Abstract
In Saccharomyces cerevisiae, the PMT, KRE2/MNT1, and MNN1 mannosyltransferase protein families catalyze the steps of the O-mannosylation pathway, sequentially adding mannoses to target proteins. We have identified members of all three families and analyzed their roles in pathogenesis of the maize smut fungus Ustilago maydis. Furthermore, we have shown that PMT4, one of the three PMT family members in U. maydis, is essential for tumor formation in Zea mays. Significantly, PMT4 seems to be required only for pathogenesis and is dispensable for other aspects of the U. maydis life cycle. We subsequently show that the deletion of pmt4 results in a strong reduction in the frequency of appressorium formation, with the few appressoria that do form lacking the capacity to penetrate the plant cuticle. Our findings suggest that the O-mannosylation pathway plays a key role in the posttranslational modification of proteins involved in the pathogenic development of U. maydis. The fact that PMT homologs are not found in plants may open new avenues for the development of fungal control strategies. Moreover, the discovery of a highly specific requirement for a single O-mannosyltransferase should aid in the identification of the proteins directly involved in fungal plant penetration, thus leading to a better understanding of plant–fungi interactions.
INTRODUCTION
In many fungal pathogens, a key step in plant infection is penetration of the host plant cuticle, an important physical barrier against plant pathogens. To overcome this barrier, fungi have developed different mechanisms ranging from penetration through natural openings, such as stomata, to the formation of appressoria (Howard and Valent, 1996; Dean, 1997; Talbot, 2003), which are specialized structures that use mechanisms including osmotic pressure, hydrolytic enzymes, and the generation of reactive oxygen intermediates to break into host plant tissue (Howard et al., 1991; Egan et al., 2007; Skamnioti and Gurr, 2007).
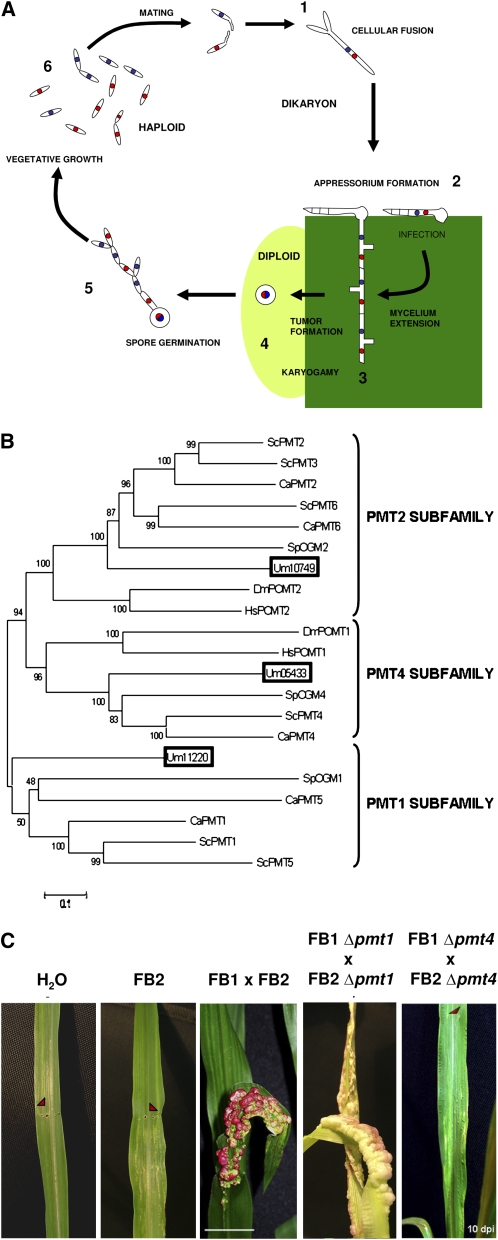
Ustilago maydis is a basidiomycete fungus with nonpathogenic haploid yeast-like and infective dikaryon filamentous stages of its life cycle. This fungus belongs to the Ustilaginales group of plant pathogens that can infect around 4000 species of angiosperms and has an important impact on agriculture. Particularly, U. maydis causes the smut disease of maize (Zea mays), one of the world's major cereal crops. This disease results in stunted plant growth leading to high economic losses (Martinez-Espinoza et al., 2002). This fact, combined with its ability to be easily cultured and genetically manipulated in the laboratory, makes U. maydis an excellent model for the molecular study of vegetal pathogenesis (Bolker, 2001; Kamper et al., 2006). In the U. maydis life cycle (Figure 1A), spore germination results in the formation of sexually differentiated, saprophytic yeast cells. Sexual compatibility is determined by the presence of two independent loci: locus a, which encodes a pheromone-receptor system, and locus b, which encodes a transcription factor composed of the bE/bW heterodimer that triggers growth of infectious hyphae (Feldbrugge et al., 2004). Mating between two sexually compatible cells leads to bE/bW complex formation, which initiates filamentation and appressorium-mediated plant penetration (Snetselaar and Mims, 1992). Once inside the plant, U. maydis colonizes plant tissues by forming hyphae, which by the end of the infection process, causes the formation of tumors in all green parts of the plant. Fungal hyphae continue to proliferate inside these tumors until finally differentiating to form diploid spores (Banuett and Herskowitz, 1996).
Figure 1.
The U. maydis PMT Family.
(A) Life cycle of U. maydis. Developmental stages of the U. maydis life cycle: 1, fusion of two haploid cells; 2, dikaryon filament invades plant cells to form the appressorium; 3, U. maydis proliferates and differentiates within tumor tissue; 4, the fungus produces diploid spores; 5, spores undergo meiosis; 6, U. maydis enters its haploid phase.
(B) Dendrogram of PMT proteins. PMT proteins of S. cerevisiae (ScPMT), S. pombe (SpOGM), C. albicans (CaPMT), D. melanogaster (DmPOMT), and H. sapiens (HsPOMT) were compared with the Um11220, Um10749, and Um05433 proteins (boxed). The black bar represents an evolutionary distance of 0.1 amino acid substitutions per site. Bootstrap support values are given at branch nodes. The phylogenetic analysis showed that a single putative U. maydis PMT protein was assigned to each of the three PMT subfamilies. A text file of the alignment used to generate this dendogram is available as Supplemental Data Set 1 online.
(C) Effects of pmt4 deletion on pathogenicity. Seven-day-old maize seedlings were inoculated with water, FB2, a cross of FB1 and FB2 wild-type strains, FB1 Δpmt1 and FB2 Δpmt1, or FB1 Δpmt4 and FB2 Δpmt4. Arrows point to the injection punctures. Ten days after infection (dpi), anthocyanin production and tumor formation were observed on plants infected with the wild-type and Δpmt1 crosses, while no infection symptoms were detected on plants inoculated with the pmt4 mutant strains. Bar = 2 cm.
Understanding the molecular basis of appressorium-based plant penetration is of great interest in the development of antifungal strategies. Fungal adhesion to hard, hydrophobic surfaces and the presence of various chemical signals are required for effective appressorium formation and plant penetration (Howard et al., 1991; Lee and Dean, 1993; Gilbert et al., 1996). Cell wall glycoproteins have been identified as fungal adhesives and have been implicated in host cell adhesion in many organisms (Gaur and Klotz, 1997; Buck and Andrews, 1999; Frieman et al., 2002; Sheppard et al., 2004). These studies show that blocking access to glycoproteins with sugar group binding compounds, such as concanavalin A, interferes with the adhesive capacity of fungal cells (Buck and Andrews, 1999). Thus, current evidence suggests that protein glycosylation plays a key role in the adhesive properties of fungal cells (Buurman et al., 1998; Huang et al., 2003).
Glycosylation is an extremely common and highly conserved type of protein modification involving the addition of carbohydrate residues to target proteins (Willer et al., 2003). It plays a critical role in determining the structure and function of numerous secreted and membrane-bound proteins. This type of protein modification is highly regulated and is mediated by the activity of protein- and site-specific enzymes. There are different types of glycosylation, the most frequent being N- and O-glycosylation, which differ from each other in the amino acid residue where the carbohydrate moieties are attached, as well as in the final structure of the carbohydrate complex. In eukaryotic cells, N- and O-glycosylation occurs within the endoplasmic reticulum and Golgi apparatus and can take place as either a cotranslational or posttranslational process (Strahl-Bolsinger et al., 1999). Both pathways are highly conserved between species from prokaryotes to humans, including plants, although they can differ in the class, number, and location of carbohydrate residues added (Burda and Aebi, 1999; Willer et al., 2003).
O-mannosylation is a type of O-glycosylation absent from plants that is characterized by the addition of mannose residues to target proteins. Over the last decade, many roles have been described for fungal O-mannosylation, including in cell wall integrity, cell morphology, and the stability, sorting, and localization of proteins (Strahl-Bolsinger et al., 1999; Hirayama et al., 2008; Lommel and Strahl, 2009). The initial O-mannosyltransferase reaction is performed by protein mannose transferases (PMTs), which are integral endoplasmic reticulum membrane proteins that add the first mannose to Ser or Thr residues of target proteins. Subsequently, as O-mannosylated proteins move into the Golgi apparatus, glycosylation continues with the addition of more mannoses in yeast or a linear heterosaccharide consisting of N-acetylglucosamine, galactose, and neuraminic acid in higher eukaryotes. The latter stage of O-mannosylation in the Golgi has been poorly characterized in most species except for Saccharomyces cerevisiae. In this yeast, the KRE2/MNT1 protein family is responsible for the addition of the second and third mannose residues (Man2-Man3) (Lussier et al., 1997a, 1997b), and MNN1 family proteins attach a fourth (Man4) and possibly additional mannose residues (Romero et al., 1999).
The PMT family was initially described in S. cerevisiae (Gentzsch and Tanner, 1996) and has been shown to be widely conserved in other fungi such as Schizosaccharomyces pombe (Willer et al., 2005) or Candida albicans (Prill et al., 2005). The PMT family can be grouped into three subfamilies: PMT1, PMT2, and PMT4, with the number of members in each subfamily varying between species. In S. cerevisiae, PMT proteins can interact to form dimers: heterodimers between PMT1 (Pmt1p and Pmt5p) and PMT2 (Pmt2p and Pmt3p) subfamily members and homodimers between the sole representative of the PMT4 subfamily (Girrbach and Strahl, 2003). PMT dimers show substrate-specific enzymatic activity. In accordance with this, it has been described that some S. cerevisisae proteins, such as Kre9p, Cts1p, Bar1p, Pir2p, and Aga2p, are exclusively O-mannosylated by the heterodimer PMT1/PMT2 (Gentzsch and Tanner, 1997), while proteins such as Kex2p, Gas1p, Axl2p, and Fus1p are modified only by PMT4 (Gentzsch and Tanner, 1997; Proszynski et al., 2004). By contrast, other proteins, such as members of the WSC family, Mid2p and Ccw5p/Pir4p, are O-mannosylated by both complexes (Ecker et al., 2003; Lommel et al., 2004). In order to determine other PMT targets, in silico studies based on the general characteristics of O-mannosylated proteins have been initiated (Hutzler et al., 2007). Mutations in PMT family proteins have been linked to defects in S. pombe morphogenesis (Willer et al., 2005), reduced pathogenicity of C. albicans (Timpel et al., 2000; Prill et al., 2005) and Cryptococcus neoformans (Olson et al., 2007; Willger et al., 2009), or, in the case of humans, to the Walter-Warburg syndrome (Beltran-Valero de Bernabe et al., 2002; Lengeler et al., 2008).
Evidence suggesting a role for N-glycosylation in U. maydis infectivity has come from work that showed that an enzyme implicated in the N-glycosylation pathway, the glucosidase II GAS1, is required for fungal pathogenicity (Schirawski et al., 2005). These observations suggest that glycosylation may be important for the pathogenic development of U. maydis. However, up to now, O-mannosylation pathway enzymes have not been characterized in phytopathogenic fungi nor has the role of protein O-mannosylation in plant pathogenesis been analyzed. Here, we describe a detailed characterization of the role of the O-mannosylation pathway in fungal phytopathogenesis, using U. maydis as a model organism. We identify several putative members of the O-mannosylation pathway in U. maydis and determine which are required for plant infectivity. Significantly, we show that the loss of one of these enzymes, PMT4, affects the ability of U. maydis to form functional appressoria and blocks plant cuticle penetration. We discuss our findings and explore the potential of this pathway as a target for antifungal strategies.
RESULTS
The U. maydis PMT Family Consists of Three Members
To identify PMT family proteins encoded by the U. maydis genome, we performed a protein BLAST search at the National Center for Biotechnology (NCBI) of the 6786 proteins defined in the MIPS U. maydis protein database using S. cerevisiae PMT proteins (Pmt1p-Pmt7p) as query sequences. We found three putative PMT protein homologs: Um11220, a protein of 956 amino acids with an amino acid identity of 43% to Sc Pmt1p (172/400; in an alignment of 400 amino acids, 172 are the same at equivalent positions); Um10749, of 787 amino acids with 46% identity (328/712) to Pmt2p; and Um05433 of 777 amino acids, with 43% identity (314/721) with respect to Pmt4p (see Supplemental Table 1 online).
Hydropathy analysis (Kyte and Doolittle, 1982) of Um11220, Um10749, and Um05433 suggest that they conserve the characteristic structure of O-mannosyltransferase proteins of two large transmembrane domains, located in their N- and C-terminal regions and separated by a central hydrophilic region (Strahl-Bolsinger and Scheinost, 1999). In addition to the topological distribution, the putative U. maydis PMT proteins conserve the number of predicted transmembrane domains with respect to S. cerevisiae PMTs: 11 for Um11220 and nine for Um10749 and Um05433 (see Supplemental Figure 1 online).
Next, we analyzed our candidate proteins for the presence of two characteristic PMT motifs: the PMT domain, a region implicated in PMT complex formation and O-mannosyltransferase activity, found within the N-terminal transmembrane region, and the MIR domain (Mannosyltransferase, IP3, RyReceptor) linked solely to transferase activity and composed of submotifs A, B, and C, located in the hydrophilic central region (Girrbach et al., 2000). We found that the PMT domain is conserved in all three putative U. maydis PMT homologs with amino acid similarities of between 53 and 74% with respect to PMT domains in other organisms. Similarly, we found regions with homology to the MIR domain submotifs in all three Um PMTs (with amino acid similarity of between 48 and 64%). Um11220, Um10749, and Um05433 all possess the conserved initial Leu-His-Ser-His sequence of submotif A, while Um05433 also has a specific seven–amino acid insertion (Tyr-Asn-Asn-Gly-Arg-Ile-Ser) within submotif A, which is present in Sc Pmt4p, Sp OGM4, and Cn PMT4 (Olson et al., 2007; see Supplemental Figure 2 online).
To analyze the phylogenetic relationships between the three putative U. maydis PMTs and their homologs in other organisms, we performed phylogenetic tree analysis using CLUSTAL and MEGA (see Methods and Supplemental Data Set 1 online). Figure 1B shows a phylogenetic tree comparing S. cerevisiae, C. albicans, S. pombe, Drosophila melanogaster, and Homo sapiens PMT families together with the three putative U. maydis proteins. Our analysis grouped each of the three putative U. maydis PMTs into one of the PMT subfamilies. Thus, consistent with their hydropathy profiles, conserved sequence motifs, and our phylogenetic analysis, we can provisionally designate Um11220, Um10749, and Um05433 as PMT1, PMT2, and PMT4, respectively.
PMT4 Is Required for Virulence on Maize
To ascertain if O-mannosylation has a role in U. maydis plant pathogenesis, we generated single deletion mutants of the putative PMT family members in FB1 (a1b1) and FB2 (a2b2) wild-type strains (see Methods). Strains carrying single deletions in either pmt1 or pmt4, as well as the combined double pmt1 pmt4 mutant, were viable and showed normal growth rates and mating (Figure 3; see Supplemental Figure 3 online). By contrast, the loss of pmt2 was lethal in both FB1 and FB2 strains. The requirement of pmt2 was confirmed by spore analysis of the diploid strain FBD11 Δpmt2/pmt2 (see Supplemental Figure 3 online). Our results concur with those previously obtained for members of the PMT2 subfamily in S. cerevisiae, C. albicans, and S. pombe (Gentzsch and Tanner, 1996; Prill et al., 2005; Willer et al., 2005) and thus reaffirm the requirement of the PMT2 subfamily for fungal viability.
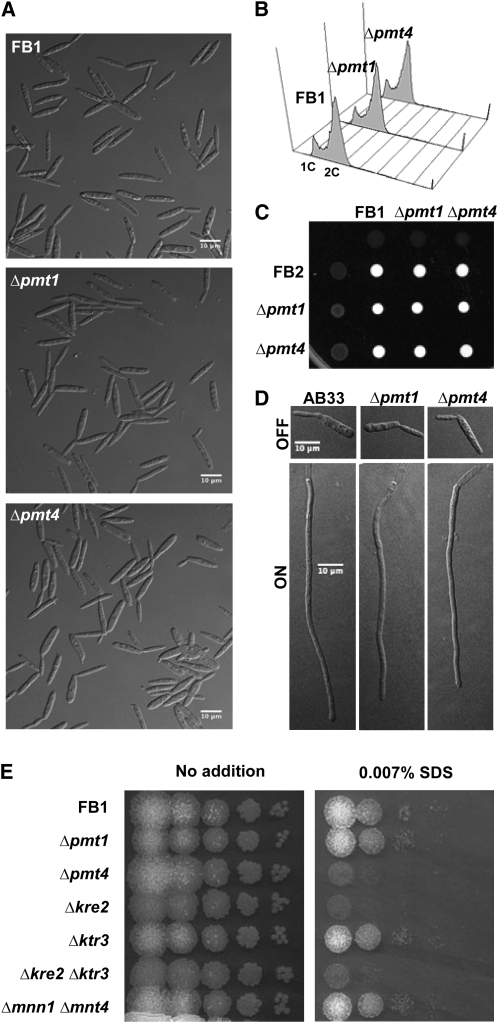
Figure 3.
U. maydis Δpmt4 Phenotypes.
(A) Cell morphology and aggregation states of wild-type (FB1), pmt1 mutant (FB1 Δpmt1), and pmt4 mutant (FB1 Δpmt4) strains. Cells were grown in 1% glucose complete medium CMD to exponential phase at 28°C.
(B) Flow cytometry analysis. The DNA content of cell populations of FB1 and FB1 Δpmt1 and FB1 Δpmt4 strains growing in CMD medium to exponential phase was measured. The relative 1C to 2C (haploid to diploid) DNA contents in pmt1 and pmt4 mutants are similar to that of the wild-type strain.
(C) Mating and dikaryont filament formation. Wild-type (FB1 and FB2), Δpmt1 (FB1 Δpmt1 and FB2 Δpmt1), and Δpmt4 (FB1 Δpmt4 and FB2 Δpmt4) strains, either as pure cultures or as a cross of compatible strains, were spotted on charcoal-containing agar plates and incubated at 25°C for 24 h. Fuzzy spots of white filaments indicate dikaryotic hyphae formation. The crosses of pmt1 and pmt4 mutants resulted in normal dikaryont formation.
(D) Polar growth. AB33 strain harboring bE/bW compatible alleles under the nar promoter and Δpmt1 and Δpmt4 derivative strains were induced to express bE/bW by switching from ammonium-containing (OFF) to nitrate-containing (ON) medium. Six hours after induction, wild-type and mutants were able to develop b-dependent filaments.
(E) SDS sensitivity of O-mannosylation pathway mutants. A series of 10-fold dilutions of FB1, Δpmt1, Δpmt4, Δkre2, Δktr3, Δkre2 Δktr3, and Δmnn1 Δmnt4 strains were spotted on YPD agar plates containing 0.007% SDS detergent and incubated at 28°C during 2 d. pmt4 and kre2 single mutants and the kre2 ktr3 double mutant showed increased sensitivity to SDS.
To test if Um PMT1 and PMT4 are required for virulence, we compared leaves from 7-d-old maize seedlings inoculated with a mixture of FB1 and FB2 wild-type strains with those inoculated with either mixtures of compatible pmt1 deletion mutants (FB1 Δpmt1 and FB2 Δpmt1) or compatible pmt4 deletion mutants (FB1 Δpmt4 and FB2 Δpmt4) (see Methods). Disease progression was evaluated 10 d after infection. The mixture of compatible Δpmt1 strains caused disease symptoms comparable to the wild-type strains (chlorosis, anthocyanin induction, and tumor formation) (Figure 1C, Table 1). By contrast, the mixture of compatible Δpmt4 strains did not show any disease symptoms, with the exception of chlorosis, which is a consequence of the inoculation process (Figure 1C). Infection assay results are shown in Table 1. Similar results were obtained 15 and 25 d after infection, ruling out the possibility that our results reflect a delay in the development of fungal pathogenesis. Significantly, we found that Δpmt4 mutants were also unable to form tumors in plants infected through the stigma, which presents a much weaker barrier to infection than leaf tissues (Snetselaar and Mims, 1993) (see Supplemental Figure 4 online).
Table 1.
Pathogenicity Assays
| Inoculum | No. of Plants | Tumor Formation (%) |
|---|---|---|
| FB1 × FB2 | 73 | 86.3 |
| FB1 Δpmt1 × FB2 Δpmt1 | 38 | 89.5 |
| FB1 Δpmt4 × FB2 Δpmt4 | 94 | 0.0 |
| FB1 × FB2 Δpmt4 | 33 | 87.9 |
| FB1 Δpmt4 × FB2 | 32 | 87.5 |
| FB1 Δkre2 × FB2 Δkre2 | 30 | 90.0 |
| FB1 Δktr3 × FB2 Δktr3 | 34 | 88.2 |
| FB1 Δkre2 Δktr3 × FB2 Δkre2 Δktr3 | 33 | 87.9 |
| FB1 Δmnn1 Δmnt4 × FB1 Δmnn1 Δmnt4 | 29 | 93.1 |
| SG200 | 84 | 91.7 |
| SG200 Δpmt4 |
75 |
0.0 |
Seven-day-old maize seedlings were inoculated with the strains indicated, and the tumor formation was scored 10 d after infection.
To test if the pmt4 alleles present in FB1 and FB2 are both functional and required for virulence, we infected plants with combinations of either FB1 Δpmt4 with the wild-type strain FB2 or FB2 Δpmt4 with the wild-type strain FB1. Infections with both combinations caused normal disease symptoms (Table 1). To exclude the possibility that our results reflect a cell fusion defect of the Δpmt4 strains, we generated a deletion of pmt4 in the solopathogenic SG200 strain (a1 mfa2 bE1bW2), which is able to infect plants without mating as it possesses both a and b loci active (Bölker et al., 1995). Deletion of pmt4 in SG200 also resulted in a total loss of infectivity (Table 1). Taken together, these results confirm that PMT4 is required for the pathogenic development of U. maydis.
Um PMT1 and PMT4 Encode Protein O-Mannosyltransferases
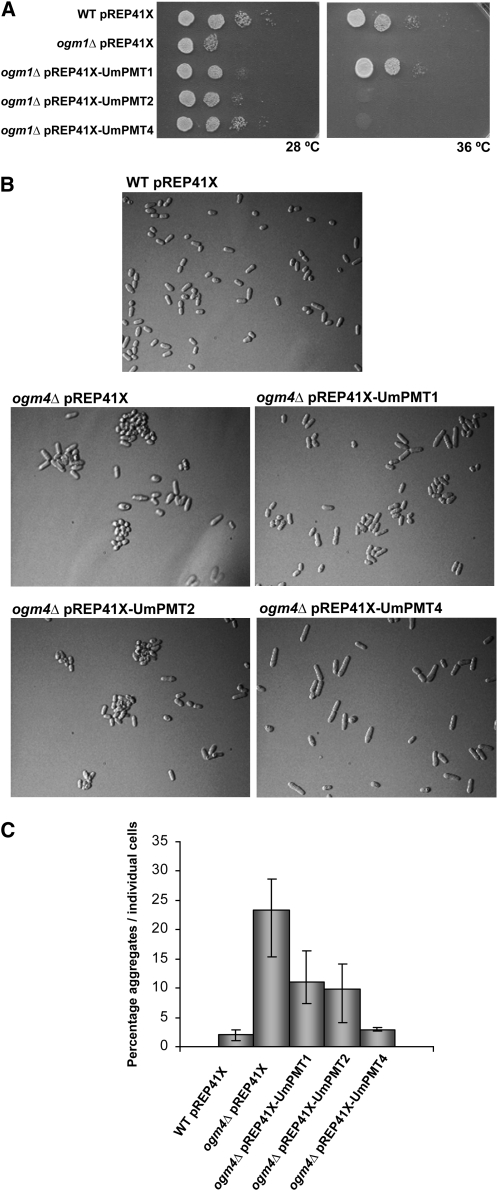
To verify the O-mannosyltransferase activity of the putative U. maydis PMT homologs, we tested them for their capacity to complement S. pombe pmt mutants ogm1Δ and ogm4Δ. For this purpose, the open reading frames of pmt1, pmt2, and pmt4 were cloned into pREP41X (LEU2 ars1+) (Maundrell, 1993) to generate the plasmids pREP41X-Umpmt1, pREP41X-Umpmt2, and pREP41X-Umpmt4 (see Methods). All three plasmids, as well as the empty vector, were transformed into S. pombe ogm1Δ, which is defective in vitro and in vivo for O-mannosyltransferase activity (Willer et al., 2005). The loss of ogm1 affects viability at 28°C and even more so at 36°C. At 28°C, U. maydis pmt1, pmt2, and pmt4 were able to partially complement the ogm1Δ mutant. However, at 36°C, only pmt1 was able to complement this phenotype, suggesting that Um PMT1 represents a functional homolog of Sp OGM1 (Figure 2A). The S. pombe ogm4Δ strain forms aggregates when grown in liquid medium. The ability of U. maydis pmt genes to complement ogm4Δ was tested by transforming the mutant ogm4Δ strain and growing transformants in EMM media at 28°C to exponential phase and then studying their cellular aggregation state by optical microscopy. Under these conditions, only pREP41X-Umpmt4 was able to completely restore the single cell growth shown by the wild-type strain (Figure 2B; see Figure 2C for quantification). These findings indicate that U. maydis PMT1 and PMT4 are functional homologs of their S. pombe equivalents, OGM1 and OGM4, respectively. Moreover, it confirms our phylogenetic-based assignment of PMT family members.
Figure 2.
U. maydis PMTs Complement S. pombe ogm1Δ and ogm4Δ Mutants.
(A) Functional complementation of S. pombe ogm1Δ. Serial dilutions of wild-type S. pombe leu1-32 strain transformed with pREP41X and the ogm1Δ strain transformed with either pREP41X (control), pREP41X-Umpmt1, pREP41X-Umpmt2, or pREP41X-Umpmt4 are shown after 3 d at 28 or 36°C. ogm1Δ cells were viable at 36°C if transformed with U. maydis pmt1. Um pmt1, pmt2, and pmt4 were able to partially complement the ogm1Δ mutant at 28°C.
(B) S. pombe ogm4Δ complementation. The ogm4Δ mutant cells form aggregates. The ogm4Δ mutants transformed with pREP41X LEU2 (empty vector) or with the vector containing either pmt1, pmt2, or pmt4 were grown to exponential phase at 28°C and photographed. pmt4 complemented the phenotype of the ogm4Δ mutant resulting in single cell growth.
(C) Quantification of pmt4 functional complementation of ogm4Δ. The quantification shows the percentage of aggregates relative to individual cells. Three independent samples were analyzed for each strain (number of cells for each sample ≥100). Error bars represent se of the mean.
PMT4 Is Required Only for the Pathogenic Phase of the U. maydis Life Cycle
The loss of PMTs from S. cerevisiae, S. pombe, and C. albicans causes several defects, such as abnormal morphology, cellular aggregation, alterations in polar growth, and hypersensitivity to antifungal drugs, among others (Proszynski et al., 2004; Prill et al., 2005; Willer et al., 2005). To determine the effects of the deletion of pmt4 on U. maydis biology, we performed an in-depth analysis of Δpmt4, looking at the different phases of the U. maydis life cycle.
The deletion of pmt4 from haploid FB1 and FB2 strains did not show any significant differences in morphology, cellular aggregation, or cell cycle progression compared with wild-type strains when grown in liquid (Figures 3A and 3B; see Supplemental Figure 5 online) or solid culture. To detect mating defects previously described in S. cerevisiae (Proszynski et al., 2004), we cocultivated a mixture of the sexually compatible strains FB1 Δpmt4 and FB2 Δpmt4 on charcoal-containing agar plates. Normal formation of dikaryon filaments, recognized as white fuzzy mycelium, was observed (Figure 3C). These results suggest that PMT4 does not have an important role in vegetative growth or mating.
To determine the role of PMT4 in polar growth, as has been described for C. albicans PMT mutants (Prill et al., 2005), we generated a pmt4 deletion in the haploid strain AB33 (Brachmann et al., 2001), which harbors the compatible bE2/bW1 genes under the control of the nitrate-inducible nar1 promoter. In nitrate-containing medium, AB33 enters the filamentation program. Under inducing conditions, AB33 Δpmt4 shows a normal polar growth in infectious hyphae when compared with the wild-type strain (Figure 3D). Thus, we can conclude that pmt4 is not required for b-dependent filament formation.
The consequences of the deletion of pmt4 on the production of secreted hydrolytic enzymes were also investigated as O-glycosylation has been shown to be required for fungal host interactions (Lehle et al., 2006). On-plate cellulase, pectinase, and amylase activity assays (see Methods) revealed no difference in the activity of these secreted enzymes between pmt4 mutant fungi and wild-type cells (see Supplemental Figure 6 online).
Finally, to establish the stress response of the Δpmt4 strain, we tested its resistance to thermal (34 and 36°C), antifungal (congo red, chlorpromazine, calcofluor white, and caffeine), oxidative (H2O2), osmotic (SDS and sorbitol), and salt-based (NaCl and CaCl2) stresses. We performed a serial dilution patch test of Δpmt4, Δpmt1, and control strains grown for 2 d at 28°C on YPD agar plates containing the compounds and conditions mentioned above. Pmt1 and pmt4 mutants did not exhibit enhanced sensitivity to any of these agents or conditions (see Supplemental Figure 7 online; data not shown) except for increased sensitivity to SDS by the Δpmt4 strain (Figure 3E). SDS sensitivity has also been observed in C. neoformans pmt4 mutants and was linked to a failure of cell wall integrity (Olson et al., 2007).
PMT4 Is the Only Putative O-Mannosyltransferase in the U. maydis Genome Essential for Pathogenesis
Our findings suggest that PMT4-mediated O-mannosylation may play a specific role in pathogenesis. In S. cerevisiae, the initial PMT-catalyzed O-mannosylation step is a prerequisite for the addition of further mannose residues by members of the KRE2/MNT1 family, which add second and third mannose residues to target proteins (Willer et al., 2003). Thus, we wanted to determine if the U. maydis equivalents of the KRE2/MNT1 family might also have a role in pathogenesis. With this aim, we searched for the putative KRE2/MNT1 family protein homologs in U. maydis using a method similar to that performed for the PMT family. The S. cerevisiae KRE2/MNT1 family comprises nine members: Kre2p, Ktr1p, Ktr2p, Ktr3p, Ktr4p, Ktr5p, Ktr6p, Ktr7p, and Yur1p. However, we found only two putative members in the U. maydis genome: Um01154 with 52% identity (in an alignment of 315 amino acids, 165 are the same at equivalent positions) with respect to Sc Kre2p; and Um01821 with 47% identity (143/290) to Sc Ktr3p (more data in Supplemental Table 1 online). We assigned Um01154 and Um01821 as putative homologs of Kre2p and Ktr3p, respectively. We generated single deletions of kre2 and ktr3 and the double Δkre2 Δktr3 mutant in FB1 and FB2 wild-type strains. To assay the virulence of these mutants, we inoculated a mixture of compatible mutants or wild-type strains on 7-d-old maize seedlings (Table 1). All mutants tested caused normal disease symptoms on plants, suggesting that U. maydis KRE2 and KTR3 are not required for virulence.
Our observation of SDS sensitivity in the U. maydis pmt4 mutant suggests that pmt4 may have a role in maintaining cell wall integrity. We were interested in knowing whether the KRE2/MNT1 family could also be involved in this process. We performed a sensitivity study of Δkre2, Δktr3, and Δkre2 Δktr3 strains to the compounds and conditions previously assayed for the PMT mutants. As shown in Figure 3E, Δkre2 mutants demonstrated enhanced sensitivity to SDS, while their reaction to other drugs was normal. As Δkre2 mutants exhibit normal infectivity, it would suggest that pmt4 mutant sensitivity to SDS is unlikely to be responsible for its pathogenic defect.
To complete the study of the U. maydis O-mannosylation pathway, we investigated the role of the MNN1 family, which adds the fourth mannose in S. cerevisiae. The Sc MNN1 family consists of six members: Mnn1p, Mnn2p, Mnn5p, Mnt2p, Mnt3p, and Mnt4p. As for the KRE2/MNT1 family, we identified only two putative members in the U. maydis genome: Um05152 with 30% (48/157) identity with respect to Mnn1p and Um05153 with 24% identity to Mnt4p (68/274), and these were therefore renamed to Um mnn1 and mnt4, respectively (see Supplemental Table 1 online). We generated single deletions of mnn1 and mnt4 and the double mutant Δmnn1 Δmnt4 in FB1 and FB2 strains. All mutants showed normal responses to the conditions previously tested for kre2 and ktr3 mutants, including SDS (double mutant in Figure 3E). To analyze the role of MNN1 family proteins in U. maydis virulence, we inoculated maize plants with mixtures of compatible mutant strains. As expected, we obtained normal tumor formation 10 d after infection (Table 1). Thus, U. maydis members of the MNN1 family are not required for pathogenesis or SDS resistance.
In summary, PMT family member PMT4 is the only identified O-mannosyltransferase enzyme that is required for U. maydis pathogenesis.
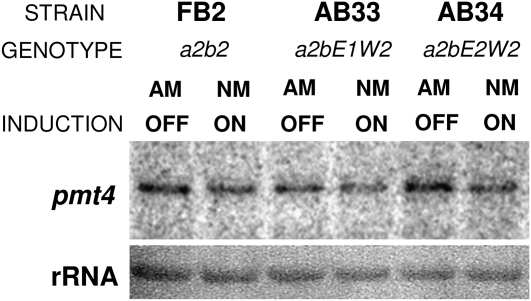
Um pmt Expression Is Not Dependent on the bE/bW Heterodimer
Our data suggest that Um pmt4 is primarily required for fungal virulence. Therefore, we looked to see if its expression, and that of other pmt family members, is regulated by the b locus–controlled pathogenicity program. We analyzed pmt gene expression by RNA gel blot. For this purpose, we used the AB33 strain, whose b-pathway becomes activated in nitrate-containing medium (Brachmann et al., 2001). We included an AB34 strain harboring the noncompatible bE2/bW2 combination (Brachmann et al., 2001) and FB2 as control strains. Our expression analysis, shown in Figure 4, suggests that pmt4 is not a b-regulated gene. Thus, we conclude that pmt4 expression is not dependent on the presence of an active bE/bW heterodimer. Analysis of pmt1 and pmt2 expression under the same conditions showed that their expression levels also did not change following b-locus activation. In addition, we analyzed pmt expression in the AB33 Δpmt4 strain because a compensatory response has been described in C. albicans pmt mutants (Cantero et al., 2007). By contrast, we found that during both yeast-like and filamentous growth of U. maydis, the expression levels of the other pmt genes are independent of the presence of pmt4 (data not shown).
Figure 4.
RNA Gel Blot Analysis of the Strains FB2, AB33, and AB34 after Induction of b-Gene Expression.
Cells of haploid FB2, AB33, and AB34 strains were grown under repressing conditions on EMM-containing ammonium (ammonium medium [AM]) as the sole nitrogen source to OD600 0.5 and then shifted to inducing conditions (nitrate medium [NM]) and incubated for 6 h. Expression was also analyzed on AM as a control. In all cases, 10 μg of total RNA were loaded per lane. 18s RNA was used as a loading control.
PMT4 Is Essential for Functional Appressorium Formation
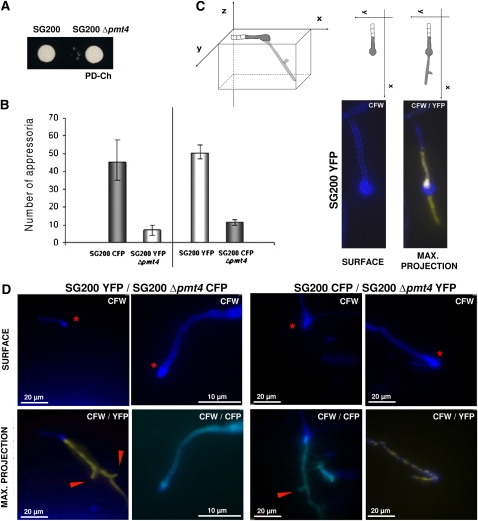
To investigate which step of pathogenic development might be affected in pmt4 mutants, we inoculated a mixture of FB1 Δpmt4 and FB2 Δpmt4 strains and observed fungal cells on the plant surface. Cells were stained with calcofluor white 15 h after plant inoculation to study filamentation and appressorium production capability. In pmt4 mutant strains, we observed normal filament formation but with a significantly lower frequency of appressorium formation. We obtained similar results comparing SG200 and SG200 Δpmt4 strains. Normal pmt4 mutant filament formation on charcoal-containing medium (see Methods) can be seen in the Figure 5A.
Figure 5.
The Deletion of pmt4 Affects Appressoria Production.
(A) Filament formation of the solopathogenic SG200 and SG200 Δpmt4 strains that were spotted on PD-charcoal (PD-Ch) plates and incubated at 25°C for 24 h. The fuzzy spots indicate filament formation.
(B) Appressoria production was reduced in pmt4 mutants. A mixture of equal numbers of cells of SG200 and SG200 Δpmt4 strains tagged with CFP and YFP under the OMA promoter were used to inoculate 7-d-old maize seedlings. After 15 h, we scored appressoria formation by scoring for cyan fluorescent protein (CFP) or yellow fluorescent protein (YFP) fluorescence. Three independent samples were analyzed. The error bars represent se. The deletion of pmt4 produced a decrease in appressoria formation.
(C) Visualization of appressorium morphology by deconvolution microscopy. Schematic XYZ drawing representing appressorium plant penetration. In the XY plane, we can see the appressorium structure on the leaf surface. Below, we showed an image without deconvolution. On the right, a series of images taken at different Z planes were reconstructed using maxim projection during deconvolution (see Methods for more details) to visualize fungal progression through plant tissue.
(D) Clamp-like cells are absent 24 h after infection in the pmt4 mutant. A mixture of equal numbers of cells for SG200 CFP and SG200 YFP Δpmt4, and SG200 YFP and SG200 CFP Δpmt4 strains were inoculated on 7-d-old maize seedlings. Twenty-four hours after infection, cells were observed by deconvolution microscopy in three channels: CFW, YFP, and CFP to identify each one of the strains. Deconvolved images of inoculated plants show that the deletion of pmt4 affects its capacity to form clamp-like cells, a structure associated with plant tissue penetration. Red asterisks indicate the site of penetration, and clamp cells in the wild type are marked with arrowheads.
To confirm these observations we coinfected plants with a mixture containing equal numbers of SG200CFP and SG200 Δpmt4 YFP cells or equal numbers of SG200YFP and SG200 Δpmt4 CFP strains. We then quantified the number of appressoria as described by Flor-Parra et al. (2006). In the quantitative comparison shown in the Figure 5B, we noticed that appressorium formation was reduced fivefold in SG200 Δpmt4 compared with SG200, irrespective of the combination analyzed. Our findings strongly suggest that Um PMT4 plays an important role in appressorium formation.
Interestingly, during the appressoria quantification process, we observed that in those cases where appressoria did form in Δpmt4 mutants, their morphology appeared abnormal (data not shown; see below). Moreover, we did not see the formation of clamp-like cells, which are typical of mycelium extensions in planta. To better visualize appressoria morphology in these mutants, we used DeltaVision deconvolution microscopy, combining a large number of optical sections from unfixed samples (see Methods). Using this method, we could visualize surface filament and appressoria morphology of wild-type U. maydis during the first stages of infection (Figure 5C). When we performed the same analysis on plants inoculated with Δpmt4 U. maydis, we found that mutant appressoria were unable to form the structures associated with plant penetration in the wild type, including clamp-like cells (Figure 5D), indicating that Δpmt4 might be defective in its ability to penetrate plant tissues.
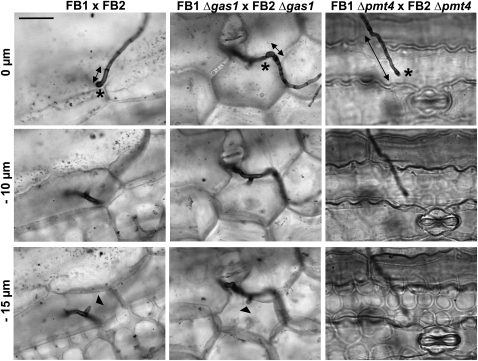
To confirm that the deletion of pmt4 affects U. maydis plant penetration, we used the Chlorazol Black E staining procedure to visualize invading hyphae (Brachmann et al., 2003). This technique has the advantage that it allows us to simultaneously visualize both appressoria and plant cells. In this experiment, we analyzed appressoria morphology 2 d after inoculation to confirm that the results seen in Figure 5 reflect a genuine failure of plant penetration. In parallel, we analyzed the Δgas1 strain, which is able to penetrate plant cuticle, but its growth arrests after surface penetration (Schirawski et al., 2005). While wild-type and Δgas1 hyphae can be seen to invade beyond the epidermal cell layer, Δpmt4 mutants were never observed to reach this stage of the infection process (Figure 6), resulting in all the Δpmt4 hyphae being localized close to the plant surface. We measured the distance from the hypha tips to the closest visible septa 2 d after infection in leaves stained with CBE. This distance correlates with the level of penetration because the new septa formed at the posterior end of the cytoplasm are probably necessary for penetration (Schirawski et al., 2005) (Figure 6). In the case of the wild-type strain, the mean distance was 6.15 ± 1.13 μm, in the gas1 mutant cells the last septum was localized 10.97 ± 3.06 μm from the tip, while in the case of the Δpmt4 strains, it was 17.62 ± 4.56 μm (Table 2). These results confirm that appressorium-mediated penetration in pmt4 mutant is defective. Taken together, our observations suggest that pmt4 plays a key role in fungal pathogenesis through roles in appressorium formation and plant penetration.
Figure 6.
Δpmt4 Mutants Are Unable to Penetrate into the Plant.
Maize seedlings were infected with a mixture of compatible FB1 and FB2, FB1 Δgas1 and FB2 Δgas1, and FB1 Δpmt4 and FB2 Δpmt4 strains. Two days after infection, leaf samples were stained with Chlorazole Black E and analyzed by light microscopy. The z axis image projections show the site of penetration (marked with an asterisk) and the hyphae invading into plant cells (narrow point of the clamp-like cells). Δgas1 mutant (identified by stronger staining of the septum closest the hyphae tip) and wild-type strains, but not Δpmt4 mutants, penetrate into the plant. Distance from hyphal tip to the closest visible septum is indicated by double-headed arrows. Bar = 15 μm.
Table 2.
Quantification of Appressoria and Plant Penetration
| Inoculum | No. of Appressorium-Mediated Penetrations/No. of Appressoria | Percentage | Distance from Hyphal Tip to the Closest Visible Septum (μm) | n |
|---|---|---|---|---|
| FB1 × FB2 | 96/97 | 98.97 | 6.15 ± 1.13 | 49 |
| FB1 Δgas1 × FB2 Δgas1 | 46/47 | 97.87 | 10.97 ± 3.06 | 29 |
| FB1 Δpmt4 × FB2 Δpmt4 |
0/73 |
0.00 |
17.62 ± 4.56 |
47 |
Maize seedlings were infected with the strains indicated above. Two days after infection, leaf samples were stained with Chlorazole Black E and analyzed by light microscopy. Analysis was based on assays of three independent pools of plants with the total number of appressoria scored shown (n).
The Inhibition of Plant ROS Production Does Not Restore the Virulence of the pmt4 Mutant
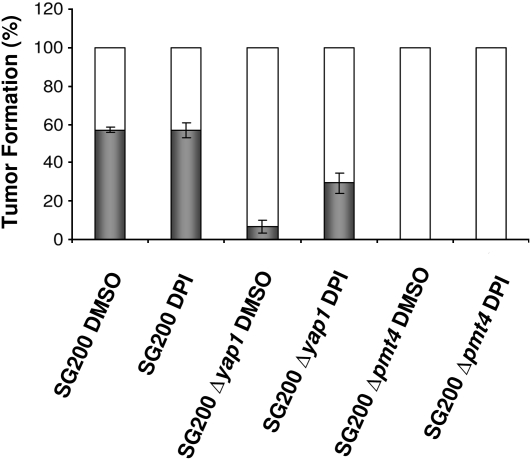
It is already known that plants respond to pathogen attack by producing, among other compounds, reactive oxygen species (ROS) at the site of attempted invasion. Due to their toxicity and importance in plant defense responses, ROS are essential for the progression of fungal infection (Wojtaszek, 1997; Apel and Hirt, 2004). U. maydis yap1, a gene involved in H2O2 detoxification, is required for virulence, and the addition of diphenyleneiodonium (DPI), an inhibitor of the plant NADPH oxidase, restores the normal infectious growth in plant tissues (Molina and Kahmann, 2007). To determine whether the infection defect shown by Δpmt4 cells is somehow related to problems in H2O2 detoxification, 0.5 μM DPI was added to the Δpmt4 cell culture during inoculation. The suppression of ROS generation by treatment with DPI did not restore the phenotype of the Δpmt4 strain as it did on the Δyap1 strain (Figure 7). Thus, consistent with the normal sensitivity to H2O2 (see Supplemental Figure 7 online), the virulence phenotype of U. maydis Δpmt4 is not caused by an enhanced sensitivity to plant ROS production.
Figure 7.
Virulence of U. maydis Δpmt4 Is Not Restored Using DPI.
Maize seedlings were inoculated with SG200 and yap1 and pmt4 mutants to which DMSO or DPI dissolved in DMSO were added. Symptoms were scored 14 d after infection. Three independent assays were performed and the average percentage of tumor formation is indicated (number of plants for each assay ≥ 30). Error bars represent se.
DISCUSSION
The fungal cell wall has historically been considered as a key target for antifungal drugs since it is in intimate contact with host cells, mediating cell interactions and eliciting host immune responses. β-Glucans and chitin, as major components of fungal cell walls, have been selected as the main targets, and many studies on their synthesis and function in fungal infection have been undertaken. Glycoproteins are also major components of the fungal cell wall, involved in its structural and cellular properties, such as cell adhesion, cell fusion, and molecular uptake (Bowman and Free, 2006). Glycoproteins have also been considered as potential targets of antifungal compounds (Ernst and Prill, 2001), and many studies of cell wall glycoproteins and the glycosylation process are now in progress to determine their role in infective mechanisms and their potential as antifungal targets.
In this study, we have analyzed the consequences of deleting all of the putative mannosyltransferases involved in the protein O-mannosylation pathway present in the U. maydis genome. Of the mannosyltransferases tested, only PMT4 was specifically required for U. maydis infectivity. Given the high level of identity between members of the three O-mannosyltransferase families and the absence of other genes with significant similarity, we conclude that PMT4 is the only O-mannosyltransferase essential for the U. maydis virulence. The deletion of pmt4 is associated with a drastic reduction in appressorium formation and, more importantly, those appressoria that do form are nonfunctional in terms of plant penetration. Thus, protein O-mannosylation can now be considered a protein modification process essential for U. maydis virulence and, as PMT family proteins are not conserved in plants (Rouabhia et al., 2005; Bowman and Free, 2006), they may represent an interesting target for future antifungal drugs.
U. maydis as a Phytopathogenic Model for Studying Protein O-Glycosylation
Fungal members of the PMT family, which are involved in the addition of the first mannose monomer to Ser/Thr residues of nascent proteins, have been best characterized in S. cerevisiae. There are seven known PMT members in S. cerevisiae, grouped into three subfamilies, PMT1, PMT2, and PMT4. By contrast, only three members have been found in the U. maydis genome, one for each of these subfamilies. Three PMT family members have also been described in S. pombe (Willer et al., 2005), C. neoformans (Olson et al., 2007; Willger et al., 2009), and Aspergillus fumigatus (Zhou et al., 2007). The increased number of PMT proteins in S. cerevisiae probably originates from a duplication event during evolution (reviewed in Dujon, 2006). In C. albicans, the PMT family contains five nonfunctionally redundant members, of which two new isoforms seem to have acquired new biological roles, such as host cell invasion (Prill et al., 2005). Our results show that U. maydis PMT proteins not only show sequence homology with three conserved PMT subfamilies, but also share their functionality and target specificity as shown by our cross-complementation assay in S. pombe. We also found that U. maydis possesses fewer genes of families implicated in subsequent rounds of O-mannosylation. In the case of the KRE2/MNT1 family, which is responsible for the addition of the second and third mannose residues to target proteins in S. cerevisiae, U. maydis contains only two members compared with the nine members described for the budding yeast. Similarly, only two members have been found belonging to the U. maydis MNN1 family, which is responsible for the addition of the fourth mannose residue, while there are six members described for S. cerevisiae. The reduced complexity of this process in U. maydis promises to make it an excellent model to better understand fungal O-mannosylation and its role in phytopathogenicity.
Biological Significance of the PMT Family
In most fungal and animal cells, protein O-mannosylation is involved in a wide variety of essential cellular processes (Lommel and Strahl, 2009). Our findings suggest that a significant part of the U.maydis protein O-mannosylation machinery is dispensable for its viability. Although the deletion of pmt2 in U. maydis is lethal, as it is in S. pombe (Willer et al., 2005), the viability of simultaneous U. maydis pmt1 and pmt4 deletions contrasts greatly to fission yeast. In S. cerevisiae, O-mannosylation determines the solubilization of misfolded proteins in the endoplasmatic reticulum (Nakatsukasa et al., 2004), the maintenance of cell polarity (Lommel et al., 2004; Proszynski et al., 2004), and the cell wall response (Gentzsch and Tanner, 1996; Lommel and Strahl, 2009). By contrast, no appreciable defects in cell morphology, cell polarity, or drug response were observed in U. maydis Δpmt1 and Δpmt4 strains. Our findings strongly suggest that protein O-mannosylation mediated by pmt1 and pmt4 is not essential for saprophytic growth in U. maydis.
Effects of Protein O-Mannosylation on Cell Wall Integrity and Pathogenesis in U. maydis
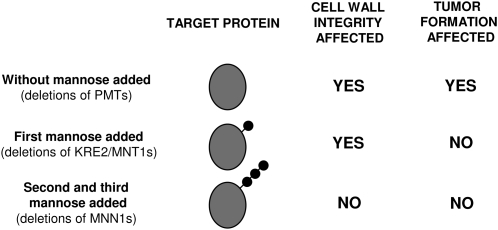
We focused our analysis toward the role of O-mannosyltransferase proteins on cell wall integrity and pathogenesis. In Figure 8, we have summarized the roles of the O-mannosylation protein families in these contexts. Individual deletions of six of the seven putative U. maydis O-mannosyltransferases, as well as double deletions of enzymes belonging to the KRE2/MNT1 and MNN1 family, did not affect cell growth. Only the deletion of pmt4 and Kre2 resulted in slight cell wall integrity defects, as revealed by sensitivity to SDS in these two mutants. Of these two genes, only pmt4 seems to be involved in pathogenesis. Thus, defects in cell wall integrity do not correlate with a defect in pathogenesis nor is the addition of the first mannose always required for virulence. By deleting any of the PMT proteins, we prevent the addition of the whole mannose moiety structure to their particular target proteins, but only those that depend on PMT4 O-mannosyltransferase seem to specifically affect pathogenesis. This finding is not surprising since specific targets of PMT4 and phenotypes exclusively depending on this O-mannosyltransferase have been described previously (Gentzsch and Tanner, 1997; Proszynski et al., 2004). Thus, we can postulate that one or more targets of Um PMT4 are important for virulence. A similar case was observed for the Um KRE2/MNT1 family proteins where deletion of kre2 but not ktr3 affected cell wall integrity. This could be because the targets of both proteins are different, and only the ones affected by KRE2 are involved in cell wall structure. Alternatively, it could be because in U. maydis, KRE2 is the enzyme primarily responsible for the addition of the second mannose, while KTR3 is involved in the addition of the third residue as has been suggested for C. albicans (Buurman et al., 1998). The fact that the deletion of genes from KRE2/MNT1 or MNN1 families does not affect pathogenesis suggests that PMT4 target proteins might only have one mannose residue added or that a single mannose is sufficient to confer to these proteins their proper tertiary structure required for functionality in the infection program.
Figure 8.
Schematic Model of the Effects on Cell Wall Integrity and Pathogenesis of Protein O-Mannosylation in U. maydis.
Deletions of pmt family members in U. maydis alter both cell wall integrity and maize tumor formation. Elimination of KRE2/MNT1 family members suggests they are essential for maintaining cell wall integrity but not for virulence. The addition of the first three mannose residues is sufficient for the fungus to exhibit normal growth and pathogenic development.
So far, PMT4 targets have been best characterized in S. cerevisiae. Of these targets, we have identified U. maydis homologs for Sc Kex2p (Um02843), Sc Gas1p (Um01640), and less clearly for Sc Axl2p (Um01289). In budding yeast, these genes have been implicated in pheromone maturation, cell wall assembly, and axial budding of haploid cells, respectively. Given that the deletion of pmt4 does not affect cell cycle progression, mating, or polar growth, it seems unlikely that these proteins are responsible for the phenotypes we observe in U. maydis, although we are currently working to test this experimentally. Sc Fus1p, Sc Opy2p, and Sc Msb2p, components of the yeast filamentous growth mitogen-activated protein kinase pathway, have recently been described as additional PMT4 targets (Yang et al., 2009). We found that U. maydis contains only some elements of the yeast filamentous growth mitogen-activated protein kinase pathway. We have identified a U. maydis homolog for Sc Msb2p (Um01513), but none were found for Sc Fus1p or Sc Opy2p. Although we cannot currently exclude a role for any of these genes without further investigation, we think that the pmt4 deletion phenotype is more likely to be caused by other, as yet unidentified, PMT4 protein targets, which are more directly implicated in pathogenic development. Thus, the identification of additional PMT4 target proteins is now a priority.
Why Are pmt4 Mutants Unable to Form a Normal Number of Appressoria and Penetrate the Plant Cuticle?
We have shown that the loss of PMT4 results in a strong decrease in the number of appressoria on the plant surface and that no fungal plant penetration occurs. We can postulate a number of different explanations. (1) Adhesion has been shown to be required for appressorium formation and has an important role in appressorium-mediated penetration. Thus, the mannan fraction of cell wall proteins that are glycosylated by PMT4 might mediate fungal adhesion to the plant surface, similar to the situation in C. albicans, in which pmt6 is required for adhesion to human epithelium (Timpel et al., 2000). (2) In other organisms, PMT mutations affect cell wall composition, increasing or decreasing the amount of β1-2 and β1-3 glucans, chitin, and glycoproteins. For example, C. albicans pmt1 mutants show increased levels of chitin (Kapteyn et al., 2000), while Ca pmt4 mutants have increased levels of β1,3-glucan compared with wild-type cells (Prill et al., 2005). Similar changes in U. maydis could affect appressorium formation by yielding either hypha without appressoria or malformed appressoria unable to penetrate the plant tissues. (3) Another possibility is that alterations in the amount of the glycosylation in cell wall proteins could affect the resistance of the fungus to pathogenesis-related proteins produced by the plant and, therefore, its virulence (Narasimhan et al., 2003). (4) A fourth possibility is that the loss of infectivity reflects a problem in the protein secretion process during appressorium formation and plant penetration. Although the production and function of secreted hydrolytic enzymes, such as cellulose, pectinase, or amylase, seem not to be affected in Um pmt4 mutants, the U. maydis genome contains ∼400 putative secreted proteins, any of which could be related to pathogenic processes or plant-fungal crosstalk (Kamper et al., 2006). The function of many of these proteins remains unknown, but it is likely that some of them could play a role in appressorium formation and host penetration. Sequence analysis of these proteins has shown that many of them are rich in Ser and Thr, which are typical residues targeted for O-mannosylation. Moreover, glycosylation is required for sorting, stability, and localization of secreted proteins. A defect in the secretion of any of these proteins caused by the loss of PMT4 could result in defects during appressorium formation and penetration into the plant.
Our results have shown that PMT4 is required for U. maydis virulence by affecting appresorium formation but is not essential for asexual growth. Significantly, while the PMT protein family is conserved in most fungi, it is not conserved in plants, making PMT4 a very attractive target for antifungal agents. Moreover, the PMT4 target protein(s) that are required for U. maydis virulence would also be potential targets for antifungal treatments. We are now initiating a search for PMT4 target proteins that could be implicated in plant pathogenesis through an in silico search as described by Hutzler et al. (2007).
METHODS
Strains, Growth Conditions, and Plasmids
Escherichia coli DH5α and pGEM-T easy (Promega) were used for cloning purposes. Schizosaccharomyces pombe strains FY527 (h-, his3-D1, leu1-32, ura4-D18, and ade6-M216; Forsburg Lab), SBY88 (isogenic to FY527 except ogm4∷hisG), and SBY90 (isogenic to FY527 except ogm1∷hisG) (Willer et al., 2005) were grown in EMM medium supplemented with the necessary amino acids and glucose (2%, w/v) (Mitchison, 1970; modified by Nurse, 1975). All Ustilago maydis strains are described in Table 3. U. maydis cells were grown at 28°C in liquid YPD (2% bactopeptone, 1% yeast extract, and 2% glucose) medium or solid YPD agar. Induction of the nar promoter in AB33 and its derivatives was done according to the protocol described by Brachmann et al. (2001). Mating assays were performed by cospotting compatible strains onto PD plates containing 1% charcoal (PD charcoal) and incubating them at 25°C for 1 to 2 d (Gillissen et al., 1992).
Table 3.
U. maydis Strains Used in This Study
| Strain | Relevant Genotype | Reference |
|---|---|---|
| FB1 | a1 b1 | Banuett and Herskowitz (1989) |
| FB2 | a2 b2 | Banuett and Herskowitz (1989) |
| FBD11 | a1 a2 b1 b2 | Banuett and Herskowitz (1989) |
| SG200 | a1 mfa2 bW2 bE1 | Bölker et al. (1995) |
| AB33 | a2 Pnar:bW2 Pnar:bE1 | Brachmann et al. (2001) |
| AB34 | a2 Pnar:bW2 Pnar:bE2 | Brachmann et al. (2001) |
| HBU13 | a1 b1 Δgas1 | Schirawski et al. (2005) |
| HBU14 | a2 b2 Δgas1 | Schirawski et al. (2005) |
| SG200CFP | a1 mfa2 bW2 bE1 POMA:CFP | Flor-Parra et al. (2006) |
| SG200YFP | a1 mfa2 bW2 bE1 POMA:YFP | Flor-Parra et al. (2006) |
| SG200 Δyap1 | a1 mfa2 bW2 bE1 Δyap1 | Molina and Kahmann (2007) |
| UMG1 | a1 b1 Δpmt1 | This work |
| UMG2 | a2 b2 Δpmt1 | This work |
| UMG3 | a1 a2 b1 b2 Δpmt2 | This work |
| UMG4 | a1 b1 Δpmt4 | This work |
| UMG5 | a2 b2 Δpmt4 | This work |
| UMG6 | a2 Pnar:bW2 Pnar:bE1 Δpmt4 | This work |
| UMG7 | a1 b1 Δkre2 | This work |
| UMG8 | a2 b2 Δkre2 | This work |
| UMG9 | a1 b1 Δktr3 | This work |
| UMG10 | a2 b2 Δktr3 | This work |
| UMG11 | a1 b1 Δkre2 Δktr3 | This work |
| UMG12 | a2 b2 Δkre2 Δktr3 | This work |
| UMG13 | a1 b1 Δmnn1 | This work |
| UMG14 | a2 b2 Δmnn1 | This work |
| UMG15 | a1 b1 Δmnt4 | This work |
| UMG16 | a2 b2 Δmnt4 | This work |
| UMG17 | a1 b1 Δmnn1 Δmnt4 | This work |
| UMG18 | a2 b2 Δmnn1 Δmnt4 | This work |
| UMG19 | a1 mfa2 bW2 bE1 Δpmt4 | This work |
| UMG20 | a1 mfa2 bW2 bE1 Δpmt4 POMA:CFP | This work |
| UMG21 | a1 mfa2 bW2 bE1 Δpmt4 POMA:YFP | This work |
| UMG22 |
a2 Pnar:bW2 Pnar:bE1 Δpmt1 |
This work |
Generation of Mutant U. maydis Strains
Deletion constructs were generated according to Kamper (2004). To generate single deletion mutants of pmt1, pmt2, pmt4, kre2, ktr3, mnn1, and mnt4 genes, fragments of the 5′ and 3′ flank of their open reading frames were generated by PCR on U. maydis FB1 genomic DNA with the following primer combinations: UmPMT1KO5-1/UmPMT1KO5-2 and UmPMT1KO3-1/UmPMT1KO3-2; UmPMT2KO5-1/UmPMT2KO5-2 and UmPMT2KO3-1/UmPMT2KO3-2; UmPMT4KO5-1/UmPMT4KO5-2 and UmPMT4KO3-1/UmPMT4KO3-2; UmKRE2KO5-1/UmKRE2KO5-2 and UmKRE2KO3-1/UmKRE2KO3-2; UmKTR3KO5-1/UmKTR3KO5-2 and UmKTR3KO3-1/UmKTR3KO3-2; UmMNN1KO5-1/UmMNN1KO5-2 and UmMNN1KO3-1/UmMNN1KO3-2; UmMNT4KO5-1/UmMNT4KO5-2 and UmMNT4KO3-1/UmMNT4KO3-2 (sequences in Supplemental Table 2 online). These fragments were digested with SfiI and ligated into the 1.9-Kb SfiI carboxin resistance cassette. To select double mutants, we used a 2.7-kb SfiI hygromycin resistance cassette as described previously (Brachmann et al., 2004).
S. pombe PMTs Complementation
Fragments containing the open reading frames of pmt1, pmt2, and pmt4 U. maydis genes were amplified by PCR using FB1 genomic DNA as template and the following primer combinations: 5′-AGGATCCATGAGTAGCCCAGCTGCTAACAGC-3′/5′-ACCCGGGTCAGTAGATGTTCCAGCTCTTACG-3′, 5′-ACTCGAGATGGCGTCGGTAGGCCAGACAGC-3′/5′-ACCCGGGTTACACGTCCTTGAGAATGACTTGTCG-3′ and 5′-AGGATCCATGGTCGACGCTACAAAAGC-3′/5′-ACCCGGGTCACTTTGCAAAGTGGAGCG-3′, respectively. These fragments were cloned into the expression vector pREP41X, obtaining the plasmids pREP41X-Umpmt1, pREP41X-Umpmt2, and pREP41X-Umpmt4. Transformed S. pombe cells were grown on EMM lacking Leu. As a control, the strains were transformed with the empty pREP41X vector. Cells were grown at 28°C in EMM liquid media supplemented with adenine, histidine, and uracil to exponential phase. Five microliters of 10-fold dilutions of each culture were then spotted onto EMM plates with the appropriate supplements and incubated for 3 d at 28 or 36°C.
Phylogenetic Analysis
PMT sequences were downloaded from the MIPS U. maydis database (http://mips.gsf.de/genre/proj/ustilago/) and NCBI (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi). Multiple sequence alignments were made with CLUSTAL 2.0.2 using the default settings. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4 (Tamura et al., 2007) with the minimum evolution algorithms using 1000 bootstrap replications. Motif analysis was performed using Pfam from Sanger Institute (http://pfam.sanger.ac.uk/).
Pathogenicity Assays
Pathogenicity assays were performed as described by Kamper et al. (2006). U. maydis cultures were grown to exponential phase and concentrated to an OD600 of 3, washed two times in water, and injected into young maize (Zea mays) seedlings (Early Golden Bantam) or directly through stigma into 2-week-old maize seedlings (var. Gaspar Flint). Tumor formation was quantified 7 to 25 d after infection. In the DPI treatment, DPI solved in DMSO was added at 0.5 μM directly to the U. maydis strains prior to inoculation. As a control, DMSO alone was added to the strains (Molina and Kahmann, 2007).
Flow Cytometry
To analyze the pmt4 mutant DNA content, we used the protocol described by Garcia-Muse et al. (2003). Cells were grown in CMD overnight at 28°C and then placed into fresh CMD medium and incubated to an OD600 of 0.8. Prior to analysis, cells were harvested, washed twice with cold water, fixed in 70% ethanol overnight, and resuspended in 50 mM sodium citrate, pH 7.5. Cellular RNA was destroyed by incubation with RNase A (0.25 mg/mL) at 50°C for 1 h and then proteinase K (1 mg/mL) was added and the cells were incubated for one more hour at 50°C.
Cells were stained at 4°C with propidium iodide (16 μg/mL). The fluorescence of 10,000 cells was measured using a FACSCalibur flow cytometer (Becton Dickinson) with a 530/30 bandpass filter.
Thermal Resistance and Drug Treatment Assays
To ascertain the effects of chemical agents on pmt mutants, we grew the strains to exponential phase in YPD at 28°C. Then, we performed a serial dilution patch test of Um pmt mutants and control strains on YPD agar plates with NaCl (to 0.25 and 0.5 M), congo red (to 15 and 30 μg/mL), chlorpromazine (50 and 75 μM), caffeine (1.5 and 3 mM), sorbitol (to 0.5, 1, and 1.5 M), calcofluor white (50 μM), SDS (0.005 to 0.01%), H2O2 (0.5 to 10 mM), and CaCl2 (100 μM) growing at 28°C for 2 d. For these sensitivity studies, analytical grade reagents from Sigma-Aldrich were used. To analyze the response to thermal stress, we incubated the YPD agar plates at 34°C for 2 d.
Enzyme Secretion Assays
Cells were growth to exponential phase in YPD at 28°C. Then, we performed a patch test of Um pmt mutants and control strains on YPD agar plates with pectin, carboxymethyl-cellulose (CMC), and starch. Pectin was precipitated with 0.1% Ruthenium Red. CMC was stained with 0.1% congo red and starch precipitates with 100% ethanol. Pectin medium contains 0.1% ammonium sulfate, 0.1% sucrose, 0.25% pectin from citrus fruit, and 25 mM phosphate buffer, pH 7. CMC medium contains 0.25% carboxymethyl-cellulose, 0.1% ammonium sulfate, 0.1% sucrose, and 25 mM phosphate buffer, pH 7. Starch medium contains 0.25% starch from potato, 0.1% ammonium sulfate, 0.1% sucrose, and 25 mM phosphate buffer, pH 7.
Calcofluor White and Chlorazol Black E Staining
To analyze the prepenetration stages of U. maydis using fluorescence microscopy, cells were stained by adding calcofluor white on infected leaves using Fluorescent Brightener 28 (Sigma-Aldrich). Postpenetration stages were studied by optical microscopy of CBE-stained leaf samples as previously described (Brachmann et al., 2003).
Microscopy
U. maydis cells were incubated in WGA staining solution (10 μg/mL WGA) for 30 min and washed in 1× PBS, pH 7.4. Cells were examined using a Leica fluorescence microscope, equipped with a PlanApo ×100 lens. Analysis of the prepenetration stages was done using a Deltavision wide-field microscope (Applied Precision). Image deconvolution was performed using z-series of between 7 and 23 focal planes, acquired at 0.5-μm intervals. Image processing was performed using Adobe Photoshop CS2 and Canvas 8.0 (Deneba).
DNA and RNA Procedures
Molecular biology techniques were used as described by Sambrook et al. (1989). U. maydis DNA was isolated following the protocol of Hoffman and Winston (1987). Standard U. maydis transformation procedure was used (Schulz et al., 1990). S. pombe molecular genetic techniques were used as described by Moreno et al. (1991). In the expression analysis, cells were washed with cold water, and total RNA was isolated with the Qiagen RNeasy mini kit, separated by formaldehyde denaturing agarose gel electrophoresis, and transferred overnight by capillary action to nylon membranes. To generate the probe for Umpmt1 a 0.429-kb fragment was generated with the primer combination UmPMT1KO3-1/5′-ACGTCCTTGAGAATGACTTGTCGG; for Umpmt2, a 0.297-kb fragment was generated with the primer combination 5′-GTACGTCGCCAAACTCGCTGCG-3′/5′-CCAGCTCTTACGCCACTTGAGG-3′; in the case of Umpmt4, a 0.303-kb fragment was generated using the primer combination 5′-UmPMT4KO3-1/5′-AAAGTGGAGCGTCCAGGATGG-3′ (see Supplemental Table 2 online). Radioactive bands were visualized and quantified using a Molecular Dynamics PhosphorImager.
Accession Numbers
U. maydis PMT sequence data can be found in the GenBank/EMBL data libraries under accession numbers XP_762320 for pmt1 (Um11220), XP_761621 for pmt2 (Um10749), XP_761580 for pmt4 (Um05433), XP_757301 for kre2 (Um01154), XP_757968 for ktr3 (Um01821), XP_761299 for mnn1 (Um05152), and XP_761300 for mnt4 (Um05153). S. cerevisiae PMT sequence data can be found under CAA64917 for Pmt1p, AAC04934 for Pmt2p, CAA58728 for Pmt3p, CAA58729 for Pmt4p, CAA98661 for Pmt5p, CAA97226 for Pmt6p, and Q06644 for Pmt7p. C. albicans PMT sequence under EEQ47067 for pmt1, EEQ44716.1 for pmt2, EEQ46011.1 for pmt4, EEQ43985 for pmt5, and EEQ45029 for pmt6. Drosophila melanogaster POMT sequence under NP_524025 for pomt1 and NP_569858 for pomt2. Finally, H. sapiens POMT sequence data can be found under NP_009102.3 for pomt1 and NP_037514.2 for pomt2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Hydropathy Profiles of U. maydis PMT1, PMT2, and PMT4 with Regard to Sc Pmt1p Using a Window of 17 Amino Acids.
Supplemental Figure 2. Conservation of PMT Sequence Motifs in U. maydis PMTs.
Supplemental Figure 3. U. maydis pmt1 pmt4 Double Mutants Are Viable, While the Loss of pmt2 Is Lethal.
Supplemental Figure 4. Effects of pmt4 Deletion on Pathogenicity in Plants Infected through Stigma.
Supplemental Figure 5. Budding Morphology and Cell Growth of Δpmt1 and Δpmt4 Strains Following WGA Staining.
Supplemental Figure 6. Assay of Hydrolytic Enzyme Secretion.
Supplemental Figure 7. Pmt4 Disruption Results in Normal Sensitivity to Reactive Oxygen Species.
Supplemental Table 1. Annotation of O-Mannosyltransferases in U. maydis.
Supplemental Table 2. Primers Used in This Study.
Supplemental Data Set 1. Alignments of PMT Proteins Produced by CLUSTAL 2.0.2.
Supplementary Material
Acknowledgments
We thank all members of the genetics area for useful discussion, José Pérez Martín and his group for technical assistance, and Angel M. Carrión and John R. Pearson for his critical reading of the manuscript. We thank all of the Ustilago community for providing strains and plasmids and S. Strahl-Bolsinger for the S. pombe strains. This work was supported by Ministerio de Ciencia e Innovación Grant BIO2007-60531. A.F.-A. and A.E.-V. were supported by fellowships from Ministerio de Ciencia e Innovación. Centro Andaluz de Biología del Desarrollo is institutionally supported by Consejo Superior de Investigaciones Científicas, Universidad Pablo de Olavide, and Junta de Andalucía.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: José I. Ibeas (joibecor@upo.es).
Online version contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Apel, K., and Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. [DOI] [PubMed] [Google Scholar]
- Banuett, F., and Herskowitz, I. (1989). Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 86 5878–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett, F., and Herskowitz, I. (1996). Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122 2965–2976. [DOI] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe, D., et al. (2002). Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am. J. Hum. Genet. 71 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker, M. (2001). Ustilago maydis–A valuable model system for the study of fungal dimorphism and virulence. Microbiology 147 1395–1401. [DOI] [PubMed] [Google Scholar]
- Bölker, M., Bohnert, H.U., Braun, K.H., Gorl, J., and Kahmann, R. (1995). Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol. Gen. Genet. 248 547–552. [DOI] [PubMed] [Google Scholar]
- Bowman, S.M., and Free, S.J. (2006). The structure and synthesis of the fungal cell wall. Bioessays 28 799–808. [DOI] [PubMed] [Google Scholar]
- Brachmann, A., Konig, J., Julius, C., and Feldbrugge, M. (2004). A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 272 216–226. [DOI] [PubMed] [Google Scholar]
- Brachmann, A., Schirawski, J., Muller, P., and Kahmann, R. (2003). An unusual MAP kinase is required for efficient penetration of the plant surface by Ustilago maydis. EMBO J. 22 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann, A., Weinzierl, G., Kamper, J., and Kahmann, R. (2001). Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42 1047–1063. [DOI] [PubMed] [Google Scholar]
- Buck, J.W., and Andrews, J.H. (1999). Attachment of the yeast Rhodosporidium toruloides is mediated by adhesives localized at sites of bud cell development. Appl. Environ. Microbiol. 65 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda, P., and Aebi, M. (1999). The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta 1426 239–257. [DOI] [PubMed] [Google Scholar]
- Buurman, E.T., Westwater, C., Hube, B., Brown, A.J., Odds, F.C., and Gow, N.A. (1998). Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 95 7670–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero, P.D., Lengsfeld, C., Prill, S.K., Subanovic, M., Roman, E., Pla, J., and Ernst, J.F. (2007). Transcriptional and physiological adaptation to defective protein-O-mannosylation in Candida albicans. Mol. Microbiol. 64 1115–1128. [DOI] [PubMed] [Google Scholar]
- Dean, R.A. (1997). Signal pathways and appressorium morphogenesis. Annu. Rev. Phytopathol. 35 211–234. [DOI] [PubMed] [Google Scholar]
- Dujon, B. (2006). Yeasts illustrate the molecular mechanisms of eukaryotic genome evolution. Trends Genet. 22 375–387. [DOI] [PubMed] [Google Scholar]
- Ecker, M., Mrsa, V., Hagen, I., Deutzmann, R., Strahl, S., and Tanner, W. (2003). O-mannosylation precedes and potentially controls the N-glycosylation of a yeast cell wall glycoprotein. EMBO Rep. 4 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M.J., Wang, Z.Y., Jones, M.A., Smirnoff, N., and Talbot, N.J. (2007). Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 104 11772–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, J.F., and Prill, S.K. (2001). O-glycosylation. Med. Mycol. 39(Suppl 1): 67–74. [PubMed] [Google Scholar]
- Feldbrugge, M., Kamper, J., Steinberg, G., and Kahmann, R. (2004). Regulation of mating and pathogenic development in Ustilago maydis. Curr. Opin. Microbiol. 7 666–672. [DOI] [PubMed] [Google Scholar]
- Flor-Parra, I., Vranes, M., Kamper, J., and Perez-Martin, J. (2006). Biz1, a zinc finger protein required for plant invasion by Ustilago maydis, regulates the levels of a mitotic cyclin. Plant Cell 18 2369–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman, M.B., McCaffery, J.M., and Cormack, B.P. (2002). Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 46 479–492. [DOI] [PubMed] [Google Scholar]
- Garcia-Muse, T., Steinberg, G., and Perez-Martin, J. (2003). Pheromone-induced G2 arrest in the phytopathogenic fungus Ustilago maydis. Eukaryot. Cell 2 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur, N.K., and Klotz, S.A. (1997). Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65 5289–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch, M., and Tanner, W. (1996). The PMT gene family: Protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J. 15 5752–5759. [PMC free article] [PubMed] [Google Scholar]
- Gentzsch, M., and Tanner, W. (1997). Protein-O-glycosylation in yeast: Protein-specific mannosyltransferases. Glycobiology 7 481–486. [DOI] [PubMed] [Google Scholar]
- Gilbert, R.D., Johnson, A.M., and Dean, R.A. (1996). Chemical signals responsible for appressorium formation in the rice blast fungus. Physiol. Mol. Plant Pathol. 48 335–346. [Google Scholar]
- Gillissen, B., Bergemann, J., Sandmann, C., Schroeer, B., Bolker, M., and Kahmann, R. (1992). A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68 647–657. [DOI] [PubMed] [Google Scholar]
- Girrbach, V., and Strahl, S. (2003). Members of the evolutionarily conserved PMT family of protein O-mannosyltransferases form distinct protein complexes among themselves. J. Biol. Chem. 278 12554–12562. [DOI] [PubMed] [Google Scholar]
- Girrbach, V., Zeller, T., Priesmeier, M., and Strahl-Bolsinger, S. (2000). Structure-function analysis of the dolichyl phosphate-mannose: Protein O-mannosyltransferase ScPmt1p. J. Biol. Chem. 275 19288–19296. [DOI] [PubMed] [Google Scholar]
- Hirayama, H., Fujita, M., Yoko-o, T., and Jigami, Y. (2008). O-mannosylation is required for degradation of the endoplasmic reticulum-associated degradation substrate Gas1*p via the ubiquitin/proteasome pathway in Saccharomyces cerevisiae. J. Biochem. 143 555–567. [DOI] [PubMed] [Google Scholar]
- Hoffman, C.S., and Winston, F. (1987). A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57 267–272. [DOI] [PubMed] [Google Scholar]
- Howard, R.J., Ferrari, M.A., Roach, D.H., and Money, N.P. (1991). Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA 88 11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, R.J., and Valent, B. (1996). Breaking and entering: Host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 50 491–512. [DOI] [PubMed] [Google Scholar]
- Huang, G., Zhang, M., and Erdman, S.E. (2003). Posttranslational modifications required for cell surface localization and function of the fungal adhesin Aga1p. Eukaryot. Cell 2 1099–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler, J., Schmid, M., Bernard, T., Henrissat, B., and Strahl, S. (2007). Membrane association is a determinant for substrate recognition by PMT4 protein O-mannosyltransferases. Proc. Natl. Acad. Sci. USA 104 7827–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper, J. (2004). A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 271 103–110. [DOI] [PubMed] [Google Scholar]
- Kamper, J., et al. (2006). Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 97–101. [DOI] [PubMed] [Google Scholar]
- Kapteyn, J.C., Hoyer, L.L., Hecht, J.E., Muller, W.H., Andel, A., Verkleij, A.J., Makarow, M., Van Den Ende, H., and Klis, F.M. (2000). The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 35 601–611. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157 105–132. [DOI] [PubMed] [Google Scholar]
- Lee, Y.H., and Dean, R.A. (1993). cAMP regulates infection structure formation in the plant pathogenic fungus Magnaporthe grisea. Plant Cell 5 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle, L., Strahl, S., and Tanner, W. (2006). Protein glycosylation, conserved from yeast to man: A model organism helps elucidate congenital human diseases. Angew. Chem. Int. Ed. Engl. 45 6802–6818. [DOI] [PubMed] [Google Scholar]
- Lengeler, K.B., Tielker, D., and Ernst, J.F. (2008). Protein-O-mannosyltransferases in virulence and development. Cell. Mol. Life Sci. 65 528–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel, M., Bagnat, M., and Strahl, S. (2004). Aberrant processing of the WSC family and Mid2p cell surface sensors results in cell death of Saccharomyces cerevisiae O-mannosylation mutants. Mol. Cell. Biol. 24 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel, M., and Strahl, S. (2009). Protein O-Mannosylation: Conserved from bacteria to humans. Glycobiology 19 816–828. [DOI] [PubMed] [Google Scholar]
- Lussier, M., Sdicu, A.M., Bussereau, F., Jacquet, M., and Bussey, H. (1997. a). The Ktr1p, Ktr3p, and Kre2p/Mnt1p mannosyltransferases participate in the elaboration of yeast O- and N-linked carbohydrate chains. J. Biol. Chem. 272 15527–15531. [DOI] [PubMed] [Google Scholar]
- Lussier, M., Sdicu, A.M., Winnett, E., Vo, D.H., Sheraton, J., Dusterhoft, A., Storms, R.K., and Bussey, H. (1997. b). Completion of the Saccharomyces cerevisiae genome sequence allows identification of KTR5, KTR6 and KTR7 and definition of the nine-membered KRE2/MNT1 mannosyltransferase gene family in this organism. Yeast 13 267–274. [DOI] [PubMed] [Google Scholar]
- Martinez-Espinoza, A.D., Garcia-Pedrajas, M.D., and Gold, S.E. (2002). The Ustilaginales as plant pests and model systems. Fungal Genet. Biol. 35 1–20. [DOI] [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123 127–130. [DOI] [PubMed] [Google Scholar]
- Mitchison, J.M. (1970). Physiological and cytological methods for Schizosaccharomyces pombe. In Methods in Cell Physiology, Vol. 4, D.M. Prescott, eds., (New York: Academic), pp. 131–165.
- Molina, L., and Kahmann, R. (2007). A Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell 19 2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194 795–823. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, K., Okada, S., Umebayashi, K., Fukuda, R., Nishikawa, S., and Endo, T. (2004). Roles of O-mannosylation of aberrant proteins in reduction of the load for endoplasmic reticulum chaperones in yeast. J. Biol. Chem. 279 49762–49772. [DOI] [PubMed] [Google Scholar]
- Narasimhan, M.L., Lee, H., Damsz, B., Singh, N.K., Ibeas, J.I., Matsumoto, T.K., Woloshuk, C.P., and Bressan, R.A. (2003). Overexpression of a cell wall glycoprotein in Fusarium oxysporum increases virulence and resistance to a plant PR-5 protein. Plant J. 36 390–400. [DOI] [PubMed] [Google Scholar]
- Nurse, P. (1975). Genetic control of cell size at cell division in yeast. Nature 256 547–551. [DOI] [PubMed] [Google Scholar]
- Olson, G.M., Fox, D.S., Wang, P., Alspaugh, J.A., and Buchanan, K.L. (2007). Role of protein O-mannosyltransferase Pmt4 in the morphogenesis and virulence of Cryptococcus neoformans. Eukaryot. Cell 6 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prill, S.K., Klinkert, B., Timpel, C., Gale, C.A., Schroppel, K., and Ernst, J.F. (2005). PMT family of Candida albicans: Five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol. Microbiol. 55 546–560. [DOI] [PubMed] [Google Scholar]
- Proszynski, T.J., Simons, K., and Bagnat, M. (2004). O-glycosylation as a sorting determinant for cell surface delivery in yeast. Mol. Biol. Cell 15 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, P.A., Lussier, M., Veronneau, S., Sdicu, A.M., Herscovics, A., and Bussey, H. (1999). Mnt2p and Mnt3p of Saccharomyces cerevisiae are members of the Mnn1p family of alpha-1,3-mannosyltransferases responsible for adding the terminal mannose residues of O-linked oligosaccharides. Glycobiology 9 1045–1051. [DOI] [PubMed] [Google Scholar]
- Rouabhia, M., Schaller, M., Corbucci, C., Vecchiarelli, A., Prill, S.K., Giasson, L., and Ernst, J.F. (2005). Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect. Immun. 73 4571–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schirawski, J., Bohnert, H.U., Steinberg, G., Snetselaar, K., Adamikowa, L., and Kahmann, R. (2005). Endoplasmic reticulum glucosidase II is required for pathogenicity of Ustilago maydis. Plant Cell 17 3532–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, B., Banuett, F., Dahl, M., Schlesinger, R., Schafer, W., Martin, T., Herskowitz, I., and Kahmann, R. (1990). The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60 295–306. [DOI] [PubMed] [Google Scholar]
- Sheppard, D.C., Yeaman, M.R., Welch, W.H., Phan, Q.T., Fu, Y., Ibrahim, A.S., Filler, S.G., Zhang, M., Waring, A.J., and Edwards, J.E., Jr. (2004). Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279 30480–30489. [DOI] [PubMed] [Google Scholar]
- Skamnioti, P., and Gurr, S.J. (2007). Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence. Plant Cell 19 2674–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snetselaar, K.M., and Mims, C.W. (1992). Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia 84 193–203. [Google Scholar]
- Snetselaar, K.M., and Mims, C.W. (1993). Infection of maize stigmas by Ustilago maydis: Light and electron microscopy. Phytopathology 83 843–850. [Google Scholar]
- Strahl-Bolsinger, S., Gentzsch, M., and Tanner, W. (1999). Protein O-mannosylation. Biochim. Biophys. Acta 1426 297–307. [DOI] [PubMed] [Google Scholar]
- Strahl-Bolsinger, S., and Scheinost, A. (1999). Transmembrane topology of pmt1p, a member of an evolutionarily conserved family of protein O-mannosyltransferases. J. Biol. Chem. 274 9068–9075. [DOI] [PubMed] [Google Scholar]
- Talbot, N.J. (2003). On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57 177–202. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Dudley, J., Nei, M., and Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24 1596–1599. [DOI] [PubMed] [Google Scholar]
- Timpel, C., Zink, S., Strahl-Bolsinger, S., Schroppel, K., and Ernst, J. (2000). Morphogenesis, adhesive properties, and antifungal resistance depend on the Pmt6 protein mannosyltransferase in the fungal pathogen candida albicans. J. Bacteriol. 182 3063–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer, T., Brandl, M., Sipiczki, M., and Strahl, S. (2005). Protein O-mannosylation is crucial for cell wall integrity, septation and viability in fission yeast. Mol. Microbiol. 57 156–170. [DOI] [PubMed] [Google Scholar]
- Willer, T., Valero, M.C., Tanner, W., Cruces, J., and Strahl, S. (2003). O-mannosyl glycans: from yeast to novel associations with human disease. Curr. Opin. Struct. Biol. 13 621–630. [DOI] [PubMed] [Google Scholar]
- Willger, S.D., Ernst, J.F., Alspaugh, J.A., and Lengeler, K.B. (2009). Characterization of the PMT gene family in Cryptococcus neoformans. PLoS One 4 e6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek, P. (1997). Oxidative burst: An early plant response to pathogen infection. Biochem. J. 322 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.Y., Tatebayashi, K., Yamamoto, K., and Saito, H. (2009). Glycosylation defects activate filamentous growth kss1 MAPK and inhibit osmoregulatory Hog1 MAPK. EMBO J. 28 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Hu, H., Zhang, L., Li, R., Ouyang, H., Ming, J., and Jin, C. (2007). O-Mannosyltransferase 1 in Aspergillus fumigatus (AfPmt1p) is crucial for cell wall integrity and conidium morphology, especially at an elevated temperature. Eukaryot. Cell 6 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.