Abstract
Background information. In the embryos of various animals, the body elongates after gastrulation by morphogenetic movements involving convergent extension. The Wnt/PCP (planar cell polarity) pathway plays roles in this process, particularly mediolateral polarization and intercalation of the embryonic cells. In ascidians, several factors in this pathway, including Wnt5, have been identified and found to be involved in the intercalation process of notochord cells.
Results. In the present study, the role of the Wnt5 genes, Hr-Wnt5α (Halocynthia roretzi Wnt5α) and Hr-Wnt5β, in convergent extension was investigated in the ascidian H. roretzi by injecting antisense oligonucleotides and mRNAs into single precursor blastomeres of various tissues, including notochord, at the 64-cell stage. Hr-Wnt5α is expressed in developing notochord and was essential for notochord morphogenesis. Precise quantitative control of its expression level was crucial for proper cell intercalation. Overexpression of Wnt5 proteins in notochord and other tissues that surround the notochord indicated that Wnt5α plays a role within the notochord, and is unlikely to be the source of polarizing cues arising outside the notochord. Detailed mosaic analysis of the behaviour of individual notochord cells overexpressing Wnt5α indicated that a Wnt5α-manipulated cell does not affect the behaviour of neighbouring notochord cells, suggesting that Wnt5α works in a cell-autonomous manner. This is further supported by comparison of the results of Wnt5α and Dsh (Dishevelled) knockdown experiments. In addition, our results suggest that the Wnt/PCP pathway is also involved in mediolateral intercalation of cells of the ventral row of the nerve cord (floor plate) and the endodermal strand.
Conclusion. The present study highlights the role of the Wnt5α signal in notochord convergent extension movements in ascidian embryos. Our results raise the novel possibility that Wnt5α functions in a cell-autonomous manner in activation of the Wnt/PCP pathway to polarize the protrusive activity that drives convergent extension.
Keywords: ascidian, convergent extension, Dishevelled (Dsh), notochord, Wnt/planar cell polarity (PCP) pathway
Abbreviations: Bra, brachyury; Ci-Wnt5, Cioni intestinalis Wnt5; Dsh, Dishevelled; FS, frame-shifted; Hr-Bra, Halocynthia roretzi Bra; Hr-Wnt5, Halocynthia roretzi Wnt5; MO, morpholino oligonucleotide; PBST, PBS with 0.2% Triton X-100; PCP, planar cell polarity; PEM, posterior end mark; pk, Prickle; YFP, yellow fluorescent protein
Introduction
In the embryos of various animals, the body elongates along the anterioposterior axis and becomes narrower across the mediolateral axis. This morphogenetic movement after gastrulation involves convergent extension, in which embryonic cells converge mediolaterally (Gerhart and Keller, 1986; Keller et al., 2000; Wallingford et al., 2002). The PCP (planar cell polarity) pathway is involved in mediolateral polarization and intercalation of embryonic cells in both invertebrates and vertebrates (Fanto and McNeill, 2004; Kiefer, 2005; Barrow, 2006; Wang and Nathans, 2007). Various players in this pathway have been identified so far, such as the Frizzled, Dsh (Dishevelled), Strabismus and pk (Prickle) proteins, although some of these factors are commonly used in the Wnt canonical pathway. Although there is no clear evidence of involvement of the Wnt signal protein in the PCP pathway of invertebrates, vertebrates utilize Wnt5 and Wnt11 to activate the PCP
pathway in convergent extension after the initiation of gastrulation (Rauch et al., 1997; Tada and Smith, 2000; Heisenberg et al., 2000; Wallingford et al., 2001; Wallingford, 2004).
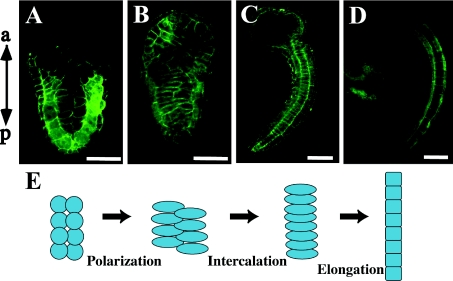
Convergent extension movement occurs in a rather simple way in ascidian embryos, in which exactly 40 notochord cells intercalate with each other in a way similar to that described in the axial mesoderm of vertebrates (Cloney, 1964; Miyamoto and Crowther, 1985; Munro and Odell, 2002a; Munro et al., 2006). These notochord cells are derived from ten blastomeres of the 110-cell stage embryo that are linearly aligned along the left–right axis (Nishida, 1987), and during gastrulation and neurulation the notochord precursors divide twice, gathering into a large single cell mass. By the initial tail-bud stage, the 40 post-mitotic cells are aligned in two bilateral lines in the frontal section (Figure 1). Then they begin intercalation to form a single file of disc-shaped notochord cells. In this process, notochord cells become motile and extend actin-rich lamellipodia (Munro and Odell, 2002a). After the mid tail-bud stage, the notochord further elongates in a post-convergent extension process, which is simply mediated by shape changes of each constituent cell, such as elongation and vacuolation (Jiang and Smith, 2007) (Figures 1C and 1D). Eventually, the notochord in hatched tadpoles serves as a hydrostatic skeleton for tail beats driven by bilateral muscles.
Figure 1. Convergent extension movement of ascidian notochord.
Confocal images of frontal section of H. roretzi stained with Alexa Fluor® 488 phalloidin to visualize cellular boundaries. (A) At the initial tail-bud stage, 40 post-mitotic notochord cells are aligned in two bilateral lines. Anterior is at the top. (B) Each notochord cell is polarized, elongates along the left–right axis and starts mediolateral intercalation. (C) The notochord is made of disc-shaped cells aligned in a single line after intercalation. (D) At the mid tail-bud stage, each notochord cell elongates in a process of post-convergent extension elongation. In consequence, the tail further elongates. (E) Representation of notochord morphogenesis. a, anterior; p, posterior. Scale bar, 100 μm.
Evidence has accumulated that the Wnt/PCP pathway is also responsible for the intercalation of ascidian notochord cells. Two Wnt genes, Hr-Wnt5α (Halocynthia roretzi Wnt5α) and Hr-Wnt5β, have been isolated from the ascidian H. roretzi (Sasakura et al., 1998; Miya and Nishida, 2002). At the neurula and tail-bud stages when convergent extension takes place, the Wnt5α gene is expressed in notochord and Wnt5β is expressed in muscle that bilaterally flanks the central notochord. When Wnt5α mRNA is overexpressed in embryos, aberrant morphogenesis of the notochord occurs and notochord cells fail to converge. Nevertheless, expression of differentiation markers of various tissues, including the notochord, is not affected, suggesting that amounts of Wnt5α have to be precisely controlled in ascidian convergent extension (Sasakura and Makabe, 2001). There has been to date no functional analysis of Wnt5β. In Ciona intestinalis embryos, when Dsh function is disrupted by the dominant-negative form, intercalation of notochord cells is prevented (Keys et al., 2002). Similarly in a pk mutant (called aimless) in Ciona savignyi, notochord cells fail to intercalate (Jiang et al., 2005). In these kinds of embryos, notochord cells initially extend motile processes in all directions, as in the wild-type, but these fail to resolve into the bipolar protrusive activity that drives convergent extension. In wild-type embryos, the Dsh and pk proteins are localized to plasma membranes of notochord cells, except for the cell membranes that face the flanking muscle cells. In the pk mutant, Dsh proteins are mislocalized into the cytoplasm, supporting that these proteins are in the same cascade (Jiang et al., 2005). However, embryos in which the PCP pathway is disrupted are able to perform the post-convergent extension process that occurs in the last phase of tail elongation, mediated by elongation of each constituent cell (Jiang and Smith, 2007). Studies of another short-tailed mutant in C. savignyi, chongmague, revealed the importance of laminin-mediated boundary formation in convergent extension (Veeman et al., 2008). In this laminin mutant, notochord cells initiate intercalation, but then migrate inappropriately to become dispersed in the tail, because of the absence of a morphological boundary around the notochord.
Microsurgical removal of various parts of embryos has revealed that the notochord has to be surrounded by other cells for its convergent extension, but no particular neighbouring tissue has been shown to be indispensable for intercalation of notochord cells (Munro and Odell, 2002b). A major unresolved question concerning convergent extension in animal development is the source of polarizing cues along the axis. The PCP pathway plays roles in the integration of extrinsic polarizing signals into the intracellular programme of cell motility. However, the precise function of the Wnt signal proteins in convergent extension remains unclear, as does the source of the Wnt signal during this process. In the present study, we investigated, in detail, the function of the Hr-Wnt5α and Hr-Wnt5β proteins in convergent extension of notochord cells of the ascidian H. roretzi, particularly focusing on mediolateral intercalation cell movement. We also examined the tissue-specific requirements of the Wnt proteins, and carried out mosaic analysis within the notochord to elucidate the cell autonomy of Wnt function.
Results
Wnt5α and Wnt5β in Halocynthia
Two Wnt5 cDNAs have been cloned previously in H. roretzi (Sasakura et al., 1998; Miya and Nishida, 2002). The amino-acid sequences of Hr-Wnt5α and Hr-Wnt5β show 53% identity. At the neurula and tail-bud stages when convergent extension takes place, the Wnt5α gene is expressed in notochord and the Wnt5β gene is expressed in muscle (Figures 2A and 2B). In the C. intestinalis genome, a single Wnt5 gene is found, and there is no Wnt11 gene (Hino et al., 2003; Hotta et al., 2003). Localization of maternal mRNA of Ci-Wnt5 (C. intestinalis Wnt5) is similar to that of Hr-Wnt5α, as it is localized to the posterior region of eggs and embryos as a member of the post-plasmic/PEM (posterior end mark) RNA (Sasakura et al., 1998; Imai et al, 2004). However, the zygotic expression pattern of Ci-Wnt5 seems to be a mixture of that of Hr-Wnt5α and Hr-Wnt5β, as Ci-Wnt5 shows a very clear expression in muscle precursors during the cleavage stages. At the neurula stage, Ci-Wnt5 is faintly expressed in both notochord and muscle in addition to the strong expression present in the tail tip epidermis (Imai et al, 2004).
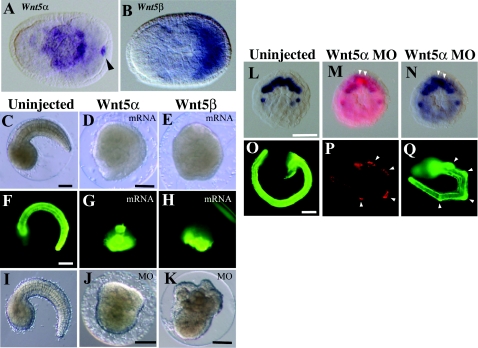
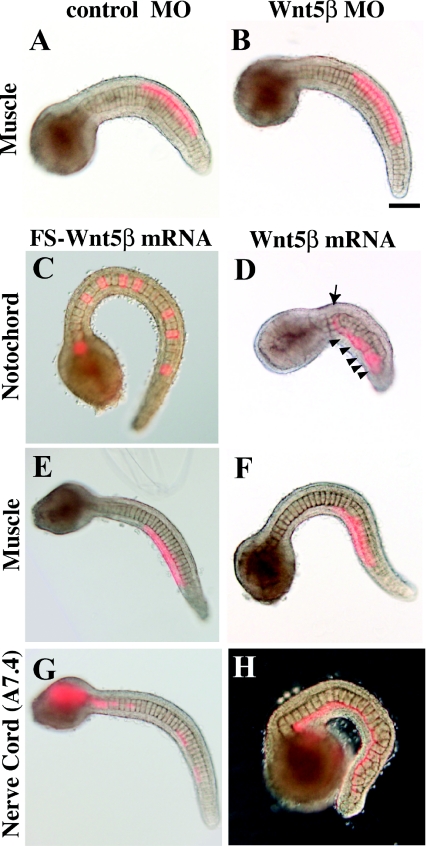
Figure 2. Effects of Wnt5 MOs and mRNAs injected into eggs.
(A, B) Expression of Hr-Wnt5α and Hr-Wnt5β genes at the neurula stage in developing notochord and muscle cells respectively. Anterior is to the left. Arrowhead indicates Hr-Wnt5α expression in the posterior pole, which is concentrated Hr-Wnt5α maternal mRNA, as it is a member of postplasmic/PEM RNAs in ascidians (Sasakura et al. 1998). (C–E) Morphology of uninjected and Wnt5α and Wnt5β mRNA-injected embryos at the tail-bud stage. (F–H) Detection of the notochord differentiation marker antigen, Not-1, in uninjected and Wnt5α and Wnt5β mRNA-injected embryos. (I–K) Morphology of uninjected, Wnt5α and Wnt5β MO-injected embryos at the tail-bud stage respectively. (L) Expression of Bra gene in ten notochord precursor blastomeres at the 110-cell stage. (M) Embryo co-injected with Wnt5α MO and a lineage tracer (rhodamine dextran) into a single notochord precursor (A7.3) blastomere of the 64-cell embryo. At the 110-cell stage, two sister blastomeres are labelled with red fluorescence (white arrowheads). (N) The same embryo normally expressed the Bra gene in notochord precursors, including the labelled cells. (O) The Not-1 antigen is expressed in notochord. The embryo was slightly overstained with the antibody, and the signal is spread in the trunk region. (P) At the tail-bud stage, several descendants of the injected blastomere can be recognized from their content of red fluorescent label. (Q) The same embryo expressed Not-1 antigen in notochord cells including the injected and labelled cells (arrowheads). Scale bar, 100 μm.
Over/mis-expression and knockdown of Wnts
The spatiotemporal expression patterns of the two Wnt5 genes suggest their involvement in convergent extension of the notochord. This is in agreement with a report that overexpression of Wnt5α mRNA disrupts the movement of notochord cells without interfering with notochord differentiation (Sasakura and Makabe, 2001). In the present study, we injected fertilized eggs with the mRNAs for Wnt5α and Wnt5β, and both of them had the effects reported previously for Wnt5α injection (Figures 2C–2H) (Sasakura and Makabe, 2001). The resulting embryos did not have recognizable tails, although they possessed differentiated notochord cells as detected with the Not-1 antigen (Nishikata and Satoh, 1990). The arrangement of notochord cells within the embryos was disorganized. These observations suggest that the Wnt5α and Wnt5β proteins can exert similar effects, although in normal embryos they are expressed in distinct tissues, notochord and muscle.
Then we attempted to inhibit the functions of the Wnt5α and Wnt5β proteins by injecting fertilized eggs with antisense MOs (morpholino oligonucleotides). Nakamura et al. (2006) reported that injected Wnt5α MO suppresses notochord formation, interfering with the early processes of notochord cell-fate specification at the cleavage stage by suppressing gene expression of a notochord-specific key transcription factor, Bra (brachyury), at the 64-cell stage. We confirmed the absence of notochord in such embryos (Figures 2I and 2J). Since MO injected into eggs abrogates the early processes of notochord cell-fate specification, we injected a single notochord precursor (A7.3) blastomere of 64-cell embryos with the MO, together with a lineage tracer (rhodamine dextran), after Bra expression had already begun (see Figure 3A). In these embryos, Bra expression was detected in ten notochord precursors at the 110-cell stage, as in normal embryos, including those labelled with Rhodamine Red fluorescence (Figures 2L–2N). At the tail-bud stage, the MO-injected descendants also showed the Not-1 differentiation marker, but the labelled cells tended to be present at the kinked positions of the tail (Figures 2O–2Q). These results suggest that Wnt5α plays roles in notochord specification and proper morphogenesis of notochord cells in early and late embryogenesis respectively.
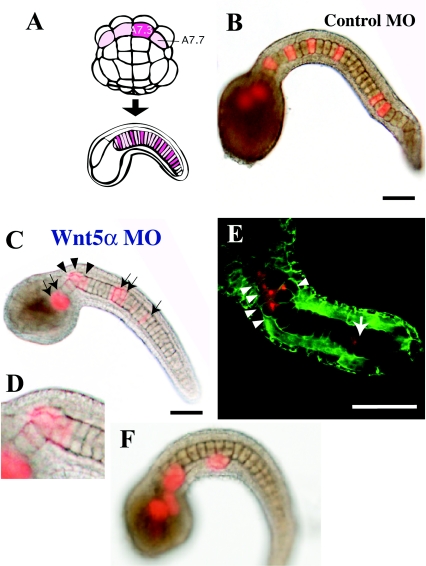
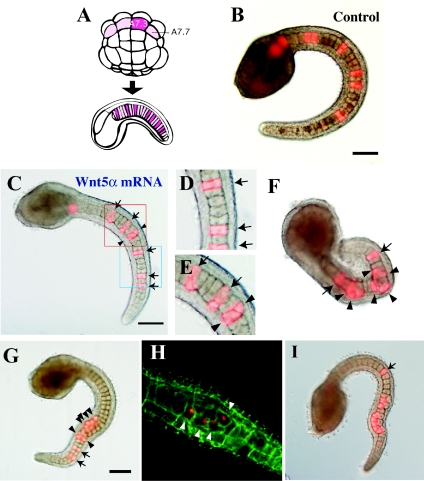
Figure 3. Effects of Wnt5α MO injected into a notochord precursor blastomere of the 64-cell embryo.
(A) Lineage illustration of notochord. Four pink blastomeres of the 64-cell embryo (vegetal view, anterior is up) give rise to the major part of the notochord in the tail. A7.3 and A7.7 are the notochord precursors. Each blastomere divides three times and gives rise to eight notochord cells randomly intercalated in the notochord. Injected blastomeres and their descendants are indicated in dark pink. (B) Tail-bud embryos co-injected with the lineage tracer and control universal MO into the A7.3 blastomere. The dark spot in the centre of each notochord cell is the nucleus. (C) Injection of Wnt5α MO. Arrows indicate normally intercalated notochord cells that are labelled with red fluorescence. Arrowheads show cells that failed to intercalate. (D) Closer view of the neck region of (C). (E) Another example of injection of Wnt5α MO observed with a confocal microscope. Cell boundaries are demarcated by green fluorescence. (F) Embryos that showed a severe phenotype. Labelled notochord cells are clustered and not incorporated into the aligned notochord. Scale bar, 100 μm.
Injection of Wnt5β MO into eggs also resulted in aberrant morphology (Figure 2K). As Wnt5β is expressed in muscle precursor cells, we injected Wnt5β MO into a single muscle precursor (B7.4) blastomere of 64-cell embryos (see Figure 5A). However, the embryos developed normally, as shown below (see Figure 6B). Therefore it is likely that Wnt5β has early roles during embryogenesis.
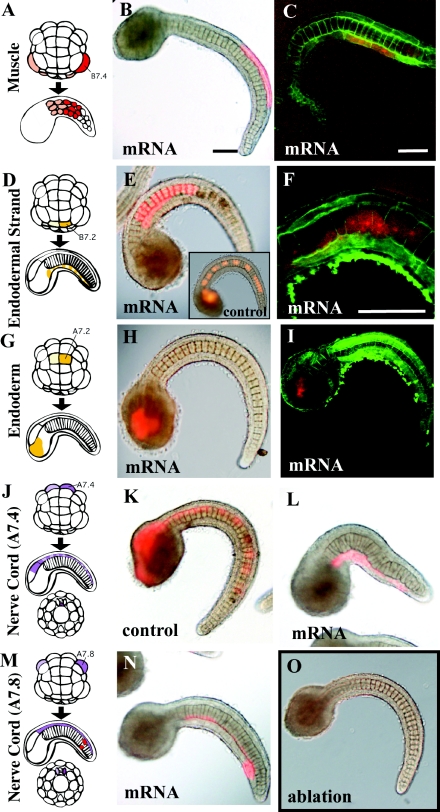
Figure 5. Effects of Wnt5α mRNA injected into various tissue precursors.
(A, D, G, J, M) Lineage illustration of muscle, endodermal strand, endoderm and nerve cord (A7.4 and A7.8) respectively (Nishida, 1987). Injected blastomeres and their descendants are indicated in dark colours. (B, E, H, K, L, N) Bright-field images are merged with fluorescent images to show the position of labelled descendant cells. (C, F, I) Confocal views. Cell boundaries are demarcated by green fluorescence. (B, C) The muscle precursor was injected. (E, F) The precursor of the endodermal strand was injected. The notochord is normal, but intercalation of the endodermal strand, which is present on this side of the notochord, was prevented as the labelled cells did not intercalate with the cells of the other bilateral side as they formed a continuous line. (F) is a confocal closer view of such a specimen. (E, insert) Control embryo. The endodermal strand is intermittently labelled in the tail. (H, I) The endoderm precursor was injected. (K, L) Control mRNA and Wnt5α mRNA were injected into the precursor of the ventral row (floor plate) of the nerve cord respectively. In the control, the nerve cord is intermittently labelled in the tail, indicating intercalation of left and right descendants. In Wnt5α mRNA-injected embryos, the labelled cells failed to intercalate as they formed a continuous line. (N) Wnt5α mRNA was injected into the precursor of the lateral row of the nerve cord in addition to two muscle cells at the tip of the tail (red). (O) Every A-line nerve cord precursor blastomere was ablated at the 64-cell stage by bursting them with injected seawater. The notochord of the embryo developed normally, indicating that the neural tube is dispensable for notochord morphogenesis. Scale bar, 100 μm.
Figure 6. Effects of Wnt5β MO and mRNA injected into various tissue precursors.
(A, B) Control and Wnt5β MO were injected into the muscle precursor (B7.4) blastomere as Wnt5β is expressed in muscle. (C–H) Control FS Wnt5β mRNA and wild-type Wnt5β mRNA were injected into the precursors of notochord (A7.3), muscle (B7.4) and the ventral row of nerve cord (A7.4). Arrow indicates a normally intercalated notochord cell. Arrowheads show cells that failed to intercalate. (G) In the control, the nerve cord is intermittently labelled in the tail, indicating intercalation of left and right descendants. (H) In Wnt5β mRNA-injected embryos, the labelled cells failed to intercalate as they formed a continuous line. Scale bar, 100 μm.
Disruption of Wnt5α in notochord lineage cells
By using the procedures described above, the role of Wnt5α in notochord morphogenesis was examined in more detail. The A7.3 or A7.7 blastomere of the 64-cell embryo gives rise exclusively after three rounds of cell division to eight post-mitotic notochord cells, which are randomly positioned along the anterior–posterior axis because of random intercalation (Figures 3A and 3B). The MO and a lineage tracer were co-injected into the A7.3 or A7.7 blastomere (Figures 3C–3F). In all embryos (n=62; Table 1), some of the descendants failed to intercalate properly, resulting in a kinked tail, although the other descendants intercalated normally. The number of notochord cells that failed to intercalate varied. In the most severe cases, several descendants were clustered and excluded from the uninjected and properly aligned notochord (Figure 3F). The variation in failure of intercalation is analysed in detail below. In contrast, every non-labelled cell intercalated successfully, as long as it was not next to labelled cells. When labelled cells are clustered, neighbouring non-labelled cells often failed to intercalate (Figure 3E; see also Figures 4H and 4I).
Table 1. Intercalation of notochord cells in embryos in which Wnt-5α/Wnt-5β/Dsh antisense MOs or mRNA were injected into specific blastomeres.
n=total number of embryos analysed. The percentage of failure is the percentage of embryos in which at least one notochord cell failed to intercalate.
| Notochord intercalation (%) | ||||
|---|---|---|---|---|
| Injected blastomeres | n | Normal | Failure | |
| Rhodamine dextran | Notochord (A7.3/A7.7) | 14 | 100 | 0 |
| Muscle (B7.4) | 2 | 100 | 0 | |
| Nerve cord (A7.4) | 2 | 100 | 0 | |
| Control MO | Notochord (A7.3/A7.7) | 6 | 100 | 0 |
| Muscle (B7.4) | 1 | 100 | 0 | |
| Wnt-5α MO | Notochord (A7.3/A7.7) | 62 | 0 | 100 |
| Wnt-5β MO | Muscle (B7.4) | 55 | 100 | 0 |
| Dsh MO | Notochord (A7.3/A7.7) | 50 | 12 | 88 |
| Muscle (B7.4) | 16 | 100 | 0 | |
| Nerve cord (A7.4) | 20 | 100 | 0 | |
| Nerve cord (A7.8) | 11 | 100 | 0 | |
| FS Wnt-5β mRNA | Notochord (A7.3/A7.7) | 18 | 72 | 28 |
| Nerve cord (A7.4) | 3 | 100 | 0 | |
| Wnt-5α mRNA | Notochord (A7.3/A7.7) | 110 | 9 | 91 |
| Muscle (B7.4) | 21 | 100 | 0 | |
| Nerve cord (A7.4) | 40 | 65 | 35 | |
| Nerve cord (A7.8) | 20 | 95 | 5 | |
| Endodermal strand (B7.2) | 13 | 100 | 0 | |
| Endoderm (A7.2) | 13 | 100 | 0 | |
| Wnt-5β mRNA | Notochord (A7.3/A7.7) | 24 | 8 | 92 |
| Muscle (B7.4) | 29 | 100 | 0 | |
| Nerve cord (A7.4) | 12 | 58 | 42 | |
| Nerve cord (A7.8) | 4 | 100 | 0 | |
Figure 4. Effects of Wnt5α mRNA injected into a notochord precursor blastomere.
(A) Lineage illustration of notochord. (B) Tail-bud embryos co-injected with the lineage tracer and control FS Wnt5α mRNA into the A7.3 blastomere. (C) Injection of Wnt5α mRNA. Arrows indicate normally intercalated notochord cells. Arrowheads show cells that failed to intercalate. (D) Closer view of the blue rectangle in (C). Three notochord cells successfully intercalated. (E) Closer view of the red rectangle in (C). The two cells marked with arrowheads failed to intercalate with each other. (F–I) Four examples of Wnt5α mRNA-injected embryos to show the wide spectrum of abnormality in intercalation. In (I), post-convergent elongation of notochord cells is taking place. Scale bar, 100 μm.
Overexpression of Wnt5α by injecting the A7.3 or A7.7 blastomere with Wnt5α mRNA essentially resulted in disorganization which was similar to that observed after knockdown of Wnt5α (91% of 110 cases; Table 1). Figure 4 shows several specimens of injected embryos (arrowheads indicate failure of intercalation and arrows indicate normal intercalation). As with MO injection, there was variation in the number of notochord cells that failed to intercalate. Notochord cells injected with universal control MO or control FS (frame-shifted) Wnt5α mRNA intercalated normally (100% and 72% normal respectively; Table 1). The results from knockdown and overexpression of Wnt5α indicated the importance of the precise control of the amount of Wnt5α protein in the developing notochord.
Wnt5α ectopic expression in surrounding tissues does not affect notochord morphogenesis
Next, we mis-expressed Wnt5α in various tissues that surround the notochord in the tail (Table 1 and Figure 5). Injection of Wnt5α mRNA into the B7.4 muscle precursor at the 64-cell stage did not affect the normal arrangement of muscle cells or notochord cells (Figures 5A–5C). The left and right B7.2 cells are the precursors of the endodermal strand. Their descendants intercalate mediolaterally to form a strand of cells aligned in a single file ventrally to the notochord (Figure 5D). The punctate labelling along the tail nerve cord in control embryos (Figure 5E, insert) substantiates this mediolateral intercalation. Injection of the mRNA into the left or right B7.2 cells did not affect notochord morphogenesis, but mediolateral intercalation of their bilateral descendants into the endodermal strand was prevented (Figures 5E and 5F). The A7.2 cell is adjacent to the notochord precursor blastomere in early embryos, but its descendants develop into trunk endoderm, and during late morphogenesis are distanced from the notochord. Ectopic expression of Wnt5α in A7.2 descendants did not affect normal embryogenesis (Figures 5G–5I).
The tail nerve cord dorsally flanks the notochord. In the ascidian, the structure of the tail nerve cord is remarkably simple, consisting of four rows of cells, namely the dorsal, ventral, left and right rows. Descendant cells of the left and right A7.4 blastomeres intercalate into the midline and form the single ventral row of the tail nerve cord (Nishida, 1987). The punctate labelling along the tail nerve cord (Figures 5J and 5K; see also Figure 6G) in control embryos substantiates this mediolateral intercalation. In contrast, descendant cells of the left and right A7.8 blastomeres give rise to the left and right rows respectively, without intercalation. Ectopic expression of Wnt5α in nerve cord did not substantially disrupt notochord morphogenesis. However, when the mRNA was injected into A7.4 blastomeres, the mediolateral intercalation of the descendant nerve cord cells was abrogated in 91% of cases, forming a continuous line of labelled cells (n=22, compare Figures 5K and 5L). In addition to the abnormality in the nerve cord, the notochord was deformed in 35% of the cases (Table 1). The failure in extension of dorsal nerve cord could indirectly affect the notochord shape, or alternatively ectopic expression of Wnt5 in the nerve cord may somehow affect notochord morphogenesis. In contrast, injection into A7.8 blastomeres did not affect nerve cord morphogenesis in 82% of cases (n=17, Figures 5M and 5N). These results suggest that Wnt5α plays a role in the intercalation of notochord cells within the notochord, and probably is not the source of polarizing cues arising outside the notochord. In the embryo shown in Figure 5(O), we ablated all of the A-line nerve cord precursor blastomeres at the 64-cell stage. The notochord of the embryo developed normally, indicating that the presence of the neural tube is dispensable for notochord morphogenesis in ascidians.
Then we evaluated the effects of Wnt5β MO. As Wnt5β is expressed in muscle cells, B7.4 blastomeres were injected with the MO. The development of the embryos appeared to be normal (Figures 6A and 6B).
Injection of Wnt5β mRNA into notochord, muscle and nerve cord blastomeres gave results similar to those of Wnt5α mRNA injection (Figures 6C–6H and Table 1), namely that notochord intercalation was affected only when Wnt5β was overexpressed in notochord blastomeres. Again, when the A7.4 blastomeres were injected with Wnt5β mRNA, mediolateral intercalation of the ventral nerve cord cells was abrogated in 55% of cases (n=11; compare Figures 6G and 6H, punctate labelling and continuous labelling respectively). Therefore Wnt5α and Wnt5β are expressed in distinct tissues, but they are interchangeable in these overexpression experiments.
Mosaic analysis of Wnt knockdown and overexpression within the notochord
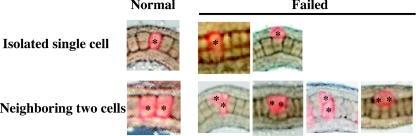
Next, the results of injection of Wnt5α MO and mRNA into notochord precursor blastomeres were analysed in more detail at the single cell level. In the experiments described above, the mixture of eight injected/labelled notochord cells and the other 32 uninjected notochord cells form a whole notochord in a mosaic manner and in random order along the anterior–posterior axis. We categorized the abnormalities associated with each notochord cell to evaluate the cell-autonomous and -non-autonomous behaviour of the cell (Figure 7 and Table 2). First, we noticed that if a single labelled notochord cell was isolated and sandwiched between unlabelled notochord cells, it was incorporated normally into the aligned notochord. In contrast, if two or more labelled notochord cells came in contact, they failed to intercalate properly. Some examples of abnormality are shown in Figure 7. The detailed proportions of abnormalities are described in Table 2. In the knockdown experiment, notochord cells intercalated normally in 77% of cases when the labelled cell happened to be isolated. In contrast, only 11% of cases showed normal intercalation when two labelled cells were in contact. Wnt5α-overexpressing notochord cells showed behaviour similar to those observed in the knockdown experiments (normal intercalation in 76% of isolated and in 21% of juxtaposed cases).
Figure 7. Mosaic analysis of Wnt knockdown and overexpression within the notochord.
A single notochord precursor blastomere was injected with Wnt5α MO or mRNA, or with Dsh MO. Examples of normal and abnormal intercalation of the labelled cells (asterisks) in the two situations where labelled cells are present in isolation or two labelled cells are adjacent. Abnormal positioning of labelled cells is categorized into two and four types respectively. The detailed frequency of each abnormality is shown is Table 2.
Table 2. Intercalation of notochord cells in embryos in which Wnt-5α/Dsh antisense MOs or mRNA were injected into notochord blastomeres.
Relative positions of labelled cells within the notochord are indicated in red. Isolated single cell: labelled cells are present in isolation. Neighbouring two cells: two labelled cells are adjacent. n=number of individual labelled notochord cells counted. Representative specimen of each normal or abnormal case is shown in Figure 7. Note that the results obtained from injection of Wnt5α MO or mRNA, or of Dsh MO, are quite similar. Concentrations of the injected solutions are indicated in parentheses.
| Isolated single cell | Neighbouring two cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercalation (%) | Intercalation (%) | |||||||||
| Normal | Failed | Normal | Failed | |||||||
| n | n | |||||||||
| Wnt-5α MO (10–20 μg/μl) | 48 | 77 | 6 | 17 | 28 | 11 | 11 | 11 | 7 | 61 |
| Total 23 | Total 89 | |||||||||
| Wnt-5α mRNA (0.8–3.6 μg/μl) | 104 | 76 | 12 | 13 | 42 | 21 | 21 | 26 | 14 | 17 |
| Total 24 | Total 79 | |||||||||
| Dsh MO (5–10 μg/μl) | 52 | 79 | 4 | 17 | 14 | 14 | 21 | 0 | 21 | 43 |
| Total 21 | Total 86 | |||||||||
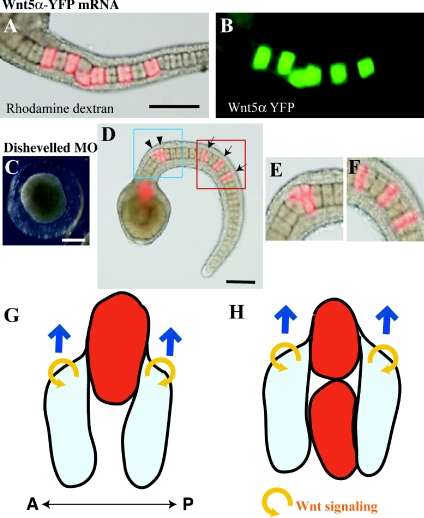
To evaluate how far the Wnt5α protein diffuses within the notochord, we injected an mRNA that encodes the Wnt5α–YFP (yellow fluorescent protein) fusion protein. As shown in Figures 8(A) and 8(B), overexpressed protein stayed close to the expressing cells, as shown in other animals (Christian, 2000).
Figure 8. Wnt5α may function in a cell-autonomous manner.
(A, B) The lineage tracer (rhodamine dextran; red) and mRNA encoding the Wnt5α–YFP fusion protein (green) were co-injected into a notochord precursor blastomere. The green signal remains close to the injected descendant cells. (C) An embryo in which Dsh MO was injected into fertilized eggs. (D) An embryo in which Dsh MO was injected into a notochord precursor blastomere. Arrows indicate normally intercalated notochord cells. Arrowheads show cells that failed to intercalate. (E, F) Closer views of the blue and red rectangles shown in (D). Scale bar, 100 μm. (G) A descendant cell of the MO- and mRNA-injected blastomere (red) is present in isolation. Flanking normal cells (light blue) may migrate on the surface of the anomalous cell. Blue arrows indicate the direction of movement of the normal cells. Yellow circular arrows refer to the autocrine Wnt5α action. Anterior is to the left. A, anterior; P, posterior. (H) When anomalous cells are closely adjacent they fail to migrate on each other. See the text for further details.
Mosaic analysis of Dsh knockdown
Then we examined the effects of suppressing Dsh function in a mosaic manner within the notochord. Dsh encodes an intracellular protein and controls PCP in a cell-autonomous manner in Drosophila (Theisen et al., 1994). It is involved in the control of convergent extension during axis elongation in vertebrates (e.g. Wallingford et al., 2000). Dsh is also essential for ascidian notochord morphogenesis and shows cell autonomy, as the dominant-negative form exerts its effect only in the notochord cells in which it is expressed (Keys et al., 2002).
First, when Dsh MO was injected into fertilized eggs, tail formation was strongly suppressed (Figure 8C). Then Dsh MO was injected into an A7.3 blastomere of the 64-cell embryo. The results of mosaic analysis were again rather similar to those obtained from the Wnt5α knockdown and overexpression experiments (Figures 8D–8F). Notochord cells intercalated normally in 79% of cases when the labelled cell was isolated, suggesting that a cell with an impaired intracellular PCP pathway can intercalate when flanked by normal cells. Accordingly, only 14% of cases showed normal intercalation when two labelled cells were adjacent (Tables 1 and 2).
Discussion
Role of the Wnt/PCP pathway in intercalation of ascidian notochord cells
We studied the role of the Wnt/PCP pathway in intercalation of ascidian notochord cells. In Halocynthia embryos, two Wnt5 genes are expressed. Wnt5α is expressed in the developing notochord. Wnt5α was overexpressed in a previous study (Sasakura and Makabe, 2001), and Wnt5α was knocked down in the present study. Together, the results of these experiments indicate that precise quantitative control of Wnt5α expression is crucial for proper cell intercalation. Therefore the function of Wnt5 in conversion extension during axis elongation is common to ascidians and vertebrates (Rauch et al., 1997; Wallingford et al., 2001). In contrast, Wnt5β is expressed in muscle precursors and its role in morphogenesis is unclear. When Wnt5β MO was injected into eggs, the embryos developed into abnormally shaped larvae, whereas we did not observe any effect when the MO was injected into muscle precursor blastomeres of 64-cell embryos. These observations suggest that Wnt5β might have similar early functions to those of Wnt5α, although the efficacy and specificity of Wnt5β MO need to be confirmed in more detailed studies. Overexpression of Wnt5β in muscle cells had no effect on the spatial arrangement of muscle and notochord cells, but over/mis-expression in notochord abrogated cell intercalation. Therefore, although Wnt5α and Wnt5β are expressed in distinct tissues, they were interchangeable in our overexpression experiments.
After the mid tail-bud stage, the notochord further elongates in a post-convergent extension process mediated by simple elongation of each constituent cell along the anterio–posterior axis. This post-convergent extension elongation was not affected in Wnt-manipulated embryos, as shown in Figure 4(I). Consistent with this observation, embryos in which the PCP pathway is interrupted demonstrate normal post-convergent extension elongation (Jiang and Smith, 2007).
Wnt5α overexpression within the notochord, but not in the surrounding tissues, affects notochord morphogenesis
To inhibit the intercalation of notochord cells, Wnt5 mRNA had to be injected into notochord precursor blastomeres. Ectopic expression in surrounding tissues flanking the central notochord, such as nerve cord, muscle and endodermal strand, essentially had no effect on notochord morphogenesis. These results suggest that Wnt5α plays its role in intercalation of notochord cells within the notochord, and is unlikely to be the source of polarizing cues arising outside the notochord. This is consistent with its expression pattern in the developing notochord and the requirement for its expression in the notochord revealed by injection of the MO, as well as with the observation that the Wnt5α–YFP fusion protein did not appear to diffuse across tissue boundaries. It is also unlikely that Wnt5β expressed in flanking muscle cells functions in convergent extension of notochord.
Wnt5α may control PCP in a cell-autonomous manner
The results of knockdown experiments appeared to indicate a cell-non-autonomous effect of Wnt5α, because the targeted cell was able to intercalate normally if it was flanked by normal cells. However, caution is required when concluding the cell non-autonomy of Wnt5α function. First, the results of overexpression are difficult to interpret according to cell-non-autonomous function of Wnt5α. Detailed observations at the single cell level gave essentially similar results in both knockdown and overexpression experiments. Most of the cells overexpressing Wnt5α were able to intercalate normally if they were flanked by normal cells, as was also observed in the knockdown experiments. It is difficult to imagine that neighbouring normal notochord cells, which themselves express Wnt5α, are able to annul the deleterious effects of overexpression of Wnt5α in the injected cells, causing Wnt5α-overexpressing cells to intercalate normally. In addition, unlabelled cells adjacent to an overexpressing labelled cell intercalated normally, regardless of whether the labelled cell was integrated into the aligned notochord or not (see Figures 4C and 4G), suggesting that overexpressed Wnt5α does not affect the morphogenetic movements of the neighbouring cells.
To overcome such difficulties in explaining what we observed in mosaic analyses, we propose an alternative model in which Wnt5α controls intercalation movement in a cell-autonomous manner (Figures 8G and 8H). When a descendant cell of the MO- or mRNA-injected blastomere is present in isolation (indicated in red), the cell loses its polarity and is unable to show proper motility for mediolateral intercalation. However, flanking normal cells (shown in light blue) may migrate on the surface of the anomalous cell. In consequence, this group of cells appears to be normally intercalated (Figure 8G). In contrast, when anomalous cells are closely adjacent they fail to migrate on each other, and consequently they fail to intercalate (Figure 8H). This explanation also resolves the contradiction of why, in the situations shown in Figures 8(G) and 8(H), the red cell succeeds in intercalation only in the case of Figure 8(G), even though the red cells are sandwiched by normal cells in both Figures 8(G) and 8(H). If Wnt functions cell-non-autonomously, the red cells would receive a Wnt signal from flanking normal cells and would intercalate with each other, even in Figure 8(H).
A cell-autonomous function of Wnt5α is consistent with the observation that the overexpressed Wnt5α–YFP fusion protein did not appear to diffuse over a long distance. The cell-autonomous model is further supported by the observation that mosaic analysis of Dsh MO-injected embryos gave results similar to those of Wnt5α disruption. As Dsh is an intracellular protein that has been shown to function in a cell-autonomous manner, the similarity in the results suggests a similarity in mode of function, namely that Wnt5α also functions in a cell-autonomous manner. The results with Dsh MO suggest that, even if a cell has an impaired intracellular PCP pathway, it can apparently intercalate when flanked by normal cells, supporting our cell-autonomous model of Wnt5α function. Thus, as shown in Figure 8, it is plausible that Wnt5α functions in an autocrine manner to polarize protrusive activity that drives cell intercalation in convergent extension morphogenesis.
Role of Wnt/PCP pathway in other tissues
Injection of the mRNAs for Wnt5α and Wnt5β into the precursor blastomere of the endodermal strand and into that of the ventral row of the tail nerve cord resulted in failure of mediolateral intercalation of the descendants of the injected cells. In contrast, injection into the precursor blastomere of the lateral row of the tail nerve cord had no effect. Therefore, Wnt/PCP-mediated intercalation at the midline may also operate in the endodermal strand and the ventral row of the tail nerve cord, resulting in alignment of cells in a single row. However, these results were obtained in ectopic expression experiments, and there is no evidence for Wnt5 expression in these tissues. Other members of the Wnt family might be expressed and function in these tissues. Indeed, it has been reported that the Wnt7 gene is specifically expressed in the dorsal and ventral rows of developing nerve cord (Sasakura and Makabe, 2000), suggesting a role in intercalation of the nerve cord cell at the midline. We have injected the ventral nerve cord precursor blastomere, A7.4, with Dsh MO, and observed malformation of the nerve cord in 13% of cases (n=23; data not shown). The proportion is relatively lower than that observed in the notochord when Dsh MO was injected into notochord precursors (88%, Table 1). Therefore further analysis is needed to address the functions of the Wnt/PCP pathway in the nerve cord and the endodermal strand.
In the present study, the role of Wnt signalling in ascidian convergent extension was investigated. Future analysis would further address the cell-autonomous model of Wnt function. A still unresolved issue concerning convergent extension in animal development is the source of the polarizing cues that define the anterior–posterior axis orientation of the extension and that presumably arise outside the converging tissues.
Materials and methods
Animals and embryos
Adults of the ascidian H. roretzi were collected near the Asamushi Research Center for Marine Biology, Aomori, Japan, and the Otsuchi International Coastal Research Center, Iwate, Japan. Naturally spawned eggs were fertilized with a suspension of non-self sperm and raised in Millipore-filtered seawater containing 50 μg/ml streptomycin sulfate and 50 μg/ml kanamycin sulfate at 9–13°C. At 13°C, embryos develop to the tail-bud stage at 18 h and into swimming tadpoles at 35 h after fertilization.
Plasmid construction for mRNA synthesis
Hr-Wnt5α (Sasakura et al., 1998; accession no. AB006608) and Hr-Wnt5β (Miya and Nishida, 2002; accession no. AB072595) mRNAs were transcribed from pBluescriptHTB (Akanuma et al. 2002), each containing an open reading frame, with the mMessage mMachine T3 kit (Ambion) and a Poly(A) Tailing kit (Ambion). FS Hr-Wnt5β, in which the cDNA has a frameshift at 470 bp and encodes a truncated protein, was provided by Dr T. Miya (Department of Biological Sciences, Tokyo Institute of Technology, Yokohama, Japan) and used as a control. To visualize Hr-Wnt5α protein distribution, cDNA of Venus–YFP (Nagai et al., 2002) was conjugated in-frame to the 3′ end of the cDNA of the Hr-Wnt5α protein-coding region. Then the construct was subcloned into the pBluescriptHTB vector and was used for mRNA synthesis. Synthetic mRNAs were dissolved in sterile distilled water at 2 μg/μl and injected into eggs and blastomeres as described previously by Miya et al. (1997).
Antisense MOs
To suppress the function of Hr-Wnt5α and Hr-Wnt5β and of Dsh, we used antisense MOs (Gene Tools). The nucleotide sequence of Hr-Wnt5α MO was 5′-ATTGTATTCTTGTCATTCCGACCAT-3′ (Nakamura et al., 2006), that of Hr-Wnt5β MO was 5′-ATAATTTCGTTTTCAAGATTCCGCA-3′, and that of Hr-Dsh (accession no. AB290435) MO was 5′-AACTATCTTGGTTTCTTCCGCCATA-3′ (Kawai et al., 2007). MOs were dissolved in sterile distilled water at 5 μg/μl and injected into eggs and blastomeres. To trace the descendants of injected blastomeres, 0.5% dextran tetramethylrhodamine (10000 Da molecular mass; Molecular Probes) was injected, together with synthetic mRNA and MO.
Visualization of cellular boundaries
Alexa Fluor® 488 phalloidin (Molecular Probes) is a drug that binds to filamentous actin and emits green fluorescence. This is used to visualize cellular boundaries during notochord formation (Munro and Odell, 2002a). Embryos were fixed in 4% paraformaldehyde dissolved in 45 mM EGTA/360 mM sucrose, buffered with 90 mM Tris/HCl (pH 6.9), for 30 min at room temperature (20°C) or for 16 h at 4°C. Embryos were rinsed four times with PBST (PBS with 0.2% Triton X-100), then stained with a solution of 5 units/ml Alexa Fluor® 488 phalloidin in PBST for 1 h at room temperature or for 16 h at 4°C. They were rinsed with PBST, mounted in 80% glycerol and observed with a fluorescence microscope (Olympus BX61) or confocal microscope (Yokogawa CSU10).
Immunostaining and in situ hybridization
Differentiation of notochord cells was monitored by staining with the Not-1 monoclonal antibody (Nishikata and Satoh, 1990; Nakatani and Nishida, 1994). Specimens were fixed at the tail-bud stage with 4% paraformaldehyde for 1 h at room temperature and then with methanol cooled to −20°C for 10–40 min. Indirect immunofluorescence was carried out by standard methods using a TSA™ fluorescein system (PerkinElmer Life Sciences). Specimens were mounted in 80% glycerol and examined under a fluorescence microscope. Whole-mount in situ hybridization was performed as described by Miya and Satoh (1997). Specimens were hybridized with a DIG (digoxigenin)-labelled Hr-Bra probe. Hr-Bra encodes the Halocynthia Bra gene, and was used to assess notochord specification (Yasuo and Satoh, 1993). The expression of Hr-Bra was monitored at the 110-cell stage.
Acknowledgements
We thank the members of the Asamushi Research Center for Marine Biology and the Otsuchi International Coastal Research Center for help in collecting live ascidian adults, and the members of the Seto Marine Biological Laboratory for help in maintaining them.
Funding
This work was supported by Grants in Aid from the Japan Society for the Promotion of Science [grant number 16107005] to H.N.; the Ministry of Education, Culture, Sports, Science and Technology, Japan [grant numbers 16770161, 18770200] to G.K.; and by a Toray Science and Technology Grant to H.N.
References
- Akanuma T., Hori S., Darras S., Nishida H. Notch signaling is involved in neurogenesis in the ascidian embryos. Dev. Genes Evol. 2002;212:459–472. doi: 10.1007/s00427-002-0264-x. [DOI] [PubMed] [Google Scholar]
- Barrow J.R. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin. Cell Dev. Biol. 2006;17:185–193. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Christian J.L. BMP, Wnt and Hedgehog signals: how far can they go? Curr. Opin. Cell Biol. 2000;2:244–249. doi: 10.1016/s0955-0674(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Cloney R.A. Development of the ascidian notochord. Acta Embryol. Morphol. Exp. 1964;7:111–130. [Google Scholar]
- Fanto M., McNeill H. Planar polarity from flies to vertebrates. J. Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Keller R. Region-specific cell activities in amphibian gastrulation. Annu. Rev. Cell Biol. 1986;2:201–229. doi: 10.1146/annurev.cb.02.110186.001221. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.P., Tada M., Rauch G.J., Saude L., Concha M.L., Geisler R., Stemple D.L., Smith J.C., Wilson S.W. Silberblick/Wnt11 activity mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hino K., Satou Y., Yagi K., Satoh N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. VI. Genes for Wnt, TGFβ, Hedgehog and JAK/STAT signaling pathways. Dev. Genes Evol. 2003;213:264–272. doi: 10.1007/s00427-003-0318-8. [DOI] [PubMed] [Google Scholar]
- Hotta K., Takahashi H., Ueno N., Gojobori T. A genome-wide survey of the genes for planar polarity signaling or convergent extension-related genes in Ciona intestinalis and phylogenetic comparisons of evolutionary conserved signaling components. Gene. 2003;317:165–185. doi: 10.1016/s0378-1119(03)00700-5. [DOI] [PubMed] [Google Scholar]
- Imai K.S., Hino K., Yagi K., Satoh N., Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- Jiang D., Smith W.C. Ascidian notochord morphogenesis. Dev. Dyn. 2007;236:1748–1757. doi: 10.1002/dvdy.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Munro E.M., Smith W.C. Ascidian prickle regulates both mediolateral and anterior–posterior cell polarity of notochord cells. Curr. Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kawai N., Iida Y., Kumano G., Nishida H. Nuclear accumulation of β-catenin and transcription of downstream genes are regulated by zygotic Wnt5α and maternal Dsh in ascidian embryos. Dev. Dyn. 2007;236:1570–1582. doi: 10.1002/dvdy.21169. [DOI] [PubMed] [Google Scholar]
- Keys D.N., Levine M., Harland R.M., Wallingford J.B. Control of intercalation is cell-autonomous in the notochord of Ciona intestinalis. Dev. Biol. 2002;246:329–340. doi: 10.1006/dbio.2002.0656. [DOI] [PubMed] [Google Scholar]
- Keller R., Davidson L., Edlund A., Elul T., Ezin M., Shook D., Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J.C. Planar cell polarity: heading in the right direction. Dev. Dyn. 2005;233:695–700. doi: 10.1002/dvdy.20359. [DOI] [PubMed] [Google Scholar]
- Miya T., Satoh N. Isolation and characterization of cDNA clones for β-tubulin genes as a molecular marker for neural cell differentiation in the ascidian embryo. Int. J. Dev. Biol. 1997;41:551–557. [PubMed] [Google Scholar]
- Miya T., Nishida H. Isolation of cDNA clones for mRNAs transcribed zygotically during cleavage in the ascidian, Halocynthia roretzi. Dev. Genes Evol. 2002;212:30–37. doi: 10.1007/s00427-001-0201-4. [DOI] [PubMed] [Google Scholar]
- Miya T., Morita K., Suzuki A., Ueno N., Satoh N. Functional analysis of an ascidian homologue of vertebrate Bmp-2/Bmp-4 suggests its role in the inhibition of neural fate specification. Development. 1997;124:5149–5159. doi: 10.1242/dev.124.24.5149. [DOI] [PubMed] [Google Scholar]
- Miyamoto D.M., Crowther R.J. Formation of the notochord in the living ascidian. J. Embryol. Exp. Morphol. 1985;86:1–17. [PubMed] [Google Scholar]
- Munro E.M., Odell G.M. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002a;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- Munro E.M., Odell G. Morphogenetic pattern formation during ascidian notochord formation is regulative and highly robust. Development. 2002b;129:1–12. doi: 10.1242/dev.129.1.1. [DOI] [PubMed] [Google Scholar]
- Munro E., Robin F., Lemaire P. Cellular morphogenesis in ascidians: how to shape a simple tadpole. Curr. Opin. Genet. Dev. 2006;16:399–405. doi: 10.1016/j.gde.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K., Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Makabe K.W., Nishida H. The functional analysis of TypeI postplasmic/PEM mRNAs in embryos of the ascidian Halocynthia roretzi. Dev. Genes Evol. 2006;216:69–80. doi: 10.1007/s00427-005-0035-6. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Nishida H. Induction of notochord during ascidian embryogenesis. Dev. Biol. 1994;166:289–299. doi: 10.1006/dbio.1994.1315. [DOI] [PubMed] [Google Scholar]
- Nishida H. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Dev. Biol. 1987;121:526–541. doi: 10.1016/0012-1606(87)90188-6. [DOI] [PubMed] [Google Scholar]
- Nishikata T., Satoh N. Specification of notochord cells in the ascidian embryos analyzed with a specific monoclonal antibody. Cell Differ. Dev. 1990;30:43–53. doi: 10.1016/0922-3371(90)90073-6. [DOI] [PubMed] [Google Scholar]
- Rauch G.J., Hammerschmidt M., Blader P., Schauerte H.E., Strahle U., Ingham P.W., McMahon A.P., Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 1997;62:227–234. [PubMed] [Google Scholar]
- Sasakura Y., Makabe K.W. Ascidian Wnt-7 gene is expressed exclusively in the tail neural tube of tailbud embryos. Dev. Genes Evol. 2000;210:641–643. doi: 10.1007/s004270000108. [DOI] [PubMed] [Google Scholar]
- Sasakura Y., Makabe K.W. Ascidian Wnt-5 gene is involved in the morphogenetic movement of notochord cells. Dev. Growth Differ. 2001;43:573–583. doi: 10.1046/j.1440-169x.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Sasakura Y., Ogasawara M., Makabe K.W. HrWnt-5: a maternally expressed ascidian Wnt gene with posterior localization in early embryos. Int. J. Dev. Biol. 1998;42:573–579. [PubMed] [Google Scholar]
- Tada M., Smith J.C. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Theisen H., Purcell J., Bennett M., Kanasagara D., Syed A., Marsh J.L. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Veeman M.T., Nakatani Y., Hendrickson C., Lin C., Smith W.C. chonmague reveals an essential roles for laminin-mediated boundary formation in chordate convergence and extension movements. Development. 2008;135:33–41. doi: 10.1242/dev.010892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J.B. Closing in on vertebrate planar polarity. Nat. Cell Biol. 2004;6:687–689. doi: 10.1038/ncb0804-687. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Rowning B.A., Vogeli K.M., Rothbacher U., Fraser S.E., Harland R.M. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Vogeli K.M., Harland R.M. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int. J. Dev. Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Wallingford J.B., Fraser S.E., Harland R.M. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wang Y., Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Yasuo H., Satoh N. Conservation of the developmental role of Brachyury in notochord formation in a urochordate, the ascidian Halocynthia roretzi. Dev. Biol. 1998;200:158–170. doi: 10.1006/dbio.1998.8958. [DOI] [PubMed] [Google Scholar]