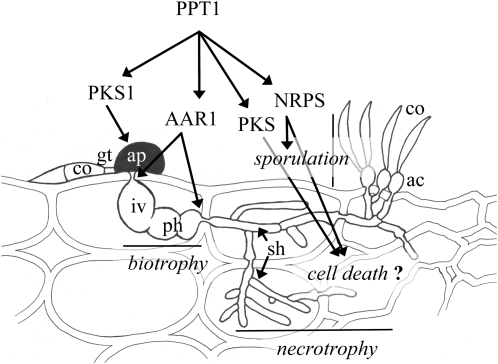
Figure 10.
4′-Phosphopantetheinyl Transferase (PPT1) Is a Central Regulator of Pathogenicity of C. graminicola.
All PKSs, NRPSs, and AAR1 require activation by PPT1. Appressoria of melanin-deficient mutants are unable to invade the host, and AAR-deficient mutants show reduced penetration competence and form biotrophic infection structures but are unable to switch to a necrotrophic lifestyle. PPT1-deficient mutants are unable to kill the host cells, possibly due to their inability to synthesize toxic PKS- and/or NRPS-dependent secondary metabolites. PPT1-deficient mutants are also unable to sporulate, probably due to their inability to synthesize siderophores by NRPSs. ac, acervulus; ap, appressorium; co, conidium; gt, germ tube; iv, infection vesicle; ph, primary hypha; sh, secondary hypha. Artwork not to scale.
To assess the role of AAR1 and Lys biosynthesis in infection structure formation and pathogenesis, we deleted the AAR1 gene of C. graminicola. Δaar1 strains differentiated melanized appressoria but showed significantly reduced penetration rates. Again, cytorrhizis experiments showed that turgor pressure of appressoria of wild-type and Δaar1 strains were similar. Interestingly, appressoria of Δaar1 strains that were able to penetrate the epidermal cell of their host plants formed biotrophic structures (i.e., infection vesicles and primary hyphae) but failed to form secondary hyphae and to switch to necrotrophic development (Figures 9B and 10). Addition of Lys to the infection droplet complemented this defect (Figure 9B), suggesting that the apoplasmic Lys concentration in maize leaves is too low to support growth of primary hyphae and to switch to necrotrophy. In agreement with this hypothesis, when Δaar1 strains were inoculated onto wounded leaves that were releasing cytoplasm containing amino acids and proteins, necrotrophic secondary hyphae formed and gave rise to typical disease symptoms. However, if C. graminicola should produce ribosomal peptide toxins as reported for the barley (Hordeum vulgare) and rye (Secale cereal) pathogen Rhynchosporium secalis (NipA) (Rohe et al., 1995) and the wheat (Triticum aestivum) pathogen Pyrenophora tritici repentis (ToxA) (Manning et al., 2007), one may consider that Lys could indirectly be required for toxin synthesis and entering of the necrotrophic lifestyle.
Comparative analyses of culture filtrates of C. graminicola wild-type and Δppt1 strains allowed us to identify several as yet unknown secondary metabolites (Figure 3). As Δppt1 strains were unable to kill host cells and to cause disease symptoms, we hypothesized that the compounds identified may act as toxins. Some of the secondary metabolites only formed by the wild-type strain; for example, orcinol, indole-3-ylacetic acid, tryptophol, colletopyrones B and C, colletoanthrone A, himanimide C, colletoquinones A and B, and colletolactone A were present in culture filtrates at concentrations allowing purifying them. However, when applied onto wounded and nonwounded maize leaves, toxic effects were not observed. This also held true when a combination of all compounds was tested. However, toxins affecting the host tissue during the infection process may not be synthesized during saprophytic growth in liquid media. Analysis of spore germination fluids of the fungus C. carbonum by plasma desorption mass spectrometry showed that synthesis and release of the host-selective HC toxin, a member of the NRP class of toxins, was coincident with maturation of appressoria. Spores incubated under conditions that did not induce appressorium formation failed to produce the toxin, indicating that toxin synthesis is regulated by infection-related morphogenesis (Weiergang et al., 1996). Future studies employing thin membranes that allow forceful penetration and formation of infection structures comparable to those normally formed in planta (Küster et al., 2008) may help identify PK or NRP toxins exclusively formed by specific infection structures.
The ability to sporulate is essential for completion of the disease cycle of C. graminicola. Δppt1 strains were unable to sporulate on infected plant tissue (Figure 6). A conditional cfwA2 mutation in A. nidulans enhanced the sporulation defects of both ΔtmpA and ΔfluG single mutants, suggesting that unidentified CfwA-dependent PKSs and/or NRPSs are involved in the production of unknown compounds required for sporulation (Soid-Raggi et al., 2006; Marquez-Fernandez et al., 2007). However, delayed sporulation has been observed in Δaar1 strains, and mutants of C. graminicola that are unable to synthesize siderophores are unable to sporulate (E. Albarouki and H.B. Deising, unpublished data). Sporulation defects in Δppt1 strains may therefore be due to various reasons, such as insufficient supply of Lys or iron in the host tissue and lack of PKS-, NRPS-, or PKS/NRPS-hybrid-dependent secondary metabolites serving as a sporulation signal.
Our analyses have shown that PPT1 affects pathogenicity of C. graminicola at different morphogenetic stages through activating enzymes of primary (AAR1) and secondary (PKS1 and possibly other PKSs and NRPSs) metabolism (Figure 10). Although this work has not identified PPT1-dependent toxic secondary metabolites, our data highlight the central role played by PPT1 in switching to destructive necrotrophic disease development.
Our findings contribute to the understanding of fungal pathogenicity. The novel fungal pathogenicity factor identified (i.e., PPTase) is not only of scientific interest but could also be applied to screen for novel lead structures in medical or agricultural pharmacology or in biotechnology. Furthermore, PPTases may also represent a promising target in novel host-induced gene silencing strategies. These RNA interference (RNAi)-based disease control strategies have recently been discovered by Schweizer et al. (2006). These authors have shown that expression of RNAi constructs targeting genes essential for pathogenic development of the powdery mildew fungus Blumeria graminis f. sp hordei and the Fusarium head blight fungus G. zeae in barley drastically reduced disease development (Schweizer et al., 2006; Gay et al., 2008). RNAi-mediated silencing of PPTase genes by either expressing RNAi constructs corresponding to conserved PPTase domains or by expressing multiple constructs corresponding to individual PPTase genes of different fungi may improve resistance to several pathogens simultaneously.
METHODS
Fungal Isolates, Culture Conditions, and Infection Assays
The wild-type strain CgM2 of Colletotrichum graminicola (Cesati) Wilson (teleomorph Glomerella graminicola [Politis]) was provided by R.L. Nicholson, Purdue University, IN.
Synthetic minimal medium contained 1% (w/v) glucose, 1 g/L Ca(NO3)2, 0.2 g/L KH2PO3, 0.25 g/L MgSO4, 0.054 g/L NaCl, and 1.5% (w/v) agarose with or without 50 μg/ml l-Lys. Synthetic complete medium [1.7 g/L yeast nitrogen base without amino acids (Becton Dickinson), 5 g/L (NH4)2SO4, 20 g/L glucose, and amino acid mixture (Treco and Lundblad, 1993)] was supplemented with 100 μM bathophenanthroline-disulfonate (Sigma-Aldrich), with or without 50 μM desferri-coprogen (EMC Microcollections) or desferri-ferrichrome (Sigma-Aldrich).
Sensitivity to reactive oxygen species was determined on potato dextrose agar (Becton Dickinson) supplemented with 0.0075% (v/v) H2O2 (Carl Roth) in darkness or with 100 μg/ml rose bengal under constant light (10 μE) at 23°C.
Infection structures were induced as described (Werner et al., 2007). Magnaporthe oryzae strain 70-15 (Fungal Genetics Stock Center) was cultured as described (Hof et al., 2007). PPTase-deficient mutants of C. graminicola and M. oryzae were grown in media supplemented with 50 mg l-Lys/L.
Saccharomyces cerevisiae Δlys5 mutant BO4421 (Mat a, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, YGL154c:kanMX4) and the reference strain BY4741 (Mat a, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) (Euroscarf) were grown at 30°C and 150 rpm in liquid YPD complete or SD minimal medium or on corresponding solidified media (Sambrook et al., 1989). BO4421 was grown in the presence of 50 mg l-Lys/L.
Fourteen-day-old whole maize (Zea maize cv Nathan) plants, leaf segments (1st to 3rd leaf, depending on the experiment) or epidermal cell layers from onion bulbs (Allium cepa cv Grano) were used to assess virulence of CgM2 and transformants. Inoculation was performed with 10-μL droplets containing 103 conidia in 0.01% (v/v) Tween 20. To inoculate wounded leaves, single pricks (DB Micro-Fine, 0.2 mm 33G Sterile Lancets; Becton Dickinson) were inserted immediately prior to inoculation. Leaf segments (8 cm) were incubated in sealed Petri dishes in a BOD 400 incubator (Uni Equip) in darkness at 23°C. Symptoms were photographed 6 DAI. Alternatively, 0.5 g mycelium of 10-d-old liquid CM cultures was washed with distilled water twice and fragmented in 15 mL sterile 0.01% (v/v) Tween 20 using an Ultra Turrax (IKA Labortechnik). Maize plants were sprayed with mycelial fragment suspensions. Mock inoculation was performed using sterile 0.01% (v/v) Tween 20. Plants were incubated in a Percival AR-75L growth chamber (12 h light; 200 μE; 70% relative humidity; 25°C). Anthracnose lesions were photographed 6 DAI.
To quantify conidiation, 10 infected leaf segments of each category were washed vigorously 6 DAI in 2 mL 0.01% (v/v) Tween 20, and conidia were counted in a Thoma chamber.
Rice plants (Oryza sativa cv CO-39) were grown as described (Hof et al., 2007). Detached leaf assays were performed with 10- to 15-cm-long segments of leaves from 21-d-old rice plants. Wounded and nonwounded leaf fragments were inoculated with washed mycelial plugs and incubated in sealed Petri dishes under rice growth conditions for 7 d.
All experiments were performed in triplicate.
Extraction of RNA and Construction of a C. graminicola cDNA Expression Library in Yeast
Total RNA was extracted from melanized mycelium (Chirgwin et al., 1979); mRNA was isolated using the Nucleotrap mRNA Purification Kit (BD Biosciences Clontech), and cDNA synthesis was performed using the Creator SMART cDNA Library Construction Kit (Clontech).
The yeast cDNA expression vector pAG300 (www.addgene.org) was constructed by inserting a SfiI cassette into the HindIII + XhoI digested plasmid pVT102U (Vernet et al., 1987). The SfiIA and SfiIB sites allowed unidirectional cloning of the cDNA library. The C. graminicola cDNA library, pcfwA (pAG300 containing the cfwA cDNA of Aspergillus nidulans), or empty vector (pAG300) were transformed into S. cerevisiae strain BO4421 using the lithium acetate procedure (Becker and Lundblad, 2001). Cells were grown on synthetic dextrose agar lacking uracil and Lys.
Targeted Inactivation of PPT1 and AAR1 of C. graminicola and of PPT1 of M. oryzae and Complementation of C. graminicola Δppt1 Strains
The complete genomic PPT1 sequence of C. graminicola, including 1232 bp of the 5′-region, was obtained by genome walking (Liu and Baird, 2001).
The Eco81I-hph cassette from pUC ATPH SI (Lu et al., 1994) was used to replace the 182-bp Eco81I fragment of C. graminicola PPT1. The resulting 4710-bp PPT1 knockout (KO) construct had 1430-bp 5′ and 709-bp 3′ homologous regions flanking the hph gene. Transformation of the KO construct into conidial protoplasts was done as described (Werner et al., 2007).
The 3.1-kb complementation vector consisted of 1232 bp of the PPT1 promoter region, the complete coding region, and the trpC terminator of A. nidulans. Transformants were plated out on selection medium [1 M sucrose, 1.7 g/L yeast nitrogen base without amino acids (Becton Dickinson), 5 g/L (NH4)2SO4, and 1.5% (w/v) agarose] that did not allow growth of Lys auxotrophs.
The AAR KO construct was assembled by double-joint-PCR (Yu et al., 2004). First, flanking regions were amplified with primers CgAARKO5′1, 5′-AAAGGGTGTTCGGTCCTCTAC-3′, and CgAARKO5′2, 5′-GATTCTATAGGAAGATCCAGGCACCGGTCATTGATCGTAGGTGCTCCGAAC-3′, and primers CgAARKO3′1, 5′-GAGGGCAAAGGAATAGAGTAGATGCCGACCTGGCGCTTTGGTCTAAGTAAC-3′, and CgAAR KO3′2, CTCCGAGACCTAGGCTAGTAAAG-3′ (sequence overlaps with the hph cassette are underlined). The hph cassette from vector pAN7-1 was amplified with primers hph1, 5′-TGACCGGTGCCTGGATCTTCCTATAGAATC-3′, and hph2, 5′-GGTCGGCATCTACTCTATTCCTTTGCCCTC-3′. In a second PCR, the products of the first step amplified in separate reactions were fused, and the complete product was amplified using nested primers CgAARKOnes1 (5′-CCATCCGTGTGGAGGAGTTG-3′) and CgAARKOnes2 (5′-AGGGAGAGGGCATACTTGACTG-3′). Transformation of the KO construct into conidial protoplasts was done as described (Werner et al., 2007).
To delete the PPT1 gene of M. oryzae, a 3135-bp DNA fragment, including the PPT1 open reading frame and ∼1000 bp upstream and downstream flanking regions, was amplified by PCR using primers MoPPT1KO1 (5′-CCCCAATACGTAGCACTA-3′) and MoPPT1KO2 (5′-GTGTTACGGATAAAACGACCG-3′) and cloned into pGEM-T Easy (Promega). An 870-bp fragment (−60 bp to 810 bp relative to ATG) was released by restriction digestion with Eco81I/StuI and replaced by the 2.6-kb hph cassette from vector pAN7-1 (Punt et al., 1987). The complete KO construct was released by NotI digestion and cloned into the PspOMI site of vector pCAMBIA0380 (GenBank accession number AF234290.1).
Agrobacterium tumefaciens–mediated transformation was performed essentially as described (Hof et al., 2007). Media were supplemented with 50 mg l-Lys/L to allow growth of Lys auxotrophs.
Expression of the PPT1:eGFP Fusion Construct in C. graminicola
The DNA sequence upstream of PPT1 was determined by genome walking (Liu and Baird, 2001).
A PCR with primers EGFP1 (5′-TTACCGGTTTGTTGGGGTCTCAAAAGGATG-3′; AgeI site underlined) and EGFP2 (5′-TTTTCGAAACTTTGATGACTCTCCCCAA-3′; Bsp 119I site underlined) yielded a 2329-bp fragment containing the PPT1 open reading frame and 1.2 kb of the promoter region. Cloning into pGEM-T Easy (Promega) resulted in plasmid pPPTfl. The unique Bsp119I site was then used to fuse a 1.5-kb PCR fragment containing the eGFP open reading frame and the TrpC terminator (primers EGFP3, 5′-TTTTCGAAATGGTGAGCAAGGGCGAG-3′, Bsp119I site underlined, and EGFP4, 5′-TTTTCGAACGCAGGACAATCGCTACAG-3′; Bsp119I site underlined) from vector pSH1.51EGFP in frame with PPT1. To introduce a resistance marker, the 2.4-kb hph cassette of pHPHAgeI (i.e., pCR2.1; Invitrogen), containing the hph gene flanked by AgeI sites, was excised and cloned into the corresponding AgeI site of the eGFP fusion vector.
The complete 5.94-kb PPT1:eGFP fusion construct was excised from pGEM-T Easy by NotI digestion, purified by gel elution, and transferred into conidial protoplasts as described (Werner et al., 2007).
DNA Extraction and Genomic DNA Gel Blot Analysis
Genomic DNA was isolated from 500 mg of vegetative mycelia of C. graminicola, following the method described (Döbbeling et al., 1997).
To perform genomic DNA gel blot analyses, 10 μg of PstI-digested DNA was separated on a 0.8% (w/v) agarose gel in TAE buffer, depurinated, and blotted onto a positively charged nylon membrane (Hybond-N+; Amersham Pharmacia Biotech) by downward alkaline capillary transfer (Brown, 1999). Specific primers CgPPT probe1 (5′-AACGTCTCAACCTCCCCC-3′), CgPPT probe2 (5′-ACGCTACAGGCAAGATCG-3′), and plasmid pPPTfl template DNA were used to generate a 511-bp alkali-labile DIG-dUTP–labeled probe (Roche Diagnostics).
To analyze transformants for the presence/absence of C. graminicola AAR1, 5 μg of Eco81I-digested genomic DNA was separated and blotted as described above. Specific primers Cg-SQ_7 (5′-ACAGCGGTCATTGACTGGAG-3′) and CgAAR-probe2-rev (5′-GTGGTGAGAGGTGCAGACAAG-3′) and the KO construct as PCR template were used to amplify a 1000-bp DIG-dUTP–labeled probe.
M. oryzae PPT1 deletions were identified using a specific 400-bp probe amplified from genomic DNA with primers MoPPT1 probe forward (5′-AGAGACTTGCAGGGACTCC-3′) and MoPPT1 probe reverse (5′-CTCTTCACCTCTGCATTCG-3′). Five micrograms of XhoI-digested genomic DNA per lane was used for DNA gel blot analysis.
Hybridization and probe detection followed the recommended protocol (Roche Diagnostics). The membrane was exposed to Hyperfilm ECL X-ray film (Amersham Pharmacia Biotech).
RT-PCR and Quantitative PCR
Gene-specific primers used to analyze expression of the PPT1 gene were PPTORF1 (5′-ATGGCCCCGACTATCATACAGTGG-3′) and PPTORF2 (5′-CTAACTTTGATGACTCTCCCCAAAAG-3′). Primers for the constitutively expressed chitin synthase gene CHSII were CHSII1 and CHSII3 (Werner et al., 2007). One-hundred nanograms of total RNA (for PPT1) or 50 ng (for CHSII) pretreated with RQ1 RNase-free DNase (Promega) were used in 10-μL RT-PCR reactions (One Step RT-PCR Kit; Qiagen). cDNA synthesis was performed at 50°C for 30 min. Cycling conditions consisted of an initial denaturation step (95°C, 3 min), followed by 40 cycles of denaturation (95°C, 30 s), annealing (60°C, 20 s), and extension (72°C, 50 s).
Quantitative PCR was performed using a MyiQ-Single Color Real-Time PCR Detection System (Bio-Rad Laboratories) equipped with iQ5 standard edition software (version 2.0.148.60623). DNA was isolated as described above with an additional phenol-chloroform extraction and precipitated (SureClean solution; Bioline).
Reactions contained 100 ng template DNA, 100 nM of each primer (CgGPD-q1, 5′-TGAACGCGAGCTAACTTGACA-3′; CgGPD-q2, 5′-GGGCATCGAAGATGGAGGA-3′), and 10 μL iQ SYBR Green Supermix (Bio-Rad Laboratories) in a final volume of 20 μL. Cycling conditions consisted of an initial denaturation (95°C, 3 min), followed by 40 cycles of denaturation (95°C, 30 s), annealing (63°C, 20 s), and extension (72°C, 15 s). Melting curve and agarose gel analyses indicated amplification of a single product. PCR efficiency was calculated using the LinRegPCR software version 7.2 (Ramakers et al., 2003). CT values of three independent replicates were used to calculate average values and standard deviations. All values are standardized to the average threshold cycle value obtained with DNA extracted from nonwounded leaves inoculated with the C. graminicola wild-type strain at 0 HAI.
α-Aminoadipate Reductase Activity (EC 1.2.1.31)
Yeast strains (BY4741, the Δlys5 mutant strain BO4421, and BO4421 transformants TcfwA, TAG2, TAG5, and TpAG300) were grown in YPD medium to the logarithmic growth phase (2 × 107 cells/mL), harvested by centrifugation (5 min, 5000g, 4°C), resuspended in 250 μL 75 mM Tris-HCl buffer, pH 8.0, and disrupted in 2.0-mL reaction tubes (Eppendorf) containing glass beads (425 to 600 μm; Sigma-Aldrich) by vortexing at 4°C for 10 min. After centrifugation (20,500g, 10 min, 4°C), supernatants were used to determine α-aminoadipate reductase activity by spectrophotometry at a wavelength of 340 nm. Reaction mixtures contained 75 mM Tris-HCl, pH 8.0, 2.5 mM DTT, 10 mM MgCl2, 10 mM l-α-aminoadipate, 10 mM ATP, 0.4 mM NADPH, and 1 mg crude protein extract/mL. Consumption of NADPH was used to quantify enzyme activity. Assays were performed at 30°C. Degradation of NADPH in the absence of α-aminoadipate served as reference.
Protein concentrations were determined with the Bio-Rad protein assay and γ-globulin as standard.
Purification of (His)6-PPT1 and the PKS100 Fragment and the 4′-Phosphopantetheinylation Assay
The C. graminicola PPT1 cDNA was cloned into the NdeI-NotI–digested expression vector pET28a (Novagen). A 300-bp PCR fragment (nucleotides 4969 to 5268) of PKS1 (Sugui and Deising, 2002) covering one 4′-phosphopantetheinylation site was cloned into the EcoRI- and NotI-digested vector pGEX-4T-1 (GE Healthcare). The plasmids were transformed into Escherichia coli BL21 DE3 (Studier and Moffatt, 1986), and expression of recombinant proteins and cell lysis were performed as described (Smith and Corcoran, 1994). PPT1 and PKS1 were N-terminally fused to a (His)6 tag and GST, respectively, separated by a thrombin cleavage site.
Proteins were purified using the ÄKTAprime system (GE Healthcare) at 4°C. The cleared cell lysate containing (His)6-PPT1 was loaded onto a HiTrap DEAE FF column equilibrated with 20 mM Tris/HCl, pH 8.0, and 1 mM DTT. The flow-through was loaded onto a HisTrap FF column equilibrated with 20 mM HEPES/NaOH, pH 7.5, 500 mM NaCl, and 20 mM imidazole. The bound (His)6-PPT1 was eluted with a linear gradient of 0 to 500 mM imidazole in the same buffer. The cell lysate containing GST-PKS100 was loaded onto a GSTrap HP column equilibrated with 20 mM HEPES/NaOH, pH 7.5, and 50 mM NaCl and eluted in the same buffer supplemented with 10 mM reduced l-glutathione (Sigma-Aldrich). Fractions containing the affinity-tagged proteins were pooled, concentrated (Amicon Ultra; Millipore), and subjected to gel filtration on a HiLoad 16/60 Superdex 75 pg column in 20 mM HEPES/NaOH, pH 7.5, 1 mM DTT, and 10 mM MgCl2. Finally, (His)6-PPT1 and GST-PKS100 were concentrated and stored at −80°C.
Protein 4′-phosphopantetheinylation reactions were performed with fluorophore-conjugated CoA-488 (Covalys) essentially as described (Yin et al., 2005). Labeling reactions were analyzed by SDS-PAGE. Fluorescence was detected using a FLA-3000 phosphor imager (Fujifilm).
For MALDI-TOF MS (Ultraflex II; Bruker Daltonics) analyses, gel slices were treated with iodoacetamide followed by a tryptic digest (Zhbanko et al., 2005).
Analysis of Fungal Metabolites
C. graminicola CgM2 was grown in HMG medium (10 g/L malt extract, 4 g/L yeast extract, and 10 g/L glucose) in a 20-liter fermenter (Biolafitte) at 22 to 24°C with agitation (130 rpm) and aeration (3 L/min). Fermentation was stopped after 5 d, when the medium had been depleted of glucose. The mycelium was separated from the culture fluid by filtration (200 × 200-mm Tiefenfilter; Pall). The culture fluid was extracted with ethyl acetate, yielding 1.8 g crude extract, which was loaded onto a column containing silica gel (Merck 60) and eluted with 100% cyclohexane followed by cyclohexane/ethylacetate (9:1, 3:1, and 1:1, v/v), followed by ethylacetate and ethylacetate/methanol (3:1 and 1:1, v/v).
Preparative HPLC (Nucleosil-7 C18; RP 18, 250 × 10 mm, 7 μm; Macherey-Nagel) with a MeCN gradient (10 to 90%) over 40 min and a flow of 20 mL/min yielded compounds 1 to 12 (Figure 3).
Extraction and analysis of siderophores were done as described (Antelo et al., 2006; Meister et al., 2007).
Spectroscopy Methods for Compound Identification
Optical rotations were measured with a Krüss P8000 polarimeter at 589 nm. UV and IR spectra were measured with a Perkin-Elmer Lambda-16 spectrophotometer and a Bruker IFS48 FTIR spectrometer, respectively. NMR spectra were recorded with a Bruker Avance II spectrometer (400 MHz); the chemical shifts were referenced to the residual solvent signal (CDCl3: δH = 7.26 ppm, δC = 77.16 ppm, CD3OD: δH = 3.31 ppm, δC = 49.00 ppm, DMSO-d6 δH = 2.50 ppm, δC = 39.52 ppm, MeCN-d3: δH = 1.94 ppm). APCIMS spectra were measured with a Hewlett Packard MSD1100 instrument. ESI-HRMS spectra were recorded on a MicroMass/Waters ESI Q-TOF mass spectrometer equipped with a LockSpray interface using NaI/CsI or trialkylamines as external reference. FAB-HRMS spectra were measured with a VG70S (Xe-FAB ionization) using m-nitrobenzyl alcohol or glycerol as matrix and PEG 300 or 600 as the reference.
Microscopy
Fluorescence microscopy was performed on a Nikon Eclipse 90i confocal laser scanning microscope (Nikon) with the following settings: excitation wavelength, 488 nm; laser light transmittance, 25% (ND4 in, ND8 out); pinhole diameter, 30 μm; lens, Plan Apo 60/1.4 oil lens.
Bright-field and interference contrast microscopy were performed with a Nikon Eclipse E600 microscope as described (Werner et al., 2007). Incipient cytorrhizis was performed with different concentrations of polyethylene glycol 6000 (PEG 6000) as described (Howard et al., 1991). Acid fuchsin and anilin blue staining were performed as described (de Neergaard, 1997). Digital images were taken with a Cyber-shot DSC-W35 camera (Sony). Image processing was done with Adobe Photoshop software.
Statistics
Calculations (t test, analysis of variance) were done with the software XLSTAT version 2009.4.02 (Addinsoft).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database under the following accession numbers: Colletotrichum graminicola PPT1, DQ028305; C. graminicola PKS1, FJ194435; and C. graminicola AAR1, FN547151. The yeast expression vector pAG300 has been deposited in the Addgene (www.addgene.org) collection (plasmid 19363).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Complementation of Growth of the S. cerevisiae Δlys5 Mutant by the PPTase Genes of A. nidulans or C. graminicola.
Supplemental Figure 2. Generation of Δppt1 Strains of C. graminicola by Homologous Recombination.
Supplemental Figure 3. Complementation of Growth of the Δppt1 Mutant of C. graminicola by the Siderophore Desferri-Ferrichrome.
Supplemental Figure 4. Detection of the Intracellular Storage Siderophore Ferricrocin in Hyphal Extracts of C. graminicola.
Supplemental Figure 5. Characteristics of Identified Compounds Produced by the Wild-Type Strain but Not by Δppt1 Strains of C. graminicola.
Supplemental Figure 6. Complementation of the Δppt1 Strains with the PPT1 Gene under Control of the Native Promoter Fully Rescues Growth Defects on Minimal Medium Lacking Lys.
Supplemental Figure 7. Generation of Δppt1 Strains of M. oryzae by Homologous Recombination.
Supplemental Figure 8. Determination of Appressorial Turgor Pressure by Incipient Cytorrhizis with PEG 6000.
Supplemental Figure 9. Generation of Δaar1 Strains of C. graminicola by Homologous Recombination.
Supplemental Table 1. 1H (400 MHz) and 13C (101 MHz) NMR Data of Colletopyrone B (CD3OD) and Colletopyrone C (CDCl3).
Supplemental Table 2. 1H (400 MHz) and 13C (101 MHz) NMR Data of Colletoquinone A (DMSO-d6 + 30% CD3OD) and Colletoquinone B (CDCl3).
Supplemental Table 3. 1H (400 MHz) and 13C (101 MHz) NMR Data of Colletoanthrone A (DMSO-d6).
Supplemental Table 4. 1H (400 MHz) and 13C (101 MHz) NMR Data of Colletolactone A (CDCl3).
Supplemental References.
Supplementary Material
Acknowledgments
Financial support by Volkswagenstiftung in the frame of the partnership program to J.A. and H.B.D. (Grant I/79 487) and by the Excellence Initiative of the State of Saxony-Anhalt to H.B.D. is gratefully acknowledged. Grant C01-1713 form SAGARPA-CONACYT to J.A. is also acknowledged. We thank A. Beutel, D. Jany, E. Vollmer, J.L. Ramos, and O. Sánchez for excellent technical support, D. Deising for artwork (Figure 10), A. Meffert and A. Schierhorn for HPLC/MS and MALDI-TOF MS analyses, H. Kolshorn and V. Sinnwell for NMR spectroscopy, N. Hanold and S. Franke for high-resolution mass spectrometry, A. Schüffler for helpful advice on the isolation of metabolites, W. Kummer and A. Mickel for help with microscopy, and J.-J. Krijger, H. Anke and T. Anke for stimulating discussions and support.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Holger B. Deising (holger.deising@landw.uni-halle.de).
Online version contains Web-only data.
References
- Antelo, L., Hof, C., Welzel, K., Eisfeld, K., Sterner, O., and Anke, H. (2006). Siderophores produced by Magnaporthe grisea in the presence and absence of iron. Z. Naturforsch. C 61 461–464. [DOI] [PubMed] [Google Scholar]
- Baker, S.E., Kroken, S., Inderbitzin, P., Asvarak, T., Li, B.Y., Shi, L., Yoder, O.C., and Turgeon, B.G. (2006). Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol. Plant Microbe Interact. 19 139–149. [DOI] [PubMed] [Google Scholar]
- Bechinger, C., Giebel, K.-F., Schnell, M., Leiderer, P., Deising, H.B., and Bastmeyer, M. (1999). Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 285 1896–1899. [DOI] [PubMed] [Google Scholar]
- Becker, D.M., and Lundblad, V. (2001). Introduction of DNA into yeast cells. In Current Protocols in Molecular Biology, F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds (New York: John Wiley & Sons), pp. 13.17.11–13.17.10. [DOI] [PubMed]
- Bergstrom, G.C., and Nicholson, R.L. (1999). The biology of corn anthracnose. Knowledge to exploit for improved management. Plant Dis. 83 596–608. [DOI] [PubMed] [Google Scholar]
- Birren, B., Fink, G., and Lander, E. (2002). Fungal Genome Initiative: White Paper Developed by the Fungal Research Community. (Cambridge, MA: Whitehead Institute Center for Genome Research).
- Brown, T. (1999). Southern blotting. In Current Protocols in Molecular Biology, F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds (New York: John Wiley & Sons), pp. 2.9.1–2.9.15.
- Chapman, D.J., and Leech, R.M. (1979). Changes in pool sizes of free amino-acids and amines in leaves and plastids of Zea mays during leaf development. Plant Physiol. 63 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., and Rutter, W.J. (1979). Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18 5294–5299. [DOI] [PubMed] [Google Scholar]
- Collemare, J., Billard, A., Böhnert, H.U., and Lebrun, M.H. (2008). Biosynthesis of secondary metabolites in the rice blast fungus Magnaporthe grisea: The role of hybrid PKS-NRPS in pathogenicity. Mycol. Res. 112 207–215. [DOI] [PubMed] [Google Scholar]
- De Jong, J.C., McCormack, B.J., Smirnoff, N., and Talbot, N.J. (1997). Glycerol generates turgor in rice blast. Nature 389 244–245. [Google Scholar]
- de Neergaard, E. (1997). Methods in Botanical Histopathology. (Copenhagen, Denmark: Kandrups Bogtrykkeri).
- Dean, R.A., et al. (2005). The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434 980–986. [DOI] [PubMed] [Google Scholar]
- Deising, H.B., Werner, S., and Wernitz, M. (2000). The role of fungal appressoria in plant infection. Microbes Infect. 2 1631–1641. [DOI] [PubMed] [Google Scholar]
- Desjardins, A.E., Manandhar, G., Plattner, R.D., Maragos, C.M., Shrestha, K., and McCormick, S.P. (2000). Occurrence of Fusarium species and mycotoxins in nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J. Agric. Food Chem. 48 1377–1383. [DOI] [PubMed] [Google Scholar]
- Döbbeling, U., Böni, R., Häffner, A., Dummer, R., and Burg, G. (1997). Method for simultaneous RNA and DNA isolation from biopsy material, culture cells, plants and bacteria. Biotechniques 22 88–90. [DOI] [PubMed] [Google Scholar]
- Ehmann, D.E., Gehring, A.M., and Walsh, C.T. (1999). Lysine biosynthesis in Saccharomyces cerevisiae: Mechanism of alpha-aminoadipate reductase (Lys2) involves posttranslational phosphopantetheinylation by Lys5. Biochemistry 38 6171–6177. [DOI] [PubMed] [Google Scholar]
- Eichhorn, H., Lessing, F., Winterberg, B., Schirawski, J., Kämper, J., Müller, P., and Kahmann, R. (2006). A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 18 3332–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtlscherer, F., Wellein, C., Mittag, M., and Schweizer, E. (2000). A novel function of yeast fatty acid synthase. Subunit alpha is capable of self-pantetheinylation. Eur. J. Biochem. 267 2666–2671. [DOI] [PubMed] [Google Scholar]
- Fudal, I., Collemare, J., Böhnert, H.U., Melayah, D., and Lebrun, M.H. (2007). Expression of Magnaporthe grisea avirulence gene ACE1 is connected to the initiation of appressorium-mediated penetration. Eukaryot. Cell 6 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuganti, C., Mendozza, M., Joulain, D., Minut, J., Pedrocchi-Fantoni, G., Piergianni, V., Servi, S., and Zucchi, G. (1996). Biogeneration and biodegradation of raspberry ketone in the fungus Beauveria bassiana. J. Agric. Food Chem. 44 3616–3617. [Google Scholar]
- Gay, A., Zimmermann, G., Nowara, D., Kumlehn, J., Schweizer, P., and Hensel, G. (2008). Triple RNAi constructs for broad-range resistance against fungal leaf diseases. Book of Abstracts, 9th International Congress of Plant Pathology 2008, Torino, Italy. J. Plant Pathol. 90 (suppl.), 267–268. [Google Scholar]
- Greenshields, D.L., Liu, G., Feng, J., Selvaraj, G., and Wei, Y. (2007). The siderophore biosynthetic gene SID1, but not the ferroxidase gene FET3, is required for full Fusarium graminearum virulence. Mol. Plant Pathol. 8 411–421. [DOI] [PubMed] [Google Scholar]
- Guzmán-de-Peña, D., Aguirre, J., and Ruiz-Herrera, J. (1998). Correlation between the regulation of sterigmatocystin biosynthesis and asexual and sexual sporulation in Emericella nidulans. Antonie Van Leeuwenhoek 73 199–205. [DOI] [PubMed] [Google Scholar]
- Haas, H., Eisendle, M., and Turgeon, B.G. (2008). Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 46 149–187. [DOI] [PubMed] [Google Scholar]
- Han, Y.-J., and Han, D.-M. (1993). Isolation and characterization of null pigment mutant in Aspergillus nidulans. Korean J. Genet. 15 1–10. [Google Scholar]
- Henson, J.M., Butler, M.J., and Day, A.W. (1999). The dark side of the mycelium: Melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 37 447–471. [DOI] [PubMed] [Google Scholar]
- Hof, C., Eisfeld, K., Welzel, K., Antelo, L., Foster, A.J., and Anke, H. (2007). Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol. Plant Pathol. 8 163–172. [DOI] [PubMed] [Google Scholar]
- Howard, R.J., and Ferrari, M.A. (1989). Role of melanin in appressorium function. Exp. Mycol. 13 403–418. [Google Scholar]
- Howard, R.J., Ferrari, M.A., Roach, D.H., and Money, N.P. (1991). Penetration of hard substances by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA 88 11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszenman-Pereyra, D., Lawrence, S., Twfieg, M.E., Price, J., and Turner, G. (2003). The npgA/cfwA gene encodes a putative 4'-phosphopantetheinyl transferase which is essential for penicillin biosynthesis in Aspergillus nidulans. Curr. Genet. 43 186–190. [DOI] [PubMed] [Google Scholar]
- Kopecká, M., and Gabriel, M. (1995). Actin cortical cytoskeleton and cell wall synthesis in regenerating protoplasts of the Saccharomyces cerevisiea actin mutant DBY 1693. Microbiology 141 1289–1299. [DOI] [PubMed] [Google Scholar]
- Krijger, J.-J., Horbach, R., Behr, M., Schweizer, P., Deising, H.B., and Wirsel, S.G.R. (2008). The yeast signal sequence trap identifies secreted proteins of the hemibiotrophic corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 21 1325–1336. [DOI] [PubMed] [Google Scholar]
- Kroken, S., Glass, N.L., Taylor, J.W., Yoder, O.C., and Turgeon, B.G. (2003). Phylogenomic analysis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proc. Natl. Acad. Sci. USA 100 15670–15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küster, S., Ludwig, N., Willers, G., Hoffmann, J., Deising, H.B., and Kiesow, A. (2008). Thin PTFE-like membranes allow characterizing germination and mechanical penetration competence of pathogenic fungi. Acta Biomater. 4 1809–1818. [DOI] [PubMed] [Google Scholar]
- Langfelder, K., Streibel, M., Jahn, B., Haase, G., and Brakhage, A.A. (2003). Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38 143–158. [DOI] [PubMed] [Google Scholar]
- Lee, B.N., Kroken, S., Chou, D.Y., Robbertse, B., Yoder, O.C., and Turgeon, B.G. (2005). Functional analysis of all nonribosomal peptide synthetases in Cochliobolus heterostrophus reveals a factor, NPS6, involved in virulence and resistance to oxidative stress. Eukaryot. Cell 4 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann, B., Mühleisen, T.W., Müller, M., Hecht, M., Weidner, G., Braun, A., Brock, M., and Brakhage, A.A. (2004). Deletion of the Aspergillus fumigatus lysine biosynthesis gene lysF encoding homoaconitase leads to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis. Arch. Microbiol. 181 378–383. [DOI] [PubMed] [Google Scholar]
- Liu, X., and Baird, W.V. (2001). Rapid amplification of genomic DNA ends by NlaIII partial digestion and polynucleotide tailing. Plant Mol. Biol. Rep. 19 261–267. [Google Scholar]
- Lu, S., Lyngholm, L., Yang, G., Bronson, C., Yoder, O.C., and Turgeon, B.G. (1994). Tagged mutations at the Tox1 locus of Cochliobolus heterostrophus by restriction enzyme-mediated integration. Proc. Natl. Acad. Sci. USA 91 12649–12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, V.A., Hardison, L.K., and Ciuffetti, L.M. (2007). Ptr ToxA interacts with a chloroplast-localized protein. Mol. Plant Microbe Interact. 20 168–177. [DOI] [PubMed] [Google Scholar]
- Marquez-Fernandez, O., Trigos, A., Ramos-Balderas, J.L., Viniegra-Gonzalez, G., Deising, H.B., and Aguirre, J. (2007). Phosphopantetheinyl transferase CfwA/NpgA is required for Aspergillus nidulans secondary metabolism and asexual development. Eukaryot. Cell 6 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, J., Weber, D., Martino, V., Sterner, O., and Anke, T. (2007). Phomopsidone, a novel depsidone from an endophyte of the medicinal plant Eupatorium arnottianum. Z. Naturforsch. C 62 11–15. [DOI] [PubMed] [Google Scholar]
- Mofid, M.R., Finking, R., and Marahiel, M.A. (2002). Recognition of hybrid peptidyl carrier proteins/acyl carrier proteins in nonribosomal peptide synthetase modules by the 4'-phosphopantetheinyl transferases AcpS and Sfp. J. Biol. Chem. 277 17023–17031. [DOI] [PubMed] [Google Scholar]
- Money, N.P., Caesar-TonThat, T.C., Frederick, B., and Henson, J.M. (1998). Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomyces graminis var. graminis. Fungal Genet. Biol. 24 240–251. [DOI] [PubMed] [Google Scholar]
- Mootz, H.D., Schorgendorfer, K., and Marahiel, M.A. (2002). Functional characterization of 4'-phosphopantetheinyl transferase genes of bacterial and fungal origin by complementation of Saccharomyces cerevisiae lys5. FEMS Microbiol. Lett. 213 51–57. [DOI] [PubMed] [Google Scholar]
- Neville, C., Murphy, A., Kavanagh, K., and Doyle, S. (2005). A 4'-phosphopantetheinyl transferase mediates non-ribosomal peptide synthetase activation in Aspergillus fumigatus. ChemBioChem 6 679–685. [DOI] [PubMed] [Google Scholar]
- Nosanchuk, J.D., and Casadevall, A. (2003). The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5 203–223. [DOI] [PubMed] [Google Scholar]
- Oberegger, H., Eisendle, M., Schrettl, M., Graessle, S., and Haas, H. (2003). 4'-Phosphopantetheinyl transferase-encoding npgA is essential for siderophore biosynthesis in Aspergillus nidulans. Curr. Genet. 44 211–215. [DOI] [PubMed] [Google Scholar]
- Oide, S., Moeder, W., Krasnoff, S., Gibson, D., Haas, H., Yoshioka, K., and Turgeon, B.G. (2006). NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell 18 2836–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaccione, D.G., Scott-Craig, J.S., Pocard, J.-A., and Walton, J.D. (1992). A cyclic peptide synthetase gene required for pathogenicity of the fungus Cochliobolus carbonum on maize. Proc. Natl. Acad. Sci. USA 89 6590–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt, P.J., Oliver, R.P., Dingemanse, M.A., Pouwels, P.H., and van den Hondel, C.A. (1987). Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56 117–124. [DOI] [PubMed] [Google Scholar]
- Ramakers, C., Ruijter, J.M., Lekanne Deprez, R.H., and Moorman, A.F.M. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339 62–66. [DOI] [PubMed] [Google Scholar]
- Rasmussen, J.B., and Hanau, R.M. (1989). Exogenous scytalone restores appressorial melanization and pathogenicity in albino mutants of Colletotrichum graminicola. Can. J. Plant Pathol. 11 349–352. [Google Scholar]
- Rohe, M., Gierlich, A., Hermann, H., Hahn, M., Schmidt, B., Rosahl, S., and Knogge, W. (1995). The race-specific elicitor, NIP1, from the barley pathogen, Rhynchosporium secalis, determines avirulence on host plants of the Rrs1 resistance genotype. EMBO J. 14 4168–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sawada, H., Sugihara, M., Takagaki, M., and Nagayama, K. (2004). Monitoring and characterization of Magnaporthe grisea isolates with decreased sensitivity to scytalone dehydratase inhibitors. Pest Manag. Sci. 60 777–785. [DOI] [PubMed] [Google Scholar]
- Scheffer, J., Chen, C., Heidrich, P., Dickman, M.B., and Tudzynski, P. (2005). A CDC42 homologue in Claviceps purpurea is involved in vegetative differentiation and is essential for pathogenicity. Eukaryot. Cell 4 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl, M., Bignell, E., Kragl, C., Sabiha, Y., Loss, O., Eisendle, M., Wallner, A., Arst, H.N.J., Haynes, K., and Haas, H. (2007). Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 3 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, P., Novara, D., and Douchkov, D. (2006). Method for increasing a fungal resistance of transgenic plants by a host-induced suppression of a pathgogenic fungi genetic expression. International Patent Number WO 2006/097465 A2.
- Smith, D.B., and Corcoran, L.M. (1994). Expression and purification of gluthathion-S-transferase fusion proteins. In Current Protocols in Molecular Biology, F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds (New York: John Wiley & Sons), pp. 16.17.11.11–16.17.17.
- Soid-Raggi, G., Sanchez, O., and Aguirre, J. (2006). TmpA, a member of a novel family of putative membrane flavoproteins, regulates asexual development in Aspergillus nidulans. Mol. Microbiol. 59 854–869. [DOI] [PubMed] [Google Scholar]
- Steiner, U., and Oerke, E.C. (2007). Localized melanization of appressoria is required for pathogenicity of Venturia inaequalis. Phytopathology 97 1222–1230. [DOI] [PubMed] [Google Scholar]
- Studier, F.W., and Moffatt, B.A. (1986). Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189 113–130. [DOI] [PubMed] [Google Scholar]
- Sugui, J.A., and Deising, H.B. (2002). Isolation of infection-specific sequence tags expressed during early stages of maize anthracnose disease development. Mol. Plant Pathol. 3 197–203. [DOI] [PubMed] [Google Scholar]
- Takeshita, N., Yamashita, S., Ohta, A., and Horiuchi, H. (2006). Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor-like domain, perform compensatory functions that are essential for hyphal tip growth. Mol. Microbiol. 59 1380–1394. [DOI] [PubMed] [Google Scholar]
- Treco, D.A., and Lundblad, V. (1993). Basic techniques of yeast genetics. In Current Protocols in Molecular Biology, F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds (New York: John Wiley & Sons), pp. 13.11.11–13.11.17.
- Tsuji, G., Kenmochi, Y., Takano, Y., Sweigard, J., Farrall, L., Furusawa, I., Horino, O., and Kubo, Y. (2000). Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol. Microbiol. 38 940–954. [DOI] [PubMed] [Google Scholar]
- Vernet, T., Dignard, D., and Thomas, D.Y. (1987). A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52 225–233. [DOI] [PubMed] [Google Scholar]
- Walton, J.D. (2006). HC-toxin. Phytochemistry 67 1406–1413. [DOI] [PubMed] [Google Scholar]
- Weiergang, I., Dunkle, L.D., Wood, K.V., and Nicholson, R.L. (1996). Morphogenic regulation of pathotoxin synthesis in Cochliobolus carbonum. Fungal Genet. Biol. 20 74–78. [DOI] [PubMed] [Google Scholar]
- Werner, S., Sugui, J.A., Steinberg, G., and Deising, H.B. (2007). A chitin synthase with a myosin-like motor domain is essential for hyphal growth, appressorium differentiation and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola. Mol. Plant Microbe Interact. 20 1555–1567. [DOI] [PubMed] [Google Scholar]
- Wilson, R.A., and Talbot, N.J. (2009). Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 7 185–195. [DOI] [PubMed] [Google Scholar]
- Yin, J., Straight, P.D., McLoughlin, S.M., Zhou, Z., Lin, A.J., Golan, D.E., Kelleher, N.L., Kolter, R., and Walsh, C.T. (2005). Genetically encoded short peptide tag for versatile protein labeling by Sfp phosphopantetheinyl transferase. Proc. Natl. Acad. Sci. USA 102 15815–15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.H., Hamari, Z., Han, K.H., Seo, J.A., Reyes-Domínguez, Y., and Scazzocchio, C. (2004). Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41 973–981. [DOI] [PubMed] [Google Scholar]
- Zhbanko, M., Zinchenko, V., Gutensohn, M., Schierhorn, A., and Klösgen, R.B. (2005). Inactivation of a predicted leader peptidase prevents photo autotrophic growth of Synechocystis sp strain PCC 6803. J. Bacteriol. 187 3071–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.