Abstract
Although sleep and exercise may seem to be mediated by completely different physiological mechanisms, there is growing evidence for clinically important relationships between these two behaviors. It is known that passive body heating facilitates the nocturnal sleep of healthy elderly people with insomnia. This finding supports the hypothesis that changes in body temperature trigger somnogenic brain areas to initiate sleep. Nevertheless, little is known about how the core and distal thermoregulatory responses to exercise fit into this hypothesis. Such knowledge could also help in reducing sleep problems associated with nocturnal shiftwork. It is difficult to incorporate physical activity into a shiftworker's lifestyle, since it is already disrupted in terms of family commitments and eating habits. A multi-research strategy is needed to identify what the optimal amounts and timing of physical activity are for reducing shiftwork-related sleep problems. The relationships between sleep, exercise and diet are also important, given the recently reported associations between short sleep length and obesity. The cardiovascular safety of exercise timing should also be considered, since recent data suggest that the reactivity of blood pressure to a change in general physical activity is highest during the morning. This time is associated with an increased risk in general of a sudden cardiac event, but more research work is needed to separate the influences of light, posture and exercise per se on the haemodynamic responses to sleep and physical activity following sleep taken at night and during the day as a nap.
Keywords: Blood pressure, Circadian rhythm, Diet, Exercise, Shiftwork, Thermoregulation
1. Introduction
Intuitively, one might consider that sleep and physical activity are separate and distinct behaviors. It would seem sensible to suggest that the physiologic mechanisms at work to ensure a relatively inactive and restful sleep are completely different from those that maintain homeostasis during intense exercise. Nevertheless, there are some important relationships between sleep and exercise that have, in fact, been appreciated throughout antiquity.
It was thought in biblical times that the physical work of a labourer promotes good sleep, and that this favorable effect is present irrespective of whether the stomach is full or not; ‘The sleep of a labouring man is sweet, whether he eats little or much’. Nevertheless, there was also a warning for the well-fed, yet idle, rich; ‘⋯but the fullness of the rich will not suffer him to sleep.’ (Ecclesiastes 5:12). Shakespeare suggested that the above relationship between exercise and good sleep was not just unidirectional. In his play Macbeth, Shakespeare stressed the importance of obtaining a good nights sleep in order to recuperate properly from prior physical work; ‘Sleep that knits up the ravell'd sleave of care, The death of each day's life, sore labour's bath' (Macbeth 2.2.46-51).
As more scientific knowledge has been gained about sleep, more links between sleep and physical activity have been postulated over and above the general and much-discussed notions that exercise is good for sleep [1], and vice versa [2]. For example, it is interesting that narcoleptic individuals have an increased propensity for sudden bouts of daytime sleep, sometimes in response to strong emotions or even exercise [3]. Sleep apnea is apparently present in a large proportion of American professional footballers [4]. One risk factor for sleep apnea is a neck that is large in circumference relative to its length [5]; this anthropometric characteristic is prevalent amongst many American footballers. There is also a growing body of evidence that the links between sleep, physical activity and the prevalence of obesity are more complicated than were previously believed. For example, a short, not a long, sleep length has been found to be associated with an increased risk of obesity [6]. Given the negative impact of a general sedentary daily lifestyle on obesity levels, such findings are surprising and are, therefore, discussed in more detail later in this review.
The aims of the present review are to summarise the various relationships that have recently been highlighted between the human behaviors of sleep and physical activity. The basic physiologic responses to these behaviors are first outlined, with particular emphasis on the thermoregulatory responses to exercise. The particular problems of disrupted sleep and physical activity patterns of the shiftworker are then discussed, which is followed by a summary of the recent research on short sleep length and obesity. Lastly, some new findings about the impact of an increase in physical activity after waking from sleep on haemodynamic responses and cardiac safety are analysed.
2. Physical activity, thermoregulation and sleep quality
The sleep–wake cycle is one of the more obvious circadian rhythms in the human. What is not apparent, until physiological measurements are obtained, is that sleep is usually initiated when body temperature is falling and individuals tend to wake up after body temperature has begun to rise [7]. These observations, taken together with other findings–that thermoregulation differs between sleep stages and that sleep is disrupted in non-thermoneutral environments [8]–suggest that there is an important link between sleep and thermoregulation.
Sleep can be divided into two main types of stages, rapid eye movement (REM) and non-REM sleep. There are approximately 4–5 non-REM/REM cycles during a typical night's sleep. Core body temperature is decreased, whilst peripheral temperature is increased, during the non-REM stages [9,10]. The REM/non-REM difference in body temperature is most apparent during the first hour of sleep [11]. Several researchers have investigated the effects of passively changing body temperature on these sleep characteristics. If a person is immersed in warm water so that there is an increase in body temperature of 1.5–2.5 °C, then the time between retiring to bed and initiation of sleep (latency) is shortened and the non-REM stages that are associated with a deep recuperative sleep (known as ‘slow-wave’ sleep stages) are enhanced [12,13]. Such changes are likely to be clinically important for individuals who generally sleep poorly, such as elderly people [14].
Given the relationships between sleep and thermoregulation, one could hypothesise that the circadian rhythm in body temperature is vital to human sleep and, vice versa, that good nocturnal sleep is an important factor governing efficient thermoregulation. Nevertheless, it has proved difficult to separate these two hypotheses [15]; i.e. does sleep mediate changes in thermoregulation or is it thermoregulation that induces sleep? Researchers have attempted to manipulate body temperature pharmacologically (using melatonin, for example) in investigations of sleep. Whilst the hypothermic effects of melatonin have been found to affect sleep propensity [16], this secretory product of the pineal gland may also have quite separate hypnotic effects [17] making it a “noisy” pharmacological intervention in sleep research. Krauchi et al. [16] used a multiple regression model to examine the relative strength of various melatonin-induced effects on sleep characteristics. In support of the notion that the hypothermic effects of melatonin are more important than the hypnotic effects, the best predictor of sleep propensity was the gradient between distal and proximal skin temperatures. These data also support the view that warming of the feet induces a rapid onset of sleep [18].
Endogenous concentrations of melatonin are affected by exercise, although there are conflicting findings regarding the direction of this effect [19]. These conflicting results could be due to differences in lighting conditions and time of day of exercise. It is also possible that the intensity of exercise, gender and age are intervening factors [20-22]. Despite the conflicting results, it is clear that exercise has effects on pineal function and that these effects are relatively short-lived. Atkinson et al. [19] concluded that more research work is needed to identify what the chronic effects of exercise training are on melatonin levels, as well as whether any such chronic effects are associated with the sleep problems that are often reported by overtrained athletes. In one study, Lucia et al. [23] examined world-class cyclists during the Tour of Spain stage race lasting 3 weeks. They found that, although urinary levels of 6-sulphatoxymelatonin (a melatonin metabolite) increased in the evening compared to the pre-stage values, generally there was a decline in 6-sulpatoxymelatonin as the race progressed.
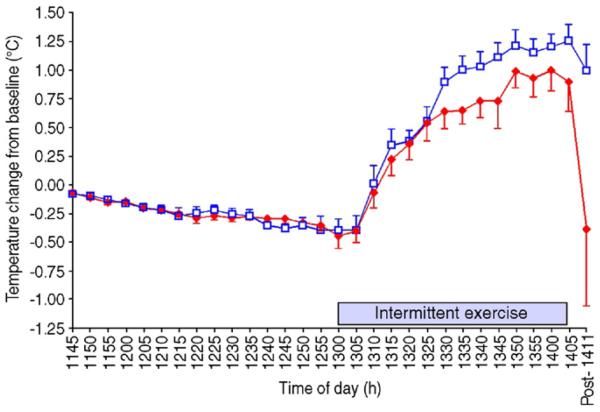
An interesting question is whether there are any interactive effects of melatonin and exercise on thermoregulation and sleep quality. Several researchers have administered melatonin prior to a bout of exercise. In two separate studies, McLellen et al. [24,25] administered 1 mg and 5 mg of melatonin to participants 2 h before, and immediately prior to, walking in an environment of 40°C and 30% relative humidity. The participants wore nuclear biological military suits during this exercise leading to uncompensatable heat stress. Melatonin had no influence on the rectal or skin temperature responses at rest or during walking in these conditions. Recently, Atkinson et al. [26] found that prior ingestion of 2.5 mg of melatonin did lower rectal temperature during a subsequent 1-h bout of intermittent running (Fig. 1). The exercise was performed in the morning in this study. No researcher has examined the presence of any such interactive effects of melatonin and exercise in the evening prior to nocturnal sleep.
Fig. 1.
The effects of prior ingestion of 2.5 mg of melatonin on the core temperature responses to subsequent intermittent exercise [26].
The results of neuroanatomical research also support the hypothesis that thermoregulatory changes regulate sleep. The most important processing of thermoregulatory information occurs in the pre-optic area/anterior hypothalamus [27]. Sleep can be initiated if this specific pre-optic area of the brain is warmed, causing neurons to increase their firing rate [28]. Moreover, similar effects can be measured if the skin is warmed, suggestive of a direct neural pathway between the periphery and somnogenic areas of the brain [29]. Although a neuroanatomical model of sleep regulation remains to be fully elucidated, the fact that warming the periphery induces sleep may lead to practical methods for sleep facilitation, such as footbaths [15]. Given that the prevalence of insomnia could be as high as 34% in some populations [14], such non-pharmacologic methods have appeal.
It is generally thought that exercise constitutes a non-pharmacologic behavior which promotes sleep, and so regular bouts of physical activity are recommended as therapy for individuals who are having difficulty in sleeping. The mechanisms for exercise being beneficial to sleep have been driven by the energy conservation and tissue restitution theories of sleep, as evidenced by the ancient quotations from the Bible and Shakespeare's Macbeth that were provided in the Introduction. It was only in the 1980s that the effects of exercise have been considered alongside the temperature down-regulation hypothesis of sleep [30]. In agreement with the results of studies on passive heating, Horne and colleagues [30,31] found that exercise-mediated hyperthermia led to increases in slow-wave sleep. It appears as though exercise late at night increases body temperature without being arousing enough to disrupt sleep, offering more support for the view that the thermoregulatory responses to exercise are more important than the alerting influences of physical activity [32]. Nevertheless, some studies of exercise have been criticised for placing too much emphasis on the amount of slow-wave sleep as an indicator of sleep quality [33]. There is also evidence that stimulation of specific motor regions of the brain leads to different EEG patterns during subsequent sleep [34]. Such a finding complicates the exploration of the effects of exercise on polysomnographic sleep measurements. In order to define fully the links between exercise-mediated changes in body temperature and subsequent sleep, it would also be important to measure the comprehensive range of core and distal body temperature changes, as well as the melatonin responses that were also measured in the studies on passive heating. Such changes should also be examined in different environmental conditions and with different populations, e.g. the elderly who often sleep poorly or shiftworkers. The study of problems specific to the latter population is associated with particular difficulties. These difficulties will now be discussed.
3. Shiftwork, sleep disruption and physical activity
About 20% of the European workforce is employed in a shiftwork schedule that involves nightwork [35]. Shiftworkers are needed in order to meet society's needs for 24-h services and emergency cover, for maintaining continuing process industries and to offset the economic effects of industrial plant shutdown. Shiftwork is no longer restricted to heavy industry but is now more common in “E-commerce” and call-centre occupations in order to meet the demands for round-the-clock retail, service and banking industries.
The general consensus of expert reviewers [35,36] is that shiftwork is associated with greater health problems than “normal” daywork. The health effects of shiftwork include a reduction in quality and quantity of sleep, chronic fatigue, anxiety and depression, adverse cardiovascular and gastrointestinal effects and reproductive effects in women. Harrington [35] identified improvements in catering and recreational facilities as factors which can ameliorate shiftwork problems in the short-term. Harrington [35] also mentioned the importance of physical fitness and activity in helping workers reduce the problems associated with shiftwork.
Many shiftworkers wish to, but cannot, perform leisure activities at the same times as dayworkers [37]. For those people participating in team activities or competitive sport, a restriction in convenient leisure-time may become one of the major factors in causing them to leave shiftwork [38,39]. For people involved in individual, rather than group, physical activities, shiftwork may not be too problematic. For example, our data collected as part of a project sponsored by the Health and Safety Executive (HSE) indicate that a reasonably-large 9% of competitive cyclists are shiftworkers [40]. Participation in other individual activities like jogging and swimming may also not be adversely affected by shiftwork. Although the opportunity for some types of activity may not be hindered by shiftwork, this activity often cannot be scheduled in the early evening, which is the time of day thought to be optimum for physiological benefit and may fit best with the family life of the shiftworker [41].
Physical activity is one of the few leisure activities which may mediate long-term favorable changes in physiologic functions as well as alter the fatigue levels of the shiftworker [42,43]. Physical activity performed at least twice a week is usually included in guidelines for improving shiftwork tolerance [35,44,45] but the usefulness of exercise during shiftwork is poorly understood and there is evidence that shiftworkers find it difficult to follow that particular piece of advice [46]. More research is needed regarding not only how leisure interests are affected by shiftwork but, conversely, how leisure activities, especially those involving exercise, affect tolerance to shiftwork and the health of the shiftworker.
It is clear that there has been no focused attempt to examine the influence of timings of physical activity on the problems associated with shiftwork. It is unknown whether these problems are due to disturbance of the circadian rhythm (the “body clock”) and/or a decreased opportunity to adopt the desired timing of lifestyle and social factors. Further, the effects that a change in physical activity might have on diet and feeding habits of shiftworkers are not known. This link is important, given the temptation of humans to overcompensate increased energy expenditure with increased energy intake.
4. Sleep disruption and eating habits
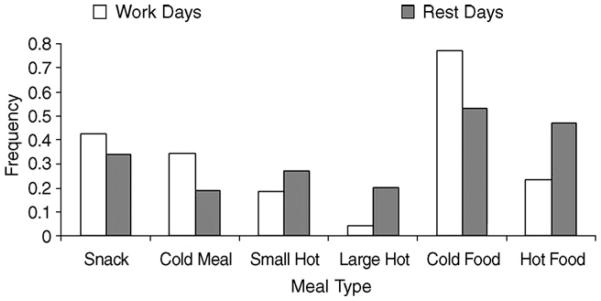
The results of previous research work by our group indicate that the timing and type of food eaten by shiftworkers are determined more by the opportunity afforded by the work schedule than by hunger [47,48] (Fig. 2). Altered eating habits are a source of concern in nightworkers, who tend to “nibble” their way through crisps and chocolate bars during the night shift rather than eat a healthy and substantial meal in the middle of it [49-51]. Indigestion is common in this group of people, and there is an increased frequency of gastrointestinal disorders and ulcers, particularly in those who have worked at night for some years [36]. There is evidence that eating at night alters the metabolic profile of the individual [52,53], and some evidence that even a small redistribution of food intake to the nighttime alters the level of blood lipids, which acts as a factor pre-disposing to cardiovascular morbidity [51].
Fig. 2.
The types of meals chosen by workers on nightshifts compared to during rest days [48].
Factors determining the decision to eat or not to eat, and what to eat, have received little study. In previous work [48], we employed a questionnaire to investigate the roles of habit, appetite, food availability, cost and time availability in subjects on rest days, during day or nightwork, and after a simulated time-zone transition. The results enabled the dominant role of time availability to be established. Even though the results obtained by us so far [48] indicate how “external” factors (work schedule, for example) can be changed to benefit nightworkers, it is unclear as to the type and timing of meal that shiftworkers would choose, if given the opportunity to do so. Advising shiftworkers to eat a more balanced diet during nightwork must be coupled with the availability of the types of meal that the workforce would respond to most favorably. What such meals might contain is unknown. Further, a “multi-level” approach [54], which studies not only the individual but also any influence of shiftworkers' eating habits at the level of their social environment and family has not been attempted. Only multi-strategy [55] and multi-level [54] research has the potential to unravel the influence of these factors, and these approaches have not yet been attempted in the area of energy expenditure and energy intake of shiftworkers.
As mentioned in the Introduction, inter-relationships between sleep and metabolism may have important implications for obesity levels in the general population. Since 1960, the proportion of US citizens reporting less than 7 h sleep per night has more than doubled to 37.1% in 2001–2002 [56]. In this same time period, the prevalence of obesity has also markedly increased to between 20% and 30% in 2002 [57]. Is there a biological reason for these parallel changes in sleep restriction and obesity levels? The results of laboratory studies involving sleep restriction have suggested marked effects on glucose tolerance and insulin sensitivity [56]. Much attention has recently been focused on the responses of ghrelin, leptin and orexin to sleep restriction. Such neuroendocrine abnormalities may affect appetite, increasing the risk of overeating and an increase in body mass index. They may also affect thermogenesis from activity other than exercise. This non-exercise thermogenesis is the most variable component of energy expenditure and can account for differential capacities of weight gain in rats [58]. Nevertheless, it is surprising how little is known about the influence of physical activity on these relationships between sleep, metabolism and body mass gain. Several epidemiologic studies (e.g. [59]) have controlled for physical activity level in the analysis of the relationship between sleep length and body mass index. Nevertheless, little is known about the impact of exercise on the results of laboratory studies involving biological markers of appetite and metabolism.
5. Sleep, exercise and cardiovascular safety
Myocardial infarction, sudden cardiac death and stroke show circadian variation with peak incidences observed between 06:00 and 12:00 h and the lowest incidences during the night-time hours [60]. A mechanism that has been postulated for this circadian variation in sudden cardiac events is the rupture of an atherosclerotic plaque leading to thrombosis [60]. Possible triggers of plaque rupture in the morning include increases in platelet aggregation, catecholamine and cortisol levels as well as increases in arterial blood pressure at this time of day [60]. There is similar circadian variation in resting blood pressure (BP) to that in the incidence of cardiovascular events. After a nocturnal nadir, blood pressure increases markedly following waking. This increase has been termed the ‘morning surge’ in blood pressure [61]. A question that is relevant to the these changes in blood pressure is whether blood pressure is inherently more responsive to physical activity at certain times of day, i.e. are the rises in BP observed after waking from a night's sleep wholly due to the increased amounts of activity during the morning period, or is there, in addition or alternatively, an increased sensitivity of BP to the effects of activity in the morning?
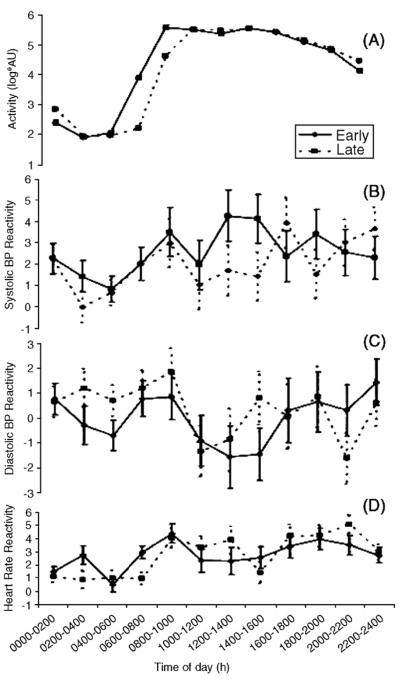
The link between 24-h changes in physical activity and fluctuations in blood pressure is thought not to be strong [62], although the strength of association seems to improve when researchers concentrate on specific periods (e.g. the morning period) within the sleep–wake cycle [63,64]. Kario et al. [63] investigated the individual “reactivity” of blood pressure to physical activity during sleep and wakefulness using a statistical index based on least squares regression methods. Recently, Jones et al. [65] used this index to examine whether the reactivity of blood pressure to physical activity varied with time of day. It was found that the increase in systolic and diastolic blood pressure in response to a given change in physical activity peaked in the morning. This 24-h profile in blood pressure reactivity was similar between groups of individuals formed on the basis of an earlier- or later-than-average wake time (Fig. 3).
Fig. 3.
The effects of time of day on the reactivity of blood pressure and heart rate to everyday activities [65]. ‘Early’ and ‘Late’ refer to subjects grouped according to whether they woke later or earlier than the median time for the pooled sample (n = 440).
The results reported by Jones et al. [65] suggest that hypertensive patients would be wise to avoid the morning period for vigorous physical exertion. This avoidance was also viewed as a ‘sensible caution’ by Floras [66], although this author maintained that less than 6% of the morning increase in blood pressure could be attributed to concurrent increases in physical activity.
Although the results of Jones et al. [65] are relevant to the above question, an experimental rather than observational study is required to unravel the influences of changes in posture, lighting condition and meal times from that of a change in activity per se. Winther et al. [67] examined platelet aggregation and fibrinolytic activity between 08:00 and 12:00 h. Exercise was systematically introduced towards the end of this period, which included previous interventions with posture. Exercise was found not to increase platelet aggregation to levels beyond that produced by the upright posture. Nevertheless, exercise per se was associated with a marked increase in fibrinolytic activity. Such an experiment could be repeated using blood pressure measurements as additional dependent variables.
Jones et al. [65] also observed a further fall and rise in systolic blood pressure reactivity in the afternoon. This finding agrees with other observations on elderly people, including those with hypertension, of a secondary rise in blood pressure in the afternoon, especially after a nap is taken [68-70]. Since some cardiac events also show a secondary peak in the late afternoon or evening, these blood pressure changes have led to the hypothesis that daytime sleep or a “siesta” is also a significant risk factor for myocardial infarction and strokes [71-73]. A direct comparison between the increases in blood pressure following nocturnal sleep and the changes after an afternoon nap would help unravel the influences of activity and posture from any endogenously-mediated difference in how blood pressure responds to activity at different times of day. Such a study, which should involve careful control of the level of activity after waking from both sleep periods, has not been attempted.
Besides the effects of increasing activity after sleep, the period before daytime sleep is taken is also relevant in terms of the associated changes in cardiovascular variables. Researchers have reported a fall in BP and HR during non-rapid eye movement (non-REM) sleep, whereas in REM sleep, blood pressure is similar to levels during waking [74]. Baroreflex sensitivity also increases during sleep [75]. The results of several studies have indicated that there are relatively abrupt falls in BP and HR in association with nighttime sleep onset [76-78]. Nevertheless, in a well-controlled study, Carrington et al. [79] found that the greatest change in blood pressure at night actually occurred between lights-off and sleep-onset (whilst the participants were supine but still awake). It would be important to ascertain whether such a phenomenon occurs during the ‘lights-off’ period prior to daytime sleep.
6. Summary
In this review, we have postulated a number of physiologic relationships between the behaviors of sleep and exercise. The transition period between physical activity and sleep onset might be important for ensuring good sleep quality. We have suggested that thermoregulatory and pineal function mechanisms are important in this relationship. Besides the lack of specific knowledge on exercise, thermoregulation and sleep, the prescription of exercise interventions to people, such as shiftworkers, who already have disrupted lifestyles and sleep, might be extremely difficult. Only a combination of biologic and socio-behavioral research strategies can explore the effectiveness and acceptability of exercise interventions on sleep. The transition between waking and onset of significant levels of physical activity might also be important in terms of cardiovascular safety. This period is associated with marked increases in haemodynamic measurements such as blood pressure as well as an increased risk of a sudden cardiac event. Nevertheless, more research work is needed to separate the influences of the multiple causes of such responses and events. Such research work would, therefore, need tight control of lighting condition, posture and diet in order to focus on any effects of exercise per se.
References
- 1.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–65. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bambaeichi E, Reilly T, Cable NT, et al. The influence of time of day and partial sleep loss on muscle strength in eumenorrheic females. Ergonomics. 2005;48:1499–511. doi: 10.1080/00140130500101437. [DOI] [PubMed] [Google Scholar]
- 3.Chakravorty SS, Rye DB. Narcolepsy in the older adult—epidemiology, diagnosis and management. Drugs Aging. 2003;20:361–76. doi: 10.2165/00002512-200320050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Stergiou GS, Vemmos KN, Pilarchopoulou KM, Synetos AG, Roussias LG, Mountokalakis TD. Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke. 2002;33:1480. doi: 10.1161/01.str.0000016971.48972.14. [DOI] [PubMed] [Google Scholar]
- 5.Katz I, Stradling J, Slutsky S, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5 pt 1):1228–31. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 6.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep. 2005;28:1217–20. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- 7.Waterhouse J, Drust B, Weinert D, Edwards B, Gregson W, Atkinson G, et al. The circadian rhythm of core temperature: origin and some implications for exercise performance. Chronobiol Int. 2005;22:205–23. doi: 10.1081/cbi-200053477. [DOI] [PubMed] [Google Scholar]
- 8.Bach V, Telliez F, Libert JP. The interaction between sleep and thermoregulation in adults and neonates. Sleep Med Rev. 2002;6:481–92. doi: 10.1053/smrv.2001.0177. [DOI] [PubMed] [Google Scholar]
- 9.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HJ, Holmes AL, Dawson D. The relationship between slow-wave activity, body temperature, and cardiac activity during nighttime sleep. Sleep. 2001;24:343–9. doi: 10.1093/sleep/24.3.343. [DOI] [PubMed] [Google Scholar]
- 11.Krauchi K, Wirz-Justice A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology. 2001;25:S92–6. doi: 10.1016/S0893-133X(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 12.Horne JA, Shackell BS. Slow-wave sleep elevations after body heating—proximity to sleep and effects of aspirin. Sleep. 1987;10:383–92. doi: 10.1093/sleep/10.4.383. [DOI] [PubMed] [Google Scholar]
- 13.Jordan J, Montgomery I, Trinder J. The effect of afternoon body heating on body temperature and slow wave sleep. Psychophysiology. 1990;27:560–6. doi: 10.1111/j.1469-8986.1990.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 14.Liao WC. Effects of passive body heating on body temperature and sleep regulation in the elderly: a systematic review. Int J Nurs Stud. 2002;39:803–10. doi: 10.1016/s0020-7489(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81–93. doi: 10.1016/S1087-0792(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 16.Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr C Physiol. 2000;278:741–8. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- 17.Luboshiszky R, Lavie P. Sleep inducing effects of exogenous melatonin administration. Sleep Med Rev. 1998;2:191–202. doi: 10.1016/s1087-0792(98)90021-1. [DOI] [PubMed] [Google Scholar]
- 18.Krauchi K, Cajochen C, Werth E. Physiology—warm feet promote the rapid onset of sleep. Nature. 1999;401(6748):36–7. doi: 10.1038/43366. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson G, Drust B, Reilly T, Waterhouse J. Relevance of melatonin to sports medicine and science. Sports Med. 2003;33:809–31. doi: 10.2165/00007256-200333110-00003. [DOI] [PubMed] [Google Scholar]
- 20.Buxton OM, LhermiteBaleriaux M, Hirschfield U, VanCauter E. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12:568–74. doi: 10.1177/074873049701200611. [DOI] [PubMed] [Google Scholar]
- 21.Barriga C, Marchena JM, Ortega E, Martin MI, Rodriguez AB. Melatonin levels and exercise in adolescent boys and girls. Biog Amines. 2000;15:643–65. [Google Scholar]
- 22.Appenzeller O, Wood SC. Peptides and exercise at high and low altitudes. Int J Sport Med. 1992;13:S135–40. doi: 10.1055/s-2007-1024618. [DOI] [PubMed] [Google Scholar]
- 23.Lucia A, Diaz B, Hoyos J, Fernandez C, Villa G, Bandres F, et al. Hormone levels of world class cyclists during the Tour of Spain stage race. Br J Sports Med. 2001;35:424–30. doi: 10.1136/bjsm.35.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLellan TM, Gannon GA, Zamecnik J. Low doses of melatonin and diurnal effects on thermoregulation and tolerance to uncompensable heat stress. J Appl Physiol. 1999;87:308–16. doi: 10.1152/jappl.1999.87.1.308. [DOI] [PubMed] [Google Scholar]
- 25.McLellan TM, Smith IF, Gannon GA. Melatonin has no effect on tolerance to uncompensable heat stress in man. Eur J Appl Physiol. 2000;83:336–43. doi: 10.1007/s004210000291. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson G, Holder A, Robertson C, Gant N, Drust B, Reilly T, et al. Effects of melatonin on the thermoregulatory responses to intermittent exercise. J Pineal Res. 2005;39:353–9. doi: 10.1111/j.1600-079X.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 27.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–61. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 28.Gong H, Szymusiak R, King J. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr C Physiol. 2000;279(6):R2079–88. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 29.Sherin JE, Shiromani PJ, McCarley RW. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 30.Horne JA, Staff LH. Exercise and sleep: body heating effects. Sleep. 1983;6:36–46. doi: 10.1093/sleep/6.1.36. [DOI] [PubMed] [Google Scholar]
- 31.Horne JA, Moore VJ. Sleep EEG effects of exercise with and without additional body cooling. Electroencephalogr Clin Neurophysiol. 1985;60:33–8. doi: 10.1016/0013-4694(85)90948-4. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor PJ, Breus MJ, Youngstedt SD. Exercise-induced increase in core temperature does not disrupt a behavioral measure of sleep. Physiol Behav. 1998;64(3):213–7. doi: 10.1016/s0031-9384(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Bergman BM, Gilliland MA. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22(1):11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 35.Harrington JM. Health effects of shiftwork and extended hours of work. Occup Environ Med. 2001;58:68–72. [Google Scholar]
- 36.Waterhouse J, Folkard S, Minors D. Shiftwork, health and safety. An overview of the scientific literature 1978–1990. Her Majesty's Stationery Office; London: 1992. HSE contract research report. [Google Scholar]
- 37.Hornberger S, Knauth P. Interindividual differences in the subjective valuation of leisure time utility. Ergonomics. 1993;36:255–64. [Google Scholar]
- 38.Herbert A. The influence of shiftwork on leisure activities. A study with repeated measurement. Ergonomics. 1983;26:565–74. doi: 10.1080/00140138308963375. [DOI] [PubMed] [Google Scholar]
- 39.Frese M, Okenek K. Reasons to leave shiftwork and psychological and psychosomatic complaints of former shiftworkers. J Appl Physiol. 1984;69:509–14. [PubMed] [Google Scholar]
- 40.Atkinson G, Reilly T. Effects of age on the circadian characteristics of physically active subjects. In: Vellas JL, Atharede JL, Garry PJ, editors. Facts and research in gerontology. Serdi Publisher; Paris: 1995. pp. 149–60. [Google Scholar]
- 41.Atkinson G, Reilly T. Circadian variation in sports performance. Sports Med. 1996;21:292–312. doi: 10.2165/00007256-199621040-00005. [DOI] [PubMed] [Google Scholar]
- 42.Reilly T, Waterhouse J, Atkinson G. Aging, rhythms of physical performance, and adjustment to changes in the sleep–activity cycle. Occup Environ Med. 1997;54:812–6. doi: 10.1136/oem.54.11.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harma MI, llmarinen J, Knauth P, Rutenfranz J, Hanninen P. Physical training intervention in shift-workers. 1. The effects of intervention on fitness, fatigue, sleep, and psychomotor symptoms. Ergonomics. 1988;31:39–50. doi: 10.1080/00140138808966647. [DOI] [PubMed] [Google Scholar]
- 44.Monk TH, Folkard S. Making shiftwork tolerable. Taylor and Francis; Basingstoke: 1992. [Google Scholar]
- 45.Knauth P, Hornberger S. Preventative and compensatory measures for shiftworkers. Occup Med. 2003;53:109–16. doi: 10.1093/occmed/kqg049. [DOI] [PubMed] [Google Scholar]
- 46.Wedderburn A, Scholarios D. Guidelines for shiftworkers: trials and errors? Ergonomics. 1993;36:211–8. [Google Scholar]
- 47.Waterhouse J, Akerstedt T, Lennernas M, Arendt J. Chronobiology and nutrition: internal and external factors. Can J Diabetes Care. 1999;23(Suppl 2):82–8. [Google Scholar]
- 48.Waterhouse J, Buckley P, Edwards B, Reilly T. Measurement of, and some reasons for, differences in eating habits between night and dayworkers. Chronobiol Int. 2003;20:1075–92. doi: 10.1081/cbi-120025536. [DOI] [PubMed] [Google Scholar]
- 49.Reinberg A, Migraine C, Apfelbaum M. Circadian and ultradian rhythms in the eating behaviour and nutrient intake of oil refinery operators (Study 2) Chronobiologia. 1979;(Suppl 1):89–102. [PubMed] [Google Scholar]
- 50.Krauchi K, Nussbaum P, Wirz-Justice A. Consumption of sweets and caffeine in the night shift: relation to fatigue. In: Horne J, editor. Sleep ′90. Pontenagel Press; Bochum: 1990. pp. 62–4. [Google Scholar]
- 51.Lennernas M, Hambraeus L, Akerstedt T. Shift related dietary intake in day and shiftworkers. Appetite. 1995;25:253–65. doi: 10.1006/appe.1995.0060. [DOI] [PubMed] [Google Scholar]
- 52.Van Cauter E, Blackman I, Roland D, Spire J-D, Refetoff S, Polonsky K. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–42. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan L, Arendt J, Owens D, Folkard S, Hampton S, Deacon S, et al. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. J Endocrinol. 1998;157:443–51. doi: 10.1677/joe.0.1570443. [DOI] [PubMed] [Google Scholar]
- 54.NIH . Toward higher levels of analysis: progress and promise in research on social and cultural dimensions of health. National Institutes of Health Publication, Office of Behavioral and Social Sciences Research; 2000. [Google Scholar]
- 55.Bryman A. Social research methods. Sage; London: 2001. [Google Scholar]
- 56.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 57.Hardman AE, Stensel DJ. Physical activity and health: The evidence explained. Routledge; London: 2003. [Google Scholar]
- 58.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity diet-induce obese and diet-resistant rats. Am J Physiol. 2006;290:396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- 59.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13 year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 60.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan NM. Morning surge in blood pressure. Circulation. 2003;107:1347. doi: 10.1161/01.cir.0000060887.83850.46. [DOI] [PubMed] [Google Scholar]
- 62.Mansoor GA, White WB, McCabe EJ, Giacco S. The relationship of electronically monitored physical activity to blood pressure, heart rate and the circadian blood pressure profile. Am J Hypertens. 2000;13:262–7. doi: 10.1016/s0895-7061(99)00147-8. [DOI] [PubMed] [Google Scholar]
- 63.Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–91. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- 64.Leary AC, Struthers AD, Donnan PT, MacDonald MT, Murphy MB. The morning surge in blood pressure and heart rate is dependent on levels of physical activity after waking. J Hypertens. 2002;20:865–70. doi: 10.1097/00004872-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Jones H, Atkinson G, Leary A, George K, Murphy M, Waterhouse J. The reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension. 2006;47:778–84. doi: 10.1161/01.HYP.0000206421.09642.b5. [DOI] [PubMed] [Google Scholar]
- 66.Floras JS. Morning activity and blood pressure—a cause for concern? J Hypertens. 2002;20:809–11. doi: 10.1097/00004872-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Winther K, Hillegass W, Tofler GH, Jimenez A, Brezinski DA, Schafer AI, et al. Effects on platelet aggregation and fibrinolytic activity during upright posture and exercise in healthy men. Am J Cardiol. 1992;70:1051–5. doi: 10.1016/0002-9149(92)90359-7. [DOI] [PubMed] [Google Scholar]
- 68.Atkinson G, Witte K, Nold G, Sasse U, Lemmer B. Effects of age on circadian blood pressure and heart rate rhythms in primary hypertensive patients. Chronobiol Int. 1994;11:35–44. doi: 10.3109/07420529409057229. [DOI] [PubMed] [Google Scholar]
- 69.Dunbar SB, Farr L. Temporal patterns of heart rate and blood pressure in elders. Nurs Res. 1996;45:43–9. doi: 10.1097/00006199-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Scorzoni D, Bazzanini F, Brunazzi MC, Chirillo F, Biondi P, Holzl A, et al. Age-related differences in blood pressure profile in essential hypertension. Chronobiol Int. 1997;14:397–407. doi: 10.3109/07420529709001460. [DOI] [PubMed] [Google Scholar]
- 71.Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stessman J. The siesta in the elderly: a new risk factor for mortality? Arch Intern Med. 1999;159:1582–6. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 72.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29(3):429–37. [PubMed] [Google Scholar]
- 73.Qureshi AI, Giles WH, Croft JB. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 74.Van de Borne P, Nguyen H, Biston P, Linkowski P, Degaute JP. Effects of wake and sleep stages on the 24-h autonomic control of blood pressure and heart rate in recumbent men. Am J Physiol. 1994;266:H548–54. doi: 10.1152/ajpheart.1994.266.2.H548. [DOI] [PubMed] [Google Scholar]
- 75.Bristow JD, Honour AJ, Pickering TG, Sleight P. Cardiovascular and respiratory changes during sleep in normal and hypertensive subjects. Cardiovasc Res. 1969;3:476–85. doi: 10.1093/cvr/3.4.476. [DOI] [PubMed] [Google Scholar]
- 76.Burgess HJ, Kleiman J, Trinder J. Cardiac activity during sleep onset. Psychophysiology. 1999;36:298–306. doi: 10.1017/s0048577299980198. [DOI] [PubMed] [Google Scholar]
- 77.Degaute JP, Van de Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 78.Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 79.Carrington MJ, Barbieri R, Colrain IM, Crowley KE, Kim Y, Trinder J. Changes in cardiovascular function during sleep onset period in young adults. J Appl Physiol. 2005;98:468–76. doi: 10.1152/japplphysiol.00702.2004. [DOI] [PubMed] [Google Scholar]