Abstract
Diabetic cardiomyopathy is a distinct primary disease process, independent of coronary artery disease, which leads to heart failure in diabetic patients. Epidemiological and clinical trial data have confirmed the greater incidence and prevalence of heart failure in diabetes. Novel echocardiographic and MR (magnetic resonance) techniques have enabled a more accurate means of phenotyping diabetic cardiomyopathy. Experimental models of diabetes have provided a range of novel molecular targets for this condition, but none have been substantiated in humans. Similarly, although ultrastructural pathology of the microvessels and cardiomyocytes is well described in animal models, studies in humans are small and limited to light microscopy. With regard to treatment, recent data with thiazoledinediones has generated much controversy in terms of the cardiac safety of both these and other drugs currently in use and under development. Clinical trials are urgently required to establish the efficacy of currently available agents for heart failure, as well as novel therapies in patients specifically with diabetic cardiomyopathy.
Keywords: diabetic cardiomyopathy, heart failure, hyperglycaemia, Type 2 diabetes
Abbreviations: ACE, angiotensin-converting enzyme; ACEI, ACE inhibitor; AGE, advanced glycosylation end-product; AngII, angiotensin II; AT1, AngII type 1 receptor; ARB, AT1 receptor blocker; BK, bradykinin; BNP, brain natriuretic peptide; BP, blood pressure; B1R, B1-receptor; B2R, B2-receptor; CHARM, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity; CHS, Cardiovascular Health Study; CTGF, connective tissue growth factor; DAG, diacylglycerol; DGK, DAG kinase; DPP-4, dipeptidyl peptidase-4; EF, ejection fraction; ERK, extracellular-signal-regulated kinase; ET, endothelin; FA, fatty acid; GLP-1, glucagon-like peptide-1; GLUT4, glucose transporter 4; HbA1c, glycated haemoglobin; HF, heart failure; HFNEF, HF with a normal EF; HIF, hypoxia-inducible factor; KLK, kallikrein; hKLK, human KLK; KKS, KLK–kinin system; LV, left ventricular; LVED, left ventricular end-diastole; LVH, LV hypertrophy; LVM, LV mass; LVMI, LVM index; MESA, Multi-Ethnic Study of Atherosclerosis; MHC, myosin heavy chain; MI, myocardial infarction; MR, magnetic resonance; MRI, MR imaging; NCX, Na2+/Ca2+ exchange; NEFA, non-esterified FA; NF-κB, nuclear factor κB; NYHA, New York Heart Association; PARP, poly(ADP-ribose) polymerase; PKC, protein kinase C; PMCA, plasma-membrane Ca2+-ATPase; QOL, quality-of-life; RAS, renin–angiotensin system; RAAS, renin–angiotensin–aldosterone system; RAGE, receptor for AGEs; ROS, reactive oxygen species; RR, relative risk; RV, right ventricular; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; SHS, Strong Heart Study; SOLVD, Studies of Left Ventricular Dysfunction; SR, sarcoplasmic reticulum; STZ, streptozotocin; TDI, tissue Doppler imaging; TGF-β1, transforming growth factor-β1; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; TZD, thiazolidinedione; UKPDS, UK Prospective Diabetes Study; VEGF, vascular endothelial growth factor; V̇O2max, maximal oxygen consumption
INTRODUCTION
The link between HF (heart failure) and diabetes is well documented, but the existence of diabetic cardiomyopathy as a distinct clinical entity continues to be the subject of debate. In 1881, Leyden commented that HF was a “frequent and noteworthy complication of diabetes mellitus” [1] and Mayer stated that “heart disease in diabetes can be traced to an abnormality in metabolism” [2]. In 1972, Rubler et al. [3] coined the term ‘diabetic cardiomyopathy’ after performing post mortem studies in diabetic patients with cardiac failure, having excluded alcohol, hypertension, and coronary and structural heart disease as possible aetiologies. Diabetic cardiomyopathy has since been the subject of much research and controversy, with evolving debate on the best management strategy for this entity. By definition, diabetic cardiomyopathy is a distinct primary disease process which develops secondary to a metabolic insult, resulting in structural and functional abnormalities of the myocardium leading to HF. We have comprehensively updated our previous review [4], taking into account the most recent developments in basic and translational research, together with an overview of the latest diagnostic and therapeutic approaches to diabetic cardiomyopathy.
EPIDEMIOLOGY
The prevalence of HF in the general population ranges from 1 to 4%, but in diabetic patients it is 12% [5], rising to 22% in those over the age of 64 years [6]. Up to a third of all patients admitted to hospital with HF have diabetes [7]. Furthermore, diabetes has a prevalence of 30% in patients with cardiac failure [8] and may be up to four times as prevalent in patients with newly diagnosed HF [9]. Diabetes is also a powerful predictor of cardiovascular morbidity and mortality, and is an independent risk factor for death in patients with established HF [10]. Diabetic patients are also more likely than non-diabetic patients to develop HF following MI (myocardial infarction), despite comparable infarct sizes [11]. The Framingham Heart Study reported a 2.4-fold increase in the incidence of HF in diabetic men and a 5.1-fold increase in diabetic women, when compared with age-matched controls [12]. This association was independent of age, hypertension, dyslipidaemia, obesity and coronary heart disease. Other large population-based studies have yielded similar results [13]. The CHS (Cardiovascular Health Study) of patients aged over 65 years showed diabetes to be associated with an increase in incident HF [14] and the SHS (Strong Heart Study) demonstrated associations between diabetes and higher LVM [LV (left ventricular) mass] and wall thickness, increased arterial stiffness and systolic dysfunction, compared with matched controls [15]. More recently, the MESA (Multi-Ethnic Study of Atherosclerosis) study used cardiac MR (magnetic resonance) to report inter-racial differences in LVM, LV volumes and LV function among diabetic patients [16].
Cross-sectional studies have consistently reported a high prevalence of HF in diabetic populations [6]. Alarmingly, one study observed 12% of Type 2 diabetic patients with HF at entry, with an annual incidence of 3.3% [17]. In a similar study over 43 months, the incidence of HF was vastly higher in diabetic (39%) compared with non-diabetic (23%) patients, with an RR (relative risk) of 1.3 for developing HF [18]. The UKPDS (UK Prospective Diabetes Study) found an increased prevalence of HF in Type 2 diabetic patients, which correlated with higher HbA1c (glycated haemoglobin) levels [19]. Indeed, for every 1% increase in HbA1c, there is an 8% increased risk of developing HF [20]. A recently published study has demonstrated an increased risk of HF in diabetic patients with retinopathy, supporting the concept of a microvascular aetiology in diabetic heart disease [21]. Sub-group analysis of the MESA cohort also revealed an association between retinal arteriolar narrowing and LV remodelling, lending weight to this argument [22].
It is not surprising, therefore, that diabetic patients are over-represented in large HF trial populations; such as SOLVD (Studies of Left Ventricular Dysfunction) [23] (diabetic patients represent 26%), ATLAS (Assessment of Treatment with Lisinopril and Survival) [24] (diabetic patients represent 19%), V-HeFT (Vasodilator-Heart Failure Trial) [25] (diabetic patients represent 20%) and RESOLVD (Randomized Evaluation of Strategies for Left Ventricular Dysfunction) [26] (diabetic patients represent 27%). In addition to overt clinical diabetes, insulin resistance and the metabolic syndrome are also independent predictors of HF [27,28]. Inflammatory markers such as CRP (C-reactive protein) and IL (interleukin)-6, as well as microalbuminuria, which are frequently elevated in individuals with the metabolic syndrome, are independently associated with incident HF [29]. This suggests that cardiomyopathy may occur in ‘pre-diabetic’ individuals. In a retrospective study of a cohort of patients with a primary diagnosis of HF, metabolic syndrome was present in 68% of patients, but, interestingly, mortality was lower in those with (44%) compared with those without (58%) the metabolic syndrome [30].
RISK FACTORS ASSOCIATED WITH THE DEVELOPMENT OF HF IN DIABETES
Hyperglycaemia
In the UKPDS, although poor glycaemic control was associated with an increased risk of HF [19], intensive glycaemic control did not reduce the risk of incident HF. A more recent study of Type 2 diabetic patients has, however, demonstrated an improvement in long-axis function (systolic strain rate) and reductions in LVM, which were associated with improvements in HbA1c and fructosamine levels [31]. These changes were independent of any BP (blood pressure)-lowering effects. In addition, there was an improvement in diastolic function during the first 3 months of follow up, which returned to baseline at 12 months. Another study by Marwick and co-workers [32] has reported a similar association between lower HbA1c and improved systolic strain. In a 10 year follow-up of the UKPDS, despite an early loss of glycaemic difference between groups receiving intensive and conventional treatment, an emergent risk reduction for MI and death from any cause was observed following intensive glucose-lowering [33], although no data was reported for HF. However, conflicting data has been generated from two recent large randomized intervention trials. The ADVANCE (Action in Diabetes and Vascular Disease) trial compared intensive with standard glycaemic control and showed a non-significant reduction in incident HF, but an increased all-cause mortality at 3.5 years [34]. Increased mortality in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial led to the discontinuation of the intensive glycaemic control arm of the study [35].
Hypertension
Hypertension is independently associated with LVH (LV hypertrophy), diastolic dysfunction, HF and cardiovascular risk [4]. In the UKPDS, lower BP, achieved by either a β-blocker or ACEI [ACE (angiotensin-converting enzyme) inhibitor], was associated with a reduced risk of incident HF compared with less intensive BP control. However, again it is interesting to note that both during and in the recent 10 year follow-up of the UKPDS, BP reduction did not demonstrate the ‘legacy effect’, i.e. there was no risk reduction during or after the trial for MI or death from any cause [36].
STRUCTURAL FEATURES OF DIABETIC CARDIOMYOPATHY AND THEIR FUNCTIONAL RELEVANCE
LVH
LVH is a powerful predictor of cardiovascular risk. The Framingham Heart Study demonstrated an increased relative risk of developing cardiovascular disease for each 50 g/m2 increment in LVMI (LVM index; RR 1.49 compared with 1.57, males compared with females) [37]. Furthermore, LVH predicts prognosis in high-risk patient groups, such as those with coronary heart disease [38], HF [39], diabetes [40], renal failure [41], hypertension [42], obesity and previous MI [43]. Pharmacological regression of LVH is associated with a reduction in cardiovascular risk [44]. The RENAAL [Reduction of Endpoints in NIDDM (non-insulin-dependent diabetes mellitus) with the Angiotensin II Antagonist Losartan] study in patients with diabetic nephropathy, found that LVH was a significant risk factor for the primary endpoints of end-stage renal failure, death or cardiovascular events [45]. Several studies have since reported an association between diabetes and LVH [46]. The SHS reported increased LVM and LV wall thickness in both diabetic men and women, and these differences remained statistically significant in multivariate analyses after adjusting for confounding factors [47]. Similar findings were reported in the CHS [48] and MESA [49], as detailed above. Interestingly, a sub-study of the MESA cohort reported an independent association between retinal arteriolar narrowing and LV remodelling, which was stronger in diabetic patients, suggesting microvascular involvement [22]. The Framingham Heart Study also reported increased LVM across all categories of glucose dysmetabolism. A recent study of Japanese Type 2 diabetic patients reported an association between insulin resistance, arterial stiffness and LVMI [using cardiac MRI (MR imaging)] [47], and this has been supported by a large population-based study in Sweden, which demonstrated associations between metabolic syndrome, insulin resistance and increased LVM and LV wall thickness [48].
Diastolic dysfunction
Diastolic function of the left ventricle is determined by its passive elastic properties, coupled with the process of active relaxation. Diastolic dysfunction is characterized by impairment of relaxation and passive filling of the left ventricle [49], and diastolic HF is said to exist when diastolic dysfunction is associated with an elevated end diastolic pressure, clinical features of HF and a normal EF (ejection fraction). This may be better termed HFNEF (HF with a normal EF), since there is emerging evidence of subtle abnormalities in regional and long-axis systolic function in some of these patients.
Functional abnormalities occur as a result of structural remodelling (concentric LVH) and result in normal or near-normal end diastolic volume, elevated LVM to volume and elevated wall thickness to chamber radius relationships respectively. Indeed, the development of diastolic dysfunction has been associated with only modest increases in LVM [47]. In diabetic cardiomyopathy with reduced LVEF, myocardial collagen deposition and AGEs (advanced glycosylation end-products) are the primary pathological processes responsible for reduced elasticity of the myocardium, whereas increased cardiomyocyte resting tension may be the predominant cause in those cases with preserved LVEF [50]. LVH and geometric remodelling on the other hand, cause an increase in passive stiffness and impaired relaxation. Consequently, the LV pressure–volume curve is shifted upward and leftward, chamber compliance is reduced, diastolic filling is altered with an elevation of the end diastolic pressure, and HF ensues.
Diastolic dysfunction is a common finding in otherwise healthy and asymptomatic diabetic patients and is thought to be the earliest detectable functional abnormality in diabetic cardiomyopathy [51]. In a study of normotensive, asymptomatic Type 2 diabetic patients with good glycaemic control, 47% were found to have diastolic dysfunction [52]. Other studies using more sensitive diagnostic methods have reported that as many as 75% of diabetic patients demonstrate abnormalities of diastolic function [53].
Systolic dysfunction
Advances in TDI (tissue Doppler imaging) have allowed for measurement of sensitive indices of regional and long-axis function of both the left and right ventricles [54]. Indeed, long-axis function is emerging as a superior prognostic indicator to EF, especially in patients with HFNEF [55]. Using these sensitive methods, several studies have demonstrated subtle abnormalities in systolic function in patients with a diagnosis of diastolic dysfunction [56–58]. This has led some to question whether diastolic dysfunction exists in isolation at all [59], whereas others have questioned the relevance of these subtle systolic abnormalities in the context of diastolic dysfunction [60]. The longitudinal fibres responsible for long-axis contraction lie in the sub-endocardium and are particularly susceptible to the effects of fibrosis, ischaemia or hypertrophy. In diabetic patients with HFNEF, long-axis systolic dysfunction is associated with a compensatory increase in radial thickening and mass, thus preserving LVEF [61]. The preservation of EF is directly related to the presence of LVH and the effect of increased muscle mass. Patients with HFNEF have significantly higher LVMI, lower LVED (left ventricular end-diastole) volume index, higher LVMI/LVED volume index ratio than patients with dilated left ventricles and a low EF. Although most studies to date have concentrated on LV function, the importance of RV (right ventricular) function should not be overlooked; RVEF has previously been shown to be an independent predictor of poor outcome [62]. Furthermore, RV diastolic dysfunction has been reported in asymptomatic patients with Type 1 diabetes [63].
DIAGNOSTIC METHODS
Although no single diagnostic test for diabetic cardiomyopathy exists, using different imaging modalities it is possible to detect the phenotypic cardiac features of this condition. Currently used diagnostic methods in clinical practice include echocardiography, cardiac MR and cardiac biomarkers such as NT-BNP [N-terminal pro-BNP (brain natriuretic peptide)].
Echocardiography
Echocardiography is an excellent non-invasive and practical imaging tool for defining cardiac structure and function and allows ‘real-time’ visualization of the cardiac cycle. Quantitative and qualitative assessment of the heart can be made with regard to LV geometry, regional wall motion, and systolic and diastolic function, in addition to valvular anatomy and function. Two-dimensional echocardiography has traditionally been the method of choice in detecting and quantifying LVH, and has been validated in the research and clinical setting. The main limitation of two-dimensional echocardiography is for patients with major distortions of LV geometry. As a result, most existing data from studies of LVH and LVM have been derived using standard two-dimensional and M-mode echocardiography. Although considered to be the ‘gold standard’ for assessing LV diastolic function, cardiac catheterization is not essential to diagnose diastolic dysfunction [63]. Pulsed-wave Doppler echocardiography is therefore the most practical and commonly used method for current assessment of diastolic function. A detailed and comprehensive diastolic study is vital in diabetic patients and should include the measurement of transmitral and pulmonary venous flow/velocities, as well as left atrial volume [64]. LVEF is the most commonly used index of LV systolic function and is derived echocardiographically. Despite being well established in clinical practice, it is important to be aware that it can be influenced by alterations in preload and afterload.
LVEF is not as sensitive for assessing contractile function in patients with HFNEF and TDI echocardiography is more capable of detecting subtle regional abnormalities in LV function. TDI allows non-invasive assessment of myocardial strain and has been shown to identify global and regional abnormalities in myocardial properties, with a high level of temporal resolution. TDI differs from conventional Doppler in that it utilizes a filter which eliminates high velocity and low amplitude signals reflected from blood cells, thereby allowing low velocity, high amplitude tissue signals to be analysed. Myocardial strain and strain rate are dimensionless indices of changes in length that reflect tissue deformation [65]. These parameters have been extensively validated in a range of different disease states, including acute ischaemic syndromes [66], and have been found to be indicative of preclinical myocardial disease in patients with Type 2 diabetes [57].
Cardiac MRI
Cardiac MRI, owing to its superior accuracy and reproducibility, is the ‘gold standard’ for measuring LVM [67]. Currently however, its use is mainly limited to research, due to costs, time constraints and the expertise required for assessment.
Cardiac biomarkers
BNP is a cardiac hormone secreted in response to ventricular volume and pressure overload. Although it is both sensitive and specific for congestive HF, it cannot reliably distinguish between systolic and diastolic HF, which limits its diagnostic use in diabetic cardiomyopathy [56,68].
MOLECULAR BASIS
Several putative factors have been considered important in the genesis of the functional and structural alterations which lead to the development and progression of diabetic cardiomyopathy (Figure 1). The molecular basis of this condition has been established primarily from studies in experimental models of diabetic cardiomyopathy [69], with few studies carried out in humans [4].
Figure 1. Summary of interactions between the myocardial and vascular changes present in diabetic hearts and their contribution to diabetic cardiomyopathy and heart failure.
Ang II R1, AT1; IGF-1, insulin-like growth factor-1.
Hyperglycaemia
Several studies, most notably the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study, have shown that hyperglycaemia is indeed a mediator of cardiovascular risk in Type 1 diabetes and that intensive diabetic therapy reduces cardiac outcomes via an impact on accelerated atherosclerosis, cardiac autonomic neuropathy and possibly cardiomyopathy [70]. In a population-based cohort study of 746 subjects stratified into normal and abnormal glucose tolerance and overt Type 2 diabetes, there was an independent increase in risk of developing systolic (2-fold) and diastolic (2.4-fold) dysfunction in patients with Type 2 diabetes, with diastolic dysfunction apparent even in those with impaired glucose tolerance [71]. Hyperglycaemia mediates its damaging effects through a series of secondary transducers which will be considered.
ROS (reactive oxygen species) and NO
Oxidative stress occurs when the production of ROS outweighs their degradation by antioxidant defences and the elevation of ROS leads to cellular damage by oxidation, disruption of vascular homoeostasis through interference with NO and, most recently, by modulation of detrimental intracellular signalling pathways. ROS have been implicated in all stages of the development of HF, from cardiac hypertrophy to fibrosis, contractile dysfunction and failure [71]. Diabetic hearts exhibit a similar picture to that of failing hearts, with numerous studies reporting increased production of ROS in a variety of animal models of diabetes [71]. Increased ROS causes cardiac dysfunction by direct damage to proteins and DNA {inducing PARP [poly(ADP-ribose) polymerase] which will be considered later}, as well as by promoting apoptosis. Furthermore, overexpression [72] or pharmacological administration [73] of the antioxidant metallothionein in rodent models of diabetes has been shown to ameliorate diabetic cardiomyopathy. Similar results have been reproduced using other antioxidants in both Type 1 and Type 2 rodent models of diabetes [74]. NADPH oxidase enzymes are a source of ROS and are involved in redox signalling by acting as catalysts for electron transfer from NADPH to molecular oxygen, resulting in the generation of free radicals [75]. They comprise multisubunit complexes, which range from NOX1 to NOX5 [75]. Through interaction with a variety of transcription factors, redox signalling influences the expression of growth-related genes and in-turn effects contractile function [76]. NADPH oxidase is increased in both failing [77] and diabetic [78] rodent hearts and this relationship is believed to be causal. In diabetic animals, up-regulation of NADPH oxidase correlates with cardiac hypertrophy and up-regulation of pro-fibrotic genes such as pro-collagen III; changes which can be ameliorated using the antioxidant Tempol [79]. ROS also react directly with NO to form peroxynitrite species, thereby inactivating the vasodilatory effect of NO, which is integral to vascular homoeostasis and endothelial function. In combination with other pathology within the endothelium [80], these changes provide an explanation for accelerated endothelial dysfunction seen in prediabetic mice [81]. Interestingly, antioxidants such as vitamin C are capable of restoring endothelial function in patients with HF [82]. Reductions in the release of NO from endothelial cells, as a consequence of ROS, have also been shown to affect ventricular relaxation in LVH [83]. Thus experimental data suggest that regimes targeting reduction of ROS or increasing antioxidant activity potentially represent novel therapeutic modalities for diabetic cardiomyopathy. However, clinical studies to date have been unable to translate these results to the clinic, with several large randomized trials failing to report a benefit of antioxidants on cardiovascular outcome in high-risk individuals [84]. The outcome of such trials in patients with diabetic cardiomyopathy however, has not been investigated.
PARP
PARP enzymes are overactivated in diabetes [85] as a reparative response to ROS-induced oxidative damage to DNA (see above). PARP inhibits GAPDH (glyceraldehyde-3-phosphate dehydrogenase), which leads to accumulation of glycolytic intermediates, which in-turn activate a series of transducers which inflict tissue damage via AGE formation and PKC (protein kinase C) activation [85]. PARP also promotes cardiac damage by activating NF-κB (nuclear factor κB) [87] and inducing overexpression of the vasoconstrictor ET (endothelin)-1 and its receptors [88]. Both hyperhexosaemia and hyperglycaemia have been shown to induce oxidative stress and up-regulate extracellular matrix and cardiomyocyte hypertrophy and these changes were effectively abolished in PARP-1 knockout (Parp−/−) mice and in rats treated with the PARP inhibitor ABA (3-aminobenzamide) [89]. PARP-driven cardiac hypertrophy and fibrosis appears to be mediated in hearts and cardiomyocytes via up-regulation of the transcriptional co-activator p300 [89]. Thus the widespread damage caused by PARP potentially creates a unique opportunity for blocking multiple sources of cardiac injury and has been shown to inhibit many pathways thought to mediate myocardial damage in diabetes [85,90]. Additionally, PARP inhibition has been shown to reverse diabetic endothelial dysfunction [91]. Again, although the experimental data appear compelling, as yet no PARP inhibitors have been developed for clinical use, due to off-target, and perhaps on-target, drug toxicity.
PKC
PKC activity is increased in both failing [92] and diabetic [93] hearts, and levels correlate with both ROS [94] and PARP [85]. PKC phosphorylates a number of proteins directly involved in cardiac excitation–contraction coupling and therefore disturbs Ca2+ handling in cardiomyocytes [95]. Transgenic mice overexpressing the PKCβ2 isoform in the myocardium develop cardiac hypertrophy, fibrosis, impairment of LV function and progressive cardiomyopathy, but these changes are reversed using a PKCβ isoform-selective inhibitor [96]. DAG (diacylglycerol) activates PKC and is phosphorylated by DGK (DAG kinase), converting it into phosphatidic acid and thereby inactivating its function in respect to DAG [97]. It has recently been shown that overexpression of the DGKζ isoform in both cultured mouse cardiomyocytes and isolated perfused hearts [98], as well as diabetic mice [99], prevents pathological activation of PKC, attenuating cardiac hypertrophy and fibrosis and improving ventricular function. Conversely, targeted overproduction of the PKCβ2 isoform in the myocardium results in LVH, multifocal fibrosis and cardiomyopathy in mice [96]. CTGF (connective tissue growth factor), which contributes to cardiac hypertrophy and fibrosis is overexpressed in concert with PKCβ2 in rodent models of diabetic cardiomyopathy [93]. Treatment with antioxidants is capable of normalizing this cardiac hypertrophy and reducing CTGF levels, therefore implicating oxidative stress as a potential mediator of PKCβ2-induced myocardial damage [100]. Inhibition of PKCα is also associated with significant improvements in cardiac function in rodent models of HF [101], in conjunction with an improved metabolic gene profile in the myocardium, as well as improved glucose utilization and diastolic function as shown by MR spectroscopy [102].
AGEs
Hyperglycaemia promotes formation of collagen types I and III in the myocardium, resulting in interstitial fibrosis, which leads to LV diastolic dysfunction; however, the mechanisms of hyperglycaemia-induced collagen production are poorly defined. The increased formation of AGEs secondary to hyperglycaemia, may alter structural proteins and lead to increased myocardial stiffness. Aminoguanidine (an inhibitor of AGE formation and protein cross-linking) has been shown to ameliorate changes in LV structure and function [103]. Hyperglycaemia induces oxidative stress which increases profibrogenic factors leading to interstitial fibrosis, a key alteration in diabetic cardiomyopathy. Oxidative stress increases expression of AGE and RAGE (receptor for AGEs) leading to an activation of NF-κB which leads to a switch in the gene expression of cardiac MHC (myosin heavy chain) from the α-MHC isoform to the β-MHC isoform altering myocardial contractility [104]. DHEA (dehydroepiandrosterone) reduces oxidative stress and in turn has been shown to reduce tissue levels of collagen I, collagen IV and fibronectin in STZ (streptozotocin)-rats and restore papillary muscle contractility [105]. Elevated levels of RAGE, NF-κB and TGF-β1 (transforming growth factor-β1) mRNA in myocardial tissue of diabetic rats was reduced significantly after administration of grape seed proanthocyanidin extract and this was associated with a decrease in the number of degenerate mitochondria and the preservation of ultrastructural alterations in LV myocardium [106]. In an in vitro study of cardiac fibroblasts exposed to a high-glucose concentration, ERK1/2 (extracellular-signal-regulated kinase 1/2) activation led to enhanced mRNA and protein expression of collagen types I and III, which was ameliorated by treatment with a blocker of ERK phosphorylation [107].
Fatty acids
Independent of the effects of hyperlipidaemia on coronary artery endothelial function, diabetic hearts have an altered metabolic phenotype, with enhanced FA (fatty acid) utilization. A recent study in db/db mice, a monogenic model of Type 2 diabetes with extreme obesity and hyperglycaemia, has demonstrated increased plasma membrane content of FA transporters [FAT/CD36 and FABPpm (membrane associated FA-binding protein)], leading to increased FA uptake and utilization in db/db cardiomyocytes [105]. This has been assumed to be driven by a range of mitochondrial mechanisms, but there was no change in CPT-1 (carnitine palmitoyltransferase-1) activity, malonyl CoA and UCP (uncoupling protein)-3 content suggesting that mitochondrial mechanisms do not contribute to elevated rates of FA oxidation in db/db hearts [105].
Dysfunctional calcium homoeostasis
Calcium is one of the principal ionic regulators in the heart and is essential for the process of excitation–contraction coupling and therefore integral to normal cardiac function. Thus, during the cardiac action potential, the cell membrane of the cardiomyocyte is depolarized and calcium enters the cell through voltage-dependent L-type calcium channels in the sarcolemma. Calcium triggers the release of further calcium ions from the SR (sarcoplasmic reticulum) store, through the RyRs (ryanodine receptors), which increase intracellular calcium and facilitate binding of calcium to myofilaments, thereby initiating cardiac contraction. For relaxation to occur, calcium ions must be removed from the cytosol, the majority of which is pumped back into the SR by SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase), while the remainder is ejected out of the cell through the sarcolemmal NCX (Na2+/Ca2+ exchange), PMCA (plasma-membrane Ca2+-ATPase) or mitochondrial calcium uniport [106]. In both Type 1 and Type 2 rodent models of diabetes, altered expression, activity and function of all transporters involved in excitation–contraction coupling, SERCA [107], NCX [108], RyR [109] and PMCA [110], as well as dysfunctional intracellular calcium signalling [111], have been reported. These findings echo calcium mishandling observed in HF [106]. Interestingly, candesartan, an ARB {AT1 [AngII (angiotensin II) type 1] receptor blocker}, has been shown to restore the contractile deficit in diabetic cardiomyopathy by stabilizing FKBP (FK506-binding protein) 12.6 and restoring calcium release through the RyR [112]. Depressed SERCA activity causes inefficient sequestration of calcium in the SR, resulting in cytosolic calcium overload, impaired relaxation and hence diastolic dysfunction [113]. Overexpression of SERCA has been shown to improve calcium handling [111] and protect against experimental diabetic cardiomyopathy [107]. In a study utilizing myocardial biopsies in seven diabetic patients with diastolic dysfunction, myofilament Ca2+ responsiveness was found to be reduced [114]. In addition to alterations in calcium homoeostasis, there is also reduced expression of mRNA and protein density of key cardiac K+ channel (Kv2.1, Kv4.2, and Kv4.3) genes in LV myocytes in experimental diabetes. This will contribute to repolarizing K+ currents and explain the susceptibility to arrhythmia in diabetic cardiomyopathy [115].
RAAS (renin–angiotensin-aldosterone system)
The involvement of the RAS (renin–angiotensin system) in HF has now begun to be defined at the molecular level in relation to HF and diabetic cardiomyopathy. AngII exerts a direct effect on cardiomyocytes through AT1 receptors [116]. Both diabetes and hyperglycaemia induce functional abnormalities in ventricular myocytes, which can be prevented by AngII blockade [117]. The mechanistic basis for this dysfunction is not clear; however, direct signalling via the AT1 receptor results in increased NADPH oxidase activity and elevation of ROS which causes oxidative damage to cardiomyocytes and endothelial cell apoptosis [117]. In diabetes an up-regulation of RAS occurs despite minimal changes in myocardial loading and increased expression of AngII in diabetic rats has been related to cardiomyocyte hypertrophy and apoptosis. Six weeks of STZ-diabetes in Ren-2 (enhanced tissue renin–angiotensin expression) rats results in impairment of both active and passive phases of diastole with interstitial fibrosis, cardiac myocyte hypertrophy and apoptosis in conjunction with increased TGF-β activity [118]. These findings provide a molecular platform for the increased benefits of ACE inhibition in diabetic patients in preventing and reversing diabetic cardiomyopathy.
AngII receptor blockers attenuate metabolic and cellular alterations in experimental diabetes [119], reduce ROS [120], restore SERCA activity and improve intracellular Ca2+ handling in HF [121]. It is therefore not surprising that angiotensin blockade has been shown to provide cardiovascular protection in diabetic patients [122]. Aldosterone antagonists also reduce cardiovascular mortality in diabetic patients with HF who exhibit elevated serum levels of collagen synthesis markers [123], suggesting that aldosterone antagonists may prevent excessive extracellular matrix turnover. As both AngII and aldosterone induce cardiac fibrosis, through enhanced accumulation of collagen and increased fibroblast proliferation [124], it has been suggested that aldosterone and glucose mediate cardiac fibrosis through stimulation of myofibroblast growth in patients with a dysregulated RAAS, a pathway of cardiac pathology which is intensified by hyperglycaemia [125].
HIF (hypoxia-inducible factor)-1 and VEGF (vascular endothelial growth factor)
An adequate angiogenic response to hypoxia is essential in protecting against myocardial injury during ischaemic events. The hypoxic stimulus is mediated largely by HIF-1, a transcriptional regulator complex which controls the expression of multiple angiogenic growth factors, of which VEGF has received particular attention [126]. The presence of hypoxia and free radicals stabilize and activate HIF-1α, which would otherwise be rapidly degraded [127]. VEGF has been shown to contribute to the development of collateral vessels [128] and is expressed in increased quantities in cardiac myocytes and arteriolar smooth muscle cells following MI in non-diabetic patients [129]. However, the expression of VEGF protein and mRNA, as well as its receptors, are significantly decreased in the myocardium of both diabetic and insulin-resistant non-diabetic rats [130]. This suggests that, in diabetic patients, the normal molecular processes which regulate angiogenesis may be impaired, although there are no direct studies to date to substantiate this. This may provide an explanation for the increased incidence of post-MI cardiac failure and enhanced mortality in diabetic patients. Interestingly, administration of TZDs (thiazolidinediones) [131] or inhibition of NADPH oxidase [132] results in an increase in circulating VEGF levels in diabetic patients and animals respectively, with restored post-ischaemic neovascularization in the latter; implicating ROS activity once more in the pathology of diabetic cardiomyopathy. Reductions in VEGF and impaired angiogenic responses have also been linked with increased levels of ET-1 in ventricles from diabetic rodents and ET receptor antagonism increases VEGF signalling, improving cardiac function [133].
The KKS [KLK (kallikrein)–kinin system]
There is now good evidence that the KKS has a profound influence on cardiac and vascular function. Tissue KLK is involved in processing vasoactive kinin peptides and appears to have dynamic roles, with reports of both protective and damaging effects on the cardiovascular system [134,135]. These effects are mediated through two distinct G-protein-coupled receptors; the BK (bradykinin) B1R (B1-receptor) and B2R (B2-receptor). Under basal conditions, BK and Lys-BK (kallidin) are the predominant kinin peptides involved in cardiovascular homoeostasis and work principally through activation of the B2R. The B1R has been shown to mediate cardiac inflammation and may therefore contribute to cardiovascular pathology [135].
A range of cardiovascular insults, including oxidative stress, inflammatory cytokines and activation of the RAS have been shown to regulate these receptors [136]. The STZ-induced diabetic rat exhibits up-regulation of both B1R and B2R [137], which may partly be explained by activation of the RAS in diabetes [134]. Paradoxically, KLK levels are reduced in diabetic animals [138] and humans [139], suggesting therefore that kinin receptor up-regulation may represent an attempted compensatory mechanism [135].
The relationship between these cardiovascular insults and levels of KLK/BK receptors is reciprocal. Alterations to B2R expression by use of transgenes in experimental models of diabetes has demonstrated protection against diabetic cardiomyopathy. BK has been shown to improve insulin stimulation of GLUT4 (glucose transporter 4) [140] via a BK B2R-induced NO pathway [141]. Following somatic hKLK (human KLK) gene delivery to STZ-induced diabetic rats, there is increased cardiac GLUT4 translocation, decreased cardiac glycogen synthesis and reduced glycogen accumulation [142], suggesting that the KKS is capable of restoring glucose utilization. Furthermore, hKLK transgenic STZ-induced diabetic rats show a reduced cardiac inflammatory response [143], increased levels of antioxidative enzymes [144], inhibition of collagen accumulation and restoration of SERCA2a-associated calcium disturbances [145]. Functionally, these changes are associated with subsequent improvement in LV filling pressure/stiffness [146].
Thus there is clearly extensive cross-talk between the KKS and the cardiovascular system. Interactive pathways between the KKS and the RAS, myocardial energy metabolism, oxidative stress, inflammation and calcium handling, are complex and dynamic with respect to the BK receptor involved. Furthermore delineation of these mechanisms may allow manipulation of this system, potentially via blockade of the RAS, utilizing readily available therapy (ACEIs, ARBs and renin inhibitors) to selectively oppose the alterations which contribute to diabetic cardiomyopathy in man.
PATHOLOGY
As a consequence of the molecular changes discussed, characteristic structural alterations develop in the cardiomyocytes and intramyocardial blood vessels of diabetic individuals, contributing to the pathogenesis of diabetic cardiomyopathy.
Cardiomyocytes
Since the cardiomyocyte is the primary functional unit of the myocardium, it has been the focus of studies investigating diabetic cardiomyopathy. Light and electron microscopic ultrastructural changes of the cardiomyocyte have been defined in a range of animal models of diabetes, including the dog [147], monkey [148], rabbit [149], mouse [150] and rat [151–154]. However, widely varying results have been reported with regard to the severity, onset and type of cardiomyocyte pathology. Hence the pathology has ranged from no change [147] to severe myocytolysis and contracture band formation [151]. With regard to onset of alterations, some studies have demonstrated significant changes within 1 week [155], whereas others only detected changes after 6 [153] and 12 weeks [151] of experimental diabetes. Possible explanations for these discrepancies, other than the duration of diabetes, include the severity of hyperglycaemia and the potential direct effect of the diabetogen used to induce diabetes. Thompson [153] induced diabetes in the Sprague–Dawley rat using a single injection of 4% alloxan monohydrate, and by light microscopy demonstrated a progressive increase in intercellular and perivascular matrix after 6 weeks of diabetes. However, this abnormality was not expressed uniformly and there was regional heterogeneity, with areas showing no increase in matrix [153]. More detailed ultrastructural assessment using electron microscopy demonstrated significant ultrastructural alterations in only 15% of the cardiomyocytes, which were characterized by: (i) loss of myofilaments with disorganization of the remaining bundles, (ii) areas of separation at the intercalated disk along the fasciae adherents, and (iii) increased lipid droplets and loss of some elements of the SR and transverse tubules [153]. A longer duration of diabetes leads to the development of more pronounced degenerative changes in larger groups of cardiomyocytes and, at 26 weeks, approx. 60% of the cells were affected, displaying large areas of free cytoplasm, dispersed proteins, contracture bands and a reduction in the frequency of SR and transverse tubules. However, interestingly, most of the mitochondria appeared normal with no evidence of swelling or lysis and morphology of the cristae remained normal, despite the presence of severe damage and disorganization of the cytoplasm with contracture band formation. This is in contrast with other ultrastructural studies, which have shown swollen and fragmented mitochondria as the most prominent finding in diabetic myocardium [149]. Indeed, mitochondrial swelling has been observed in rats after 7 days of hyperglycaemia [155], as well as in dogs [156]. A number of other studies have reported disruption and loss of mitochondria in more advanced stages of diabetic cardiomyopathy [150–152].
Studies in humans are limited. Fischer et al. [157] undertook detailed ultrastructural studies of tissue plugs from the anterior apical segment of the heart in 145 patients undergoing CABG (coronary artery bypass grafting). Subjects were divided into three groups: ‘overtly diabetic’, ‘chemically diabetic’ (representing those with impaired glucose tolerance) and ‘euglycaemic/non-diabetic’ patients. The study showed cardiomyocyte hypertrophy and interstitial fibrosis in all except two samples. In addition, a spectrum of other myocardial lesions were observed in all three groups of patients, ranging from mitochondrial degeneration and fatty infiltration of the myofibrils to contraction band formation, perivascular and interstitial oedema and myocytolysis. The study thus failed to detect specific alterations in the cardiomyocytes of diabetic patients, as all changes were also present in non-diabetic patients [157]. The one ultrastructural alteration which distinguished diabetic from non-diabetic patients was capillary basement membrane thickening, which will be discussed below. However, it is important to note that the criteria used to group the patients was not precise and overlap between the groups could have resulted from ambiguous terminology, dividing patients into ‘overtly diabetic’ and ‘chemically diabetic’.
With regard to the size of cardiomyocytes, contrasting studies in diabetic patients and animal models demonstrate both cardiomyocyte hypertrophy and atrophy. Cardiomyocyte apoptosis has been demonstrated in the diabetic myocardium of animal models [158], as well as in humans [159]. In STZ-induced diabetic rats [160], TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) detected 3-OH strand breaks resulting from DNA breakdown. The nuclear marker DAPI (4′,6-diamidino-2-phenylindole) was used to counterstain DNA and cells defined as apoptotic if the nuclei showed a sharply demarcated morphology and were labelled by the TUNEL assay, were increased 3.3-fold in diabetic rats, although the overall incidence of this abnormality was very low [160]. However, it is important to note that this method is not specific for detecting those cells which do eventually undergo apoptosis and a more accurate means to detect actual cardiomyocyte apoptosis is by electron microscopy, but is rarely employed. A study of human diabetic myocardium has shown an 85-fold increase in cardiomyocyte apoptosis [159]. Such apoptotic myocyte loss may be one of the mechanisms contributing to the poor prognosis in diabetic patients after MI [161]. Several reports have shown that inhibition of myocardial cell death by antioxidants or inhibitors of apoptosis-specific signalling pathways, leads to significant prevention of diabetic cardiomyopathy [162]. Table 1 summarizes all of the structural changes which have been reported in diabetic cardiomyopathy.
Table 1. Summary of the major structural changes in experimental and human diabetic cardiomyopathy.
↑ indicates increase; ↓ indicates decrease; = indicates no difference. BB, BioBreeding; ZDF, Zucker diabetic fatty.
| Diabetes type | Model | Cardiac mass | Cardiomyocyte diameter | Cardiomyocyte loss | Mitochondria | SR | Apoptosis | Intramyocardial lipid | Fibrosis |
|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | STZ rat | =[151] or ↓[166,201] | ↓[166,201] | ↑[151] | ↑[151,155,201] | ↑[151,155] | ↑[158] | ↑[151,155] | =[201] or ↑[166] |
| Alloxan rat | =[153] | ↑[153] | =[153] | ↑[153] | ↑[153] | ||||
| BB Wistar rat | ↓[152] | ↑[152] | ↑[152] | ↑[152] | ↑[152] | ↑[152] | |||
| Type 2 diabetes | KK mice | ↑[163] | ↑[163] | ↑[163] | ↑[163] | ||||
| ZDF rat | =but after insulin↑[202] | ↑[202] | ↑[203] | ↑[202] | |||||
| ob/ob Mice | ↑[150,204] | ↑[204] or ↓[150] | ↑[150] | ↑[150] | =[204] or ↑[150] | =[204] | |||
| Type 1 and 2 diabetes | Humans | ↑[205,206] | ↑[207,208] or ↓[209] | =?[157] | =?[157] | =?[157] | ↑[159] | =?[157] | =[157] or ↑[208] |
Myocardial microvessels
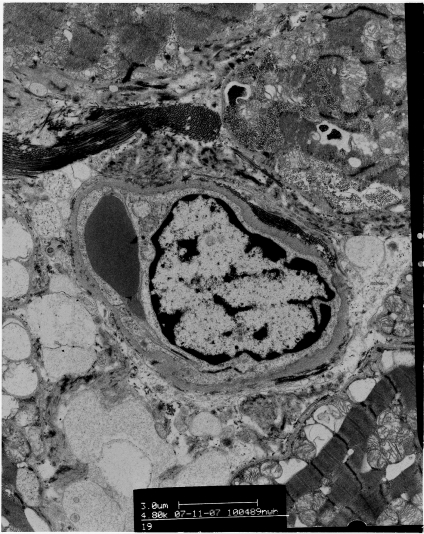
Although most structural studies of the myocardial vasculature have concentrated on coronary arteries and intramural arterioles, few studies have assessed myocardial capillary structure in either clinical or experimental diabetes. Some reports show significant ultrastructural changes in cardiomyocytes, in the absence of any vascular change [152,163], whereas others report vascular changes as the only specific myocardial alteration in the diabetic heart [157]. The aforementioned study by Thompson [153] was unable to detect any structural abnormalities in the myocardial capillaries during the first 6 weeks of diabetes. At 26 weeks, however, a 3-fold increase in thickening of the endothelial cytoplasm, with endothelial cell hypertrophy and cytoplasmic bridging led to complete obstruction of the lumen. The capillary basement membrane showed moderate, irregular thickening with occasional areas of duplication, with all changes distributed focally throughout the papillary muscle [153]. Thickening of the capillary basement membrane has been reported in mice [150], rats [164] and humans [157,165]. Thompson [166] further quantified the effect of diabetes on different components of the myocardial microvasculature in rats, by demonstrating a decrease in mean capillary diameter by 11% after 12 weeks and 22% after 26 weeks of diabetes. Moreover, stereological analysis showed a significant progressive decrease in capillary density, which after 26 weeks of diabetes reached 77% of the value seen in non-diabetic age-matched control rats [166]. Ultrastructural signs of angiogenesis have also been reported and are characterized by alterations in endothelial and pericyte shape [164]. A study of human diabetic myocardium [157] found two characteristic abnormalities in myocardial capillaries: endothelial swelling and/or degeneration and thickening of the capillary basement membrane (Figure 2). This is the pathological hallmark of diabetic microangiopathy and occurred in an uneven, patchy, segmental manner [157]. Diabetic myocardial capillary microangiopathy has also been shown by others [167,168]. Table 2 summarizes the microvascular alterations seen in models of diabetic cardiomyopathy.
Figure 2. Electronmicrograph of a myocardial capillary from a diabetic patient, demonstrating luminal occlusion with basement membrane thickening.
Table 2. Summary of the major microvascular changes in experimental and human diabetic cardiomyopathy.
BB, BioBreeding; ZDF, Zucker diabetic fatty.
| Diabetes type | Model | Microvascular changes |
|---|---|---|
| Type 1 diabetes | STZ rat | Decreased capillary diameter and density [166] |
| Narrow capillary lumen with endothelial swelling [164] | ||
| Basement membrane thickening [201] | ||
| Alloxan rat | Decreased capillary lumen [153] | |
| Endothelial swelling [153] | ||
| Basement membrane thickening [153] | ||
| BB Wistar rat | No changes [152] | |
| Type 2 diabetes | KK mice | No changes [163] |
| ZDF | Perivascular fibrosis [202] | |
| ob/ob Mice | Basement membrane thickening [150] | |
| Type 1 and 2 diabetes | Humans | Basement membrane thickening [157,165] |
TREATMENT
Sulfonylureas
Of the relatively little published data on the use of sulfonylureas in diabetic HF, two studies have reported conflicting findings in terms of outcome [169,170]. A recent meta-analysis has indicated that these drugs are not associated with an increase in cardiovascular events [171,172]. Clearly, further studies are needed to determine the benefits and possible harm of these drugs in diabetic patients with HF.
Metformin
Metformin was previously contraindicated in patients with HF in the U.S. due to the theoretical risk of lactic acidosis; its use is still strongly cautioned. Evidence to support the use of metformin in HF comes from three cohort studies in which metformin therapy was compared with other OHAs (oral hypoglycaemic agents) and insulin respectively [171]. Metformin treatment was associated with a reduction in all-cause mortality and all-cause hospital admissions. In addition to its modest insulin-sensitizing properties, metformin has been shown in an animal model to inhibit cardiac hypertrophy [173].
TZDs
The TZDs [PPARγ (peroxisome-proliferator-activated receptor γ) receptor agonists] are primarily insulin-sensitizing agents, but in addition to their antihyperglycaemic action, these drugs also exert beneficial effects on the myocardium, vascular endothelium, lipid profile and BP with additive anti-inflammatory and profibrinolytic effects [174]. They have been shown in both animal and human studies to improve myocardial metabolism and glucose handling [175]. In a 6 month double-blind, randomized, multicentre study comparing pioglitazone with glyburide in patients with Type 2 diabetes, systolic dysfunction and NYHA (New York Heart Association) functional class II/III HF, although pioglitazone was associated with a higher incidence of hospitalization for HF there was no increase in cardiovascular mortality or worsening cardiac function (by echocardiography) [176].
Thus the increased risk of HF is due to fluid retention via increased renal retention of sodium and subsequent plasma volume expansion and there is no evidence to suggest that TZDs may be harmful to the myocardium. In contrast, animal and experimental studies have demonstrated favourable effects on the myocardium such as reduced cardiac hypertrophy and improved systolic and diastolic function [177]. Results from clinical trials assessing TZD use and cardiac function however, is limited by both number and validity. Hence, the use of TZDs in HF is restricted to those in NYHA functional class I–II [178]. Two recent meta-analyses of seven and 19 randomized controlled trials of TZDs (pioglitazone or rosiglitazone), reported an increase in the risk of HF in diabetic and pre-diabetic subjects [RR of 1.72 and HR (hazard ratio) 1.41 respectively] [179,180], but there was no increase in the risk of cardiovascular mortality in these studies. Rosiglitazone has been associated with an increased risk of MI [177]. In the PROactive study, although pioglitazone was associated with a significant increase in serious HF (10.8% compared with 7.5% over 3 years, P<0.0001), there was no increase in mortality or cardiovascular events compared with placebo [181]. Thus the overall cardiovascular benefits derived from pioglitazone are thought to outweigh such risks [182]. In the RECORD (Rosaglitazone Evaluated for Cardiovascular Outcomes and Regulation of glycemia in Diabetes) study, rosiglitazone-treated patients had a slightly increased risk of incident HF (1.7 compared with 0.8%, P=0.006) after a mean treatment period of 3.8 years [183].
Insulin
Insulin use in diabetic patients has also been associated with an increased risk of HF and death [171]. Cohort studies in diabetic populations with HF have yielded conflicting results. In the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) study, the risk of death was greater in diabetic patients treated with insulin compared with those not treated with insulin [184]. These findings contrast with those of UKPDS which did not report an increase in mortality with insulin use [171].
GLP-1 (glucagon-like peptide-1) analogues
GLP-1 is an ‘incretin’ hormone which stimulates post-prandial insulin secretion and improves insulin sensitivity. GLP-1 analogues exert their cardiovascular benefit indirectly by inducing weight loss and improving glycaemic profile and endothelial function [185]. There is some promising data from a trial of GLP-1 analogues in patients following successful coronary intervention for acute MI whereby the treated group demonstrated a significantly greater improvement in LVEF (29±2% compared with 39±2%, P<0.01) with concomitant improvements in global and regional wall motion [186]. The same group conducted a trial of GLP-1 analogues in patients with advanced HF and reported significant improvements in LVEF, V̇O2max (maximal oxygen consumption), 6 min walk distance and standardized QOL (quality-of-life) score [187]. Clearly, long-term data are required for this class of drugs to determine their efficacy and safety in HF.
DPP-4 (dipeptidyl peptidase-4) inhibitors
The DPP-4 inhibitors or gliptins are a group of drugs which increase incretin levels by inhibiting the enzyme DPP-4. Of note, DPP-4 is expressed on the endothelium of myocardial venules and paradoxically has been shown to be reduced in experimental diabetes [164]. These agents are relatively new and, as for the GLP-1 analogues, one may predict a long-term benefit on cardiovascular and all-cause mortality. Long-term morbidity and mortality trials are currently underway, but it will be several years before the data is reported.
Metabolic modulators
These agents, previously used as anti-anginals, address the imbalance in myocyte energy efficiency by facilitating the switch from NEFA (non-esterified FA; ‘free FA’) to glucose metabolism. Trimetazidine, one such agent currently available in Europe, inhibits an enzyme involved in the β-oxidation of NEFAs and has been shown to improve LVEF, NYHA functional class and QOL in patients with HF [188]. Similarly, perhexiline has demonstrated substantial improvements in LVEF, V̇O2max and QOL, but is associated with a risk of hepatotoxicity and peripheral neuropathy [189]. Ranolazine, is another drug with potential as a metabolic modulator [190]. It reduces intracellular Na+ and diastolic Ca2+ overload, thus improving diastolic function, but it has been associated with prolongation of the QT interval.
The RAAS
The role of the RAAS in the pathogenesis of diabetic cardiomyopathy has been described above and forms the rationale for treatment with ACEIs and ARBs in diabetic cardiomyopathy. Both ACE inhibition and treatment with ARBs reduce total and cardiovascular mortality in diabetic patients [191,192]. In addition, there was a reduction in cardiovascular events in large studies such as RENAAL [45], HOPE (Heart Outcomes Prevention Evaluation) [193] and IDNT (Irbesartan Diabetic Nephropathy Trial) [194], the effect being more pronounced in diabetic compared with non-diabetic patients. Furthermore, in the CHARM study [184], candesartan was associated with a 40% reduction in the diagnosis of new onset diabetes and similarly, in the SOLVD study, treatment with enalapril reduced the incidence of diabetes compared with placebo [23]. A meta-analysis of the major HF trials found an RR of 0.84 for death in diabetic patients treated with ACEIs [195]. The CHARM and Val-HeFT [196] studies also demonstrated mortality and morbidity benefits with ARBs which were similar in diabetic and non-diabetic patients.
β-Blockers
β-Blockers are now well-established in the treatment of HF. Concerns in diabetic patients with regard to the increased risk of hypoglycaemia, dyslipidaemia and insulin resistance has meant that diabetic patients with HF are less likely to be discharged from hospital on a β-blocker than their non-diabetic counterparts [197]. However, the GEMINI (Clinical Utility of Amlodipine/Atorvastatin to Improve Concomitant Cardiovascular Risk Factors of Hypertension and Dyslipidemia) study compared the use of carvedilol with metoprolol in patients already receiving ACEIs or ARBs [198] and demonstrated that carvedilol improved insulin resistance and had no effect on HbA1c, whereas metoprolol worsened HbA1c and did not improve insulin resistance. Although to date there has not been a study of β-blockers in diabetic patients with HF, up to a quarter of patients in the major β-blocker trials in HF were diabetic [199,200]. Subgroup analysis of these trials demonstrated significant mortality and morbidity benefits in diabetic patients. In a meta-analysis of these trials, the overall RR of mortality in diabetic patients was 0.84 compared with 0.72 in non-diabetic patients.
CONCLUSIONS
Considerable progress has been made to unravel the mechanistic basis for the increased frequency of HF in diabetic individuals. Functional consequences such as diastolic and then systolic dysfunction and failure correlate with glycaemic control. These functional alterations are preceded by a variety of structural, molecular and cellular changes, many of which are present in asymptomatic diabetic individuals and experimental models of diabetes. Moreover, alterations to myocardial and vascular integrity appear to initiate during a pre-diabetic stage. Although the aetiology of diabetic cardiomyopathy is complex and multifactorial, we have begun mapping a molecular, structural and functional basis for this condition and the debate regarding its existence as a distinct clinical entity is at an end. At present however, translational studies are lacking and it remains under-diagnosed and inadequately treated. Although hyperglycaemia appears to drive the pathogenesis of diabetic cardiomyopathy, intensive glycaemic control in diabetic patients has not translated into meaningful morbidity and mortality benefits, indeed recent results suggest the possibility of worsening outcomes. Novel diagnostic techniques to identify patients earlier, together with the development of more targeted therapeutic strategies exploiting recent experimental data, may lead to improved outcome in patients with diabetic cardiomyopathy.
ACKNOWLEDGEMENTS
We acknowledge the NIHR (National Institute for Health Research) Manchester Biomedical Research Centre.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Leyden E. Asthma and diabetes mellitus. Zeutschr. Klin. Med. 1881;3:358–364. [Google Scholar]
- 2.Mayer J. Ueber den zusammenhang des diabetes mellitus miterkrankungen des herzens. Zeutschr. Klin. Med. 1888;14:212–239. [Google Scholar]
- 3.Rubler S., Dlugash J., Yuceoglu Y. Z., Kumral T., Branwood A. W., Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 4.Hayat S. A., Patel B., Khattar R. S., Malik R. A. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin. Sci. 2004;107:539–557. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 5.Thrainsdottir I. S., Aspelund T., Thorgeirsson G., Gudnason V., Hardarson T., Malmberg K., Sigurdsson G., Rydén L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28:612–616. doi: 10.2337/diacare.28.3.612. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni A. G., Hundley W. G., Massing M. W., Bonds D. E., Burke G. L., Goff D. C., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 7.Reis S. E., Holubkov R., Edmundowicz D., McNamara D. M., Zell K. A., Detre K. M., Feldman A. M. Treatment of patients admitted to the hospital with congestive heart failure: specialty-related disparities in practice patterns and outcomes. J. Am. Coll. Cardiol. 1997;30:733–738. doi: 10.1016/s0735-1097(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Solal A., Beauvais F., Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. J. Card. Failure. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Amato L., Paolisso G., Cacciatore F., Ferrara N., Ferrara P., Canonico S., Varricchio M., Rengo F. Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab. 1997;23:213–218. [PubMed] [Google Scholar]
- 10.Berry C., Brett M., Stevenson K., McMurray J. J., Norrie J. Nature and prognostic importance of abnormal glucose tolerance and diabetes in acute heart failure. Heart. 2008;94:296–304. doi: 10.1136/hrt.2006.110999. [DOI] [PubMed] [Google Scholar]
- 11.Mak K. H., Moliterno D. J., Granger C. B., Miller D. P., White H. D., Wilcox R. G., Califf R. M., Topol E. J. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 1997;30:171–179. doi: 10.1016/s0735-1097(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 12.Kannel W. B., Hjortland M., Castelli W. P. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 13.Bertoni A. G., Tsai A., Kasper E. K., Brancati F. L. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791–2795. doi: 10.2337/diacare.26.10.2791. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener J. S., Arnold A. M., Aurigemma G. P., Polak J. F., Tracy R. P., Kitzman D. W., Gardin J. M., Rutledge J. E., Boineau R. C. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J. Am. Coll. Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 15.Devereux R. B., Roman M. J., Paranicas M., O'Grady M. J., Lee E. T., Welty T. K., Fabsitz R. R., Robbins D., Rhoades E. R., Howard B. V. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 16.Bertoni A. G., Goff D. C., Jr, D'Agostino R. B., Jr, Liu K., Hundley W. G., Lima J. A., Polak J. F., Saad M. F., Szklo M., Tracy R. P., Siscovick D. S. Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2006;29:588–594. doi: 10.2337/diacare.29.03.06.dc05-1501. [DOI] [PubMed] [Google Scholar]
- 17.Nichols G. A., Hillier T. A., Erbey J. R., Brown J. B. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 18.Aronow W. S., Ahn C. Incidence of heart failure in 2737 older persons with and without diabetes mellitus. Chest. 1999;115:867–868. doi: 10.1378/chest.115.3.867. [DOI] [PubMed] [Google Scholar]
- 19.Stratton I. M., Adler A. I., Neil H. A., Matthews D. R., Manley S. E., Cull C. A., Hadden D., Turner R. C., Holman R. R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br. Med. J. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iribarren C., Karter A. J., Go A. S., Ferrara A., Liu J. Y., Sidney S., Selby J. V. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 21.Cheung N., Wang J. J., Rogers S. L., Brancati F., Klein R., Sharrett A. R., Wong T. Y. Diabetic retinopathy and risk of heart failure. J. Am. Coll. Cardiol. 2008;51:1573–1578. doi: 10.1016/j.jacc.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 22.Cheung N., Bluemke D. A., Klein R., Sharrett A. R., Islam F. M., Cotch M. F., Klein B. E., Criqui M. H., Wong T. Y. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2007;50:48–55. doi: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 24.Komajda M., Wimart M. C., Thibout E. The ATLAS study (Assessment of Treatment with Lisinopril and Survival); justification and objectives. Arch. Mal. Coeur Vaiss. 1994;87:45–50. [PubMed] [Google Scholar]
- 25.Cohn J. N., Archibald D. G., Ziesche S., Archibald D. G., Ziesche S., Franciosa J. A., Harston W. E., Tristani F. E., Dunkman W. B., Jacobs W., Francis G. S., Flohr K. H., et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N. Engl. J. Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 26.McKelvie R. S., Yusuf S., Pericak D., Avezum A., Burns R. J., Probstfield J., Tsuyuki R. T., White M., Rouleau J., Latini R., et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 27.Ingelsson E., Arnlov J., Lind L., Sundstrom J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92:1409–1413. doi: 10.1136/hrt.2006.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingelsson E., Sundstrom J., Arnlov J., Zethelius B., Lind L. Insulin resistance and risk of congestive heart failure. JAMA, J. Am. Med. Assoc. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 29.Bahrami H., Bluemke D. A., Kronmal R., Bertoni A. G., Lloyd-Jones D. M., Shahar E., Szklo M., Lima J. A. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Hassan S. A., Deswal A., Bozkurt B., Aguilar D., Mann D. L., Pritchett A. M. The metabolic syndrome and mortality in an ethnically diverse heart failure population. J. Card. Failure. 2008;14:590–595. doi: 10.1016/j.cardfail.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Andersen N. H., Poulsen S. H., Poulsen P. L., Knudsen S. T., Helleberg K., Hansen K. W., Dinesen D. S., Eiskjaer H., Flyvbjerg A., Mogensen C. E. Effects of blood pressure lowering and metabolic control on systolic left ventricular function in Type II diabetes mellitus. Clin. Sci. 2006;111:53–59. doi: 10.1042/CS20050367. [DOI] [PubMed] [Google Scholar]
- 32.Fang Z. Y., Schull-Meade R., Downey M., Prins J., Marwick T. H. Determinants of subclinical diabetic heart disease. Diabetologia. 2005;48:394–402. doi: 10.1007/s00125-004-1632-z. [DOI] [PubMed] [Google Scholar]
- 33.Holman R. R., Paul S. K., Bethel M. A., Matthews D. R., Neil H. A. 10-Year follow-up of intensive glucose control in Type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 34.Patel A., MacMahon S., Chalmers J., Neal B., Billot L., Woodward M., Marre M., Cooper M., Glasziou P., Grobbee D., et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 35.Gerstein H. C., Miller M. E., Byington R. P., Goff D. C., Jr, Bigger J. T., Buse J. B., Cushman W. C., Genuth S., Ismail-Beigi F., Grimm R. H., Jr, et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holman R. R., Paul S. K., Bethel M. A., Neil H. A., Matthews D. R. Long-term follow-up after tight control of blood pressure in Type 2 diabetes. N. Engl. J. Med. 2008;359:1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 37.Levy D., Garrison R. J., Savage D. D., Kannel W. B., Castelli W. P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 38.Yotsukura M., Suzuki J., Yamaguchi T., Sasaki K., Koide Y., Mizuno H., Yoshino H., Ishikawa K. Prognosis following acute myocardial infarction in patients with ECG evidence of left ventricular hypertrophy prior to infarction. J. Electrocardiol. 1998;31:91–99. doi: 10.1016/s0022-0736(98)90039-5. [DOI] [PubMed] [Google Scholar]
- 39.Quinones M. A., Greenberg B. H., Kopelen H. A., Koilpillai C., Limacher M. C., Shindler D. M., Shelton B. J., Weiner D. H. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2000;35:1237–1244. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 40.Boner G., Cooper M. E., McCarroll K., Brenner B. M., de Zeeuw D., Kowey P. R., Shahinfar S., Dickson T., Crow R. S., Parving H. H. Adverse effects of left ventricular hypertrophy in the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. Diabetologia. 2005;48:1980–1987. doi: 10.1007/s00125-005-1893-1. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Gomez J. M., Verde E., Perez-Garcia R. Blood pressure, left ventricular hypertrophy and long-term prognosis in hemodialysis patients. Kidney Int. Suppl. 1998;68:S92–S98. doi: 10.1046/j.1523-1755.1998.06820.x. [DOI] [PubMed] [Google Scholar]
- 42.Eguchi K., Ishikawa J., Hoshide S., Ishikawa S., Pickering T. G., Schwartz J. E., Homma S., Shimada K., Kario K. Differential impact of left ventricular mass and relative wall thickness on cardiovascular prognosis in diabetic and nondiabetic hypertensive subjects. Am. Heart J. 2007;154:79.e9–79.e15. doi: 10.1016/j.ahj.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 43.de Simone G., Gottdiener J. S., Chinali M., Maurer M. S. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur. Heart J. 2008;29:741–747. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 44.Pierdomenico S. D., Lapenna D., Cuccurullo F. Regression of echocardiographic left ventricular hypertrophy after 2 years of therapy reduces cardiovascular risk in patients with essential hypertension. Am. J. Hypertens. 2008;21:464–470. doi: 10.1038/ajh.2008.2. [DOI] [PubMed] [Google Scholar]
- 45.Brenner B. M., Cooper M. E., de Zeeuw D., Keane W. F., Mitch W. E., Parving H. H., Remuzzi G., Snapinn S. M., Zhang Z., Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 46.Danielsen R., Nordrehaug J. E., Lien E., Vik-Mo H. Subclinical left ventricular abnormalities in young subjects with long-term type 1 diabetes mellitus detected by digitized M-mode echocardiography. Am. J. Cardiol. 1987;60:143–146. doi: 10.1016/0002-9149(87)91001-0. [DOI] [PubMed] [Google Scholar]
- 47.Ozasa N., Furukawa Y., Morimoto T., Tadamura E., Kita T., Kimura T. Relation among left ventricular mass, insulin resistance, and hemodynamic parameters in type 2 diabetes. Hypertens. Res. 2008;31:425–432. doi: 10.1291/hypres.31.425. [DOI] [PubMed] [Google Scholar]
- 48.Sundstrom J., Arnlov J., Stolare K., Lind L. Blood pressure-independent relations of left ventricular geometry to the metabolic syndrome and insulin resistance: a population-based study. Heart. 2008;94:874–878. doi: 10.1136/hrt.2007.121020. [DOI] [PubMed] [Google Scholar]
- 49.Zile M. R., Baicu C. F., Gaasch W. H. Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 50.van Heerebeek L., Hamdani N., Handoko M. L., Falcao-Pires I., Musters R. J., Kupreishvili K., Ijsselmuiden A. J., Schalkwijk C. G., Bronzwaer J. G., Diamant M., et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 51.Karamitsos T. D., Karvounis H. I., Dalamanga E. G., Papadopoulos C. E., Didangellos T. P., Karamitsos D. T., Parharidis G. E., Louridas G. E. Early diastolic impairment of diabetic heart: the significance of right ventricle. Int. J. Cardiol. 2007;114:218–223. doi: 10.1016/j.ijcard.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Zabalgoitia M., Ismaeil M. F., Anderson L., Maklady F. A. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am. J. Cardiol. 2001;87:320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 53.Boyer J. K., Thanigaraj S., Schechtman K. B., Perez J. E. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Ha J. W., Lee H. C., Kang E. S., Ahn C. M., Kim J. M., Ahn J. A., Lee S. W., Choi E. Y., Rim S. J., Oh J. K., Chung N. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571–1576. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svealv B. G., Olofsson E. L., Andersson B. Ventricular long-axis function is of major importance for long-term survival in patients with heart failure. Heart. 2008;94:284–289. doi: 10.1136/hrt.2006.106294. [DOI] [PubMed] [Google Scholar]
- 56.Fang Z. Y., Schull-Meade R., Leano R., Mottram P. M., Prins J. B., Marwick T. H. Screening for heart disease in diabetic subjects. Am. Heart J. 2005;149:349–354. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Fang Z. Y., Yuda S., Anderson V., Short L., Case C., Marwick T. H. Echocardiographic detection of early diabetic myocardial disease. J. Am. Coll. Cardiol. 2003;41:611–617. doi: 10.1016/s0735-1097(02)02869-3. [DOI] [PubMed] [Google Scholar]
- 58.Yu C. M., Lin H., Yang H., Kong S. L., Zhang Q., Lee S. W. Progression of systolic abnormalities in patients with ‘isolated’ diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 59.Maciver D. H., Townsend M. A novel mechanism of heart failure with normal ejection fraction. Heart. 2008;94:446–449. doi: 10.1136/hrt.2006.114082. [DOI] [PubMed] [Google Scholar]
- 60.Aurigemma G. P., Zile M. R., Gaasch W. H. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 61.Fang Z. Y., Leano R., Marwick T. H. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin. Sci. 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 62.Zornoff L. A., Skali H., Pfeffer M. A., St John Sutton M., Rouleau J. L., Lamas G. A., Plappert T., Rouleau J. R., Moyé L. A., Lewis S. J., et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J. Am. Coll. Cardiol. 2002;39:1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 63.Zile M. R., Gaasch W. H., Carroll J. D., Feldman M. D., Aurigemma G. P., Schaer G. L., Ghali J. K., Liebson P. R. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 64.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J. Am. Coll. Cardiol. 2006;48:1548–1551. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Edvardsen T., Gerber B. L., Garot J., Bluemke D. A., Lima J. A., Smiseth O. A. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50–56. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 66.Gotte M. J., van Rossum A. C., Twisk J. W. R., Kuijer J. P. A., Marcus J. T., Visser C. A. Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. J. Am. Coll. Cardiol. 2001;37:808–817. doi: 10.1016/s0735-1097(00)01186-4. [DOI] [PubMed] [Google Scholar]
- 67.Chuang M. L., Beaudin R. A., Riley M. F., Mooney M. G., Mannin W. J., Douglas P. S., Hibberd M. G. Three-dimensional echocardiographic measurement of left ventricular mass: comparison with magnetic resonance imaging and two-dimensional echocardiographic determinations in man. Int. J. Cardiac Imaging. 2000;16:347–357. doi: 10.1023/a:1026540809758. [DOI] [PubMed] [Google Scholar]
- 68.Maisel A. S., McCord J., Nowak R. M., Hollander J. E., Wu A. H., Duc P., Omland T., Storrow A. B., Krishnaswamy P., Abraham W. T., et al. Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J. Am. Coll. Cardiol. 2003;41:2010–2017. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 69.Shen X., Bornfeldt K. E. Mouse models for studies of cardiovascular complications of type 1 diabetes. Ann. N. Y. Acad. Sci. 2007;1103:202–217. doi: 10.1196/annals.1394.004. [DOI] [PubMed] [Google Scholar]
- 70.Retnakaran R., Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 2008;371:1790–1799. doi: 10.1016/S0140-6736(08)60767-9. [DOI] [PubMed] [Google Scholar]
- 71.Wold L. E., Ceylan-Isik A. F., Fang C. X., Yang X., Li S. Y., Sreejayan N., Privratsky J. R., Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-ribose) polymerase and myosin heavy chain isozyme. Free Radical Biol. Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Liang Q., Carlson E. C., Donthi R. V., Kralik P. M., Shen X., Epstein P. N. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–181. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 73.Cai L., Wang Y., Zhou G., Chen T., Song Y., Li X., Kang Y. J. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006;48:1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 74.Ye G., Metreveli N. S., Donthi R. V., Xia S., Xu M., Carlson E. C., Epstein P. N. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 75.Seddon M., Looi Y. H., Shah A. M. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao W. D., Liu Y., Marban E. Selective effects of oxygen free radicals on excitation-contraction coupling in ventricular muscle. Implications for the mechanism of stunned myocardium. Circulation. 1996;94:2597–2604. doi: 10.1161/01.cir.94.10.2597. [DOI] [PubMed] [Google Scholar]
- 77.Li J.-M., Gall N. P., Grieve D. J., Chen M., Shah A. M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 78.Wold L. E., Ceylan-Isik A. F., Fang C. X., Yang X., Li S. Y., Sreejayan N., Privratsky J. R., Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-ribose) polymerase and myosin heavy chain isozyme. Free Radical Biol. Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 79.Ritchie R. H., Quinn J. M., Cao A. H., Drummond G. R., Kaye D. M., Favaloro J. M., Proietto J., Delbridge L. M. The antioxidant tempol inhibits cardiac hypertrophy in the insulin-resistant GLUT4-deficient mouse In vivo. J. Mol. Cell. Cardiol. 2007;42:1119–1128. doi: 10.1016/j.yjmcc.2007.03.900. [DOI] [PubMed] [Google Scholar]
- 80.Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J. Clin. Invest. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan E. R., Walker S. J., Ezzat V. A., Wheatcroft S. B., Li J. M., Shah A. M., Kearney M. T. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1311–E1319. doi: 10.1152/ajpendo.00299.2007. [DOI] [PubMed] [Google Scholar]
- 82.Hornig B., Arakawa N., Kohler C., Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 83.MacCarthy P. A., Grieve D. J., Li J. M., Dunster C., Kelly F. J., Shah A. M. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation. 2001;104:2967–2974. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- 84.Yusuf S., Dagenais G., Pogue J., Bosch J., Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 85.Du X., Matsumura T., Edelstein D., Rossetti L., Zsengellér Z., Szabó C., Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reference deleted.
- 87.Szabo C. PARP as a drug target for the therapy of diabetic cardiovascular dysfunction. Drug News Perspect. 2002;15:197–205. doi: 10.1358/dnp.2002.15.4.840052. [DOI] [PubMed] [Google Scholar]
- 88.Minchenko A. G., Stevens M. J., White L., Abatan O. I., Komjáti K., Pacher P., Szabó C., Obrosova I. G. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 89.Chiu J., Farhangkhoee H., Xu B. Y., Chen S., George B., Chakrabarti S. PARP mediates structural alterations in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2008;45:385–393. doi: 10.1016/j.yjmcc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Yang Z., Zingarelli B., Szabo C. Effect of genetic disruption of poly (ADP-ribose) synthetase on delayed production of inflammatory mediators and delayed necrosis during myocardial ischemia-reperfusion injury. Shock. 2000;13:60–66. doi: 10.1097/00024382-200013010-00011. [DOI] [PubMed] [Google Scholar]
- 91.Soriano F. G., Pacher P., Mabley J., Liaudet L., Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ. Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 92.Bowling N., Walsh R. A., Song G., Estridge T., Sandusky G. E., Fouts R. L., Mintze K., Pickard T., Roden R., Bristow M. R., et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 93.Way K. J., Isshiki K., Suzuma K., Yokota T., Zvagelsky D., Schoen F. J., Sandusky G. E., Pechous P. A., Vlahos C. J., Wakasaki H., King G. L. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C β2 activation and diabetes. Diabetes. 2002;51:2709–2718. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 94.Koya D., King G. L. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 95.Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., et al. PKCα regulates cardiac contractility and propensity toward heart failure. Nat. Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 96.Wakasaki H., Koya D., Schoen F. J., Jirousek M. R., Ways D. K., Hoit B. D., Walsh R. A., King G. L. Targeted overexpression of protein kinase C β2 isoform in myocardium causes cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Topham M. K., Prescott S. M. Diacylglycerol kinases: regulation and signaling roles. Thrombosis Haemostasis. 2002;88:912–918. [PubMed] [Google Scholar]
- 98.Harada M., Takeishi Y., Arimoto T., Niizeki T., Kitahara T., Goto K., Walsh R. A., Kubota I. Diacylglycerol kinase ζ attenuates pressure overload-induced cardiac hypertrophy. Circ. J. 2007;71:276–282. doi: 10.1253/circj.71.276. [DOI] [PubMed] [Google Scholar]
- 99.Bilim O., Takeishi Y., Kitahara T., Arimoto T., Niizeki T., Sasaki T., Goto K., Kubota I. Diacylglycerol kinase ζ inhibits myocardial atrophy and restores cardiac dysfunction in streptozotocin-induced diabetes mellitus. Cardiovasc. Diabetol. 2008;7:2. doi: 10.1186/1475-2840-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia Z., Kuo K.-H., Nagareddy P. R., Wang F., Guo Z., Guo T., Jiang J., McNeill J. aH. N-acetylcysteine attenuates PKCβ2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc. Res. 2007;73:770–782. doi: 10.1016/j.cardiores.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 101.Hambleton M., Hahn H., Pleger S. T., Kuhn M. C., Klevitsky R., Carr A. N., Kimball T. F., Hewett T. E., Dorn G. W., 2nd, Koch W. J., Molkentin J. D. Pharmacological- and gene therapy-based inhibition of protein kinase Cα/β enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arikawa E., Ma R. C. W., Isshiki K., Luptak I., He Z., Yasuda Y., Maeno Y., Patti M. E., Weir G. C., Harris R. A., et al. Effects of insulin replacements, inhibitors of angiotensin, and PKCβ's actions to normalize cardiac gene expression and fuel metabolism in diabetic rats. Diabetes. 2007;56:1410–1420. doi: 10.2337/db06-0655. [DOI] [PubMed] [Google Scholar]
- 103.Montagnani M. Diabetic cardiomyopathy: how much does it depend on AGE? Br. J. Pharmacol. 2008;154:725–726. doi: 10.1038/bjp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aragno M., Mastrocola R., Medana C., Catalano M. G., Vercellinatto I., Danni O., Boccuzzi G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology. 2006;147:5967–5974. doi: 10.1210/en.2006-0728. [DOI] [PubMed] [Google Scholar]
- 105.Carley A. N., Severson D. L. What are the biochemical mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice? Cardiovasc. Drugs Ther. 2008;22:83–89. doi: 10.1007/s10557-008-6088-9. [DOI] [PubMed] [Google Scholar]
- 106.Bers D. M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 107.Trost S. U., Belke D. D., Bluhm W. F., Meyer M., Swanson E., Dillmann W. H. Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166–1171. doi: 10.2337/diabetes.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 108.Hattori Y., Matsuda N., Kimura J., Ishitani T., Tamada A., Gando S., Kemmotsu O., Kanno M. Diminished function and expression of the cardiac Na+-Ca2+ exchanger in diabetic rats: implication in Ca2+ overload. J. Physiol. 2000;527:85–94. doi: 10.1111/j.1469-7793.2000.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira L., Matthes J., Schuster I., Valdivia H. H., Herzig S., Richard S., Gómez A. M. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 110.Golfman L., Dixon I. M., Takeda N., Lukas A., Dakshinamurti K., Dhalla N. S. Cardiac sarcolemmal Na+-Ca2+ exchange and Na+-K+ ATPase activities and gene expression in alloxan-induced diabetes in rats. Mol. Cell. Biochem. 1998;188:91–101. [PubMed] [Google Scholar]
- 111.Vetter R., Rehfeld U., Reissfelder C., Weiss W., Wagner K. D., Günther J., Hammes A., Tschöpe C., Dillmann W., Paul M. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. FASEB J. 2002;16:1657–1659. doi: 10.1096/fj.01-1019fje. [DOI] [PubMed] [Google Scholar]
- 112.Yaras N., Bilginoglu A., Vassort G., Turan B. Restoration of diabetes-induced abnormal local Ca2+ release in cardiomyocytes by angiotensin II receptor blockade. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H912–H920. doi: 10.1152/ajpheart.00824.2006. [DOI] [PubMed] [Google Scholar]
- 113.Shishehbor M. H., Hoogwerf B. J., Schoenhagen P., Marso S. P., Sun J. P., Li J., Klein A. L., Thomas J. D., Garcia M. J. Relation of hemoglobin A1c to left ventricular relaxation in patients with type 1 diabetes mellitus and without overt heart disease. Am. J. Cardiol. 2003;91:1514–1517. doi: 10.1016/s0002-9149(03)00414-4. [DOI] [PubMed] [Google Scholar]
- 114.Jweied E. E., McKinney R. D., Walker L. A., Brodsky I., Geha A. S., Massad M. G., Buttrick P. M., de Tombe P. P. Depressed cardiac myofilament function in human diabetes mellitus. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2478–H2483. doi: 10.1152/ajpheart.00638.2005. [DOI] [PubMed] [Google Scholar]
- 115.Qin D., Huang B., Deng L., El-Adawi H., Ganguly K., Sowers J. R., El-Sherif N. Downregulation of K+ channel genes expression in type I diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2001;283:549–553. doi: 10.1006/bbrc.2001.4825. [DOI] [PubMed] [Google Scholar]
- 116.Dostal D. E., Rothblum K. N., Chernin M. I., Cooper G. R., Baker K. M. Intracardiac detection of angiotensinogen and renin: a localized renin-angiotensin system in neonatal rat heart. Am. J. Physiol. 1992;263:C838–C850. doi: 10.1152/ajpcell.1992.263.4.C838. [DOI] [PubMed] [Google Scholar]
- 117.Privratsky J. R., Wold L. E., Sowers J. R., Quinn M. T., Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 118.Connelly K. A., Kelly D. J., Zhang Y., Prior D. L., Martin J., Cox A. J., Thai K., Feneley M. P., Tsoporis J., White K. E., et al. Functional, structural and molecular aspects of diastolic heart failure in the diabetic (mRen-2)27 rat. Cardiovasc. Res. 2007;76:280–291. doi: 10.1016/j.cardiores.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 119.Raimondi L., De Paoli P., Mannucci E., Lonardo G., Sartiani L., Banchelli G., Pirisino R., Mugelli A., Cerbai E. Restoration of cardiomyocyte functional properties by angiotensin II receptor blockade in diabetic rats. Diabetes. 2004;53:1927–1933. doi: 10.2337/diabetes.53.7.1927. [DOI] [PubMed] [Google Scholar]
- 120.Fiordaliso F., Cuccovillo I., Bianchi R., Bai A., Doni M., Salio M., De Angelis N., Ghezzi P., Latini R., Masson S. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79:121–129. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 121.Liu X., Suzuki H., Sethi R., Tappia P. S., Takeda N., Dhalla N. S. Blockade of the renin-angiotensin system attenuates sarcolemma and sarcoplasmic reticulum remodeling in chronic diabetes. Ann. N. Y. Acad. Sci. 2006;1084:141–154. doi: 10.1196/annals.1372.003. [DOI] [PubMed] [Google Scholar]
- 122.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 123.Zannad F., Alla F., Dousset B., Perez A., Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES) Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 124.McEwan P. E., Gray G. A., Sherry L., Webb D. J., Kenyon C. J. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis In vivo. Circulation. 1998;98:2765–2773. doi: 10.1161/01.cir.98.24.2765. [DOI] [PubMed] [Google Scholar]
- 125.Neumann S., Huse K., Semrau R., Diegeler A., Gebhardt R., Buniatian G. H., Scholz G. H. Aldosterone and D-glucose stimulate the proliferation of human cardiac myofibroblasts in vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Cox S. R., Morita T., Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 127.Richard D. E., Berra E., Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J. Biol. Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 128.Abaci A., Oguzhan A., Kahraman S., Eryol N. K., Unal S., Arinç H., Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 129.Shinohara K., Shinohara T., Mochizuki N., Mochizuki Y., Sawa H., Kohya T., Fujita M., Fujioka Y., Kitabatake A., Nagashima K. Expression of vascular endothelial growth factor in human myocardial infarction. Heart Vessels. 1996;11:113–122. doi: 10.1007/BF01745169. [DOI] [PubMed] [Google Scholar]
- 130.Chou E., Suzuma I., Way K. J., Opland D., Clermont A. C., Naruse K., Suzuma K., Bowling N. L., Vlahos C. J., Aiello L. P., King G. L. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 131.Emoto M., Anno T., Sato Y., Tanabe K., Okuya S., Tanizawa Y., Matsutani A., Oka Y. Troglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3-L1 adipocytes. Diabetes. 2001;50:1166–1170. doi: 10.2337/diabetes.50.5.1166. [DOI] [PubMed] [Google Scholar]
- 132.Ebrahimian T. G., Heymes C., You D., Blanc-Brude O., Mees B., Waeckel L., Duriez M., Vilar J., Brandes R. P., Levy B. I., et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am. J. Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jesmin S., Zaedi S., Shimojo N., Iemitsu M., Masuzawa K., Yamaguchi N., Mowa C. N., Maeda S., Hattori Y., Miyauchi T. Endothelin antagonism normalizes VEGF signaling and cardiac function in STZ-induced diabetic rat hearts. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1030–E1040. doi: 10.1152/ajpendo.00517.2006. [DOI] [PubMed] [Google Scholar]
- 134.Tschope C., Schultheiss H. P., Walther T., Tschope C., Schultheiss H.-P., Walther T. Multiple interactions between the renin-angiotensin and the kallikrein-kinin systems: role of ACE inhibition and AT1 receptor blockade. J. Cardiovasc. Pharmacol. 2002;39:478–487. doi: 10.1097/00005344-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 135.Tschope C., Westermann D. Development of diabetic cardiomyopathy and the kallikrein-kinin system–new insights from B1 and B2 receptor signaling. Biol. Chem. 2008;389:707–711. doi: 10.1515/BC.2008.082. [DOI] [PubMed] [Google Scholar]
- 136.Spillmann F., Van Linthout S., Schultheiss H. P., Tschöpe C. Cardioprotective mechanisms of the kallikrein-kinin system in diabetic cardiopathy. Curr. Opin. Nephrol. Hypertension. 2006;15:22–29. doi: 10.1097/01.mnh.0000199009.56799.2b. [DOI] [PubMed] [Google Scholar]
- 137.Cloutier F., Couture R. Pharmacological characterization of the cardiovascular responses elicited by kinin B(1) and B(2) receptor agonists in the spinal cord of streptozotocin-diabetic rats. Br. J. Pharmacol. 2000;130:375–385. doi: 10.1038/sj.bjp.0703319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tschope C., Walther T., Yu M., Reinecke A., Koch M., Seligmann C., Heringer S. B., Pesquero J. B., Bader M., Schultheiss H., Unger T. Myocardial expression of rat bradykinin receptors and two tissue kallikrein genes in experimental diabetes. Immunopharmacology. 1999;44:35–42. doi: 10.1016/s0162-3109(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 139.Pelikanova T., Pinsker P., Smrckova I., Stribrna L., Dryakova M. Decreased urinary kallikrein with hyperglycemia in patients with short-term insulin-dependent diabetes mellitus. J. Diabetes Compl. 1998;12:264–272. doi: 10.1016/s1056-8727(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 140.Rett K., Wicklmayr M., Dietze G. J., Haring H. U. Insulin-induced glucose transporter (GLUT1 and GLUT4) translocation in cardiac muscle tissue is mimicked by bradykinin. Diabetes. 1996;45(Suppl. 1):S66–S69. doi: 10.2337/diab.45.1.s66. [DOI] [PubMed] [Google Scholar]
- 141.Shiuchi T., Cui T. X., Wu L., Nakagami H., Takeda-Matsubara Y., Iwai M., Horiuchi M. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension. 2002;40:329–334. doi: 10.1161/01.hyp.0000028979.98877.0c. [DOI] [PubMed] [Google Scholar]
- 142.Montanari D., Yin H., Dobrzynski E., Agata J., Yoshida H., Chao J., Chao L. Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes. 2005;54:1573–1580. doi: 10.2337/diabetes.54.5.1573. [DOI] [PubMed] [Google Scholar]
- 143.Tschope C., Walther T., Escher F., Spillmann F., Du J., Altmann C., Schimke I., Bader M., Sanchez-Ferrer C. F., Schultheiss H. P., Noutsias M. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J. 2005;19:2057–2059. doi: 10.1096/fj.05-4095fje. [DOI] [PubMed] [Google Scholar]
- 144.Mikrut K., Paluszak J., Kozlik J., Sosnowski P., Krauss H., Grzeskowiak E. The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res. Clin. Prac. 2001;51:79–85. doi: 10.1016/s0168-8227(00)00222-9. [DOI] [PubMed] [Google Scholar]
- 145.Tschope C., Spillmann F., Rehfeld U., Koch M., Westermann D., Altmann C., Dendorfer A., Walther T., Bader M., Paul M., et al. Improvement of defective sarcoplasmic reticulum Ca2+ transport in diabetic heart of transgenic rats expressing the human kallikrein-1 gene. FASEB J. 2004;18:1967–1969. doi: 10.1096/fj.04-1614fje. [DOI] [PubMed] [Google Scholar]
- 146.Tschope C., Walther T., Koniger J., Spillmann F., Westermann D., Escher F., Pauschinger M., Pesquero J. B., Bader M., Schultheiss H. P., Noutsias M. Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J. 2004;18:828–835. doi: 10.1096/fj.03-0736com. [DOI] [PubMed] [Google Scholar]
- 147.Regan T. J., Wu C. F., Yeh C. K., Oldewurtel H. A., Haider B. Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ. Res. 1981;49:1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- 148.Haider B., Yeh C. K., Thomas G., Oldewurtel H. A., Lyons M. M., Regan T. J. Influence of diabetes on the myocardium and coronary arteries of rhesus monkey fed an atherogenic diet. Circ. Res. 1981;49:1278–1288. doi: 10.1161/01.res.49.6.1278. [DOI] [PubMed] [Google Scholar]
- 149.Bhimji S., Godin D. V., McNeill J. H. Myocardial ultrastructural changes in alloxan-induced diabetes in rabbits. Acta Anat. (Basel) 1986;125:195–200. doi: 10.1159/000146161. [DOI] [PubMed] [Google Scholar]
- 150.Giacomelli F., Wiener J. Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab. Invest. 1979;40:460–473. [PubMed] [Google Scholar]
- 151.Jackson C. V., McGrath G. M., Tahiliani A. G., Vadlamudi R. V., McNeill J. H. A functional and ultrastructural analysis of experimental diabetic rat myocardium. Manifestation of a cardiomyopathy. Diabetes. 1985;34:876–883. doi: 10.2337/diab.34.9.876. [DOI] [PubMed] [Google Scholar]
- 152.Hsiao Y. C., Suzuki K., Abe H., Toyota T. Ultrastructural alterations in cardiac muscle of diabetic BB Wistar rats. Virchows Arch. A Pathol. Anat. Histopathol. 1987;411:45–52. doi: 10.1007/BF00734513. [DOI] [PubMed] [Google Scholar]
- 153.Thompson E. W. Structural manifestations of diabetic cardiomyopathy in the rat and its reversal by insulin treatment. Am. J. Anat. 1988;182:270–282. doi: 10.1002/aja.1001820308. [DOI] [PubMed] [Google Scholar]
- 154.Tarach J. S. Histomorphological and ultrastructural studies of a rat myocardium in experimental conditions. Z Mikrosk Anat. Forsch. 1976;90:1145–1157. [PubMed] [Google Scholar]
- 155.Reinila A., Akerblom H. K. Ultrastructure of heart muscle in short-term diabetic rats: influence of insulin treatment. Diabetologia. 1984;27:397–402. doi: 10.1007/BF00304857. [DOI] [PubMed] [Google Scholar]
- 156.Koltai M. Z., Balogh I., Wagner M., Pogatsa G. Diabetic myocardial alterations in ultrastructure and function. Exp. Pathol. 1984;25:215–221. doi: 10.1016/s0232-1513(84)80023-7. [DOI] [PubMed] [Google Scholar]
- 157.Fischer V. W., Barner H. B., Larose L. S. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum. Pathol. 1984;15:1127–1136. doi: 10.1016/s0046-8177(84)80307-x. [DOI] [PubMed] [Google Scholar]
- 158.Dyntar D., Eppenberger-Eberhardt M., Maedler K., Pruschy M., Eppenberger H. M., Spinas G. A., Donath M. Y. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–2113. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 159.Frustaci A., Kajstura J., Chimenti C., Jakoniuk I., Leri A., Maseri A., Nadal-Ginard B., Anversa P. Myocardial cell death in human diabetes. Circ. Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 160.Dyntar D., Sergeev P., Klisic E., Ambuhl P., Schaub M. C., Donath M. Y. High glucose alters cardiomyocyte contacts and inhibits myofibrillar formation. J. Clin. Endocrinol. Metab. 2006;91:1961–1967. doi: 10.1210/jc.2005-1904. [DOI] [PubMed] [Google Scholar]
- 161.Backlund T., Palojoki E., Saraste A., Eriksson A., Finckenberg P., Kytö V., Lakkisto P., Mervaala E., Voipio-Pulkki L. M., Laine M., Tikkanen I. Sustained cardiomyocyte apoptosis and left ventricular remodelling after myocardial infarction in experimental diabetes. Diabetologia. 2004;47:325–330. doi: 10.1007/s00125-003-1311-5. [DOI] [PubMed] [Google Scholar]
- 162.Cai L., Kang Y. J. Cell death and diabetic cardiomyopathy. Cardiovasc. Toxicol. 2003;3:219–228. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- 163.Saito K., Nishi S., Kashima T., Tanaka H. Histologic and ultrastructural studies on the myocardium in spontaneously diabetic KK mice: a new animal model of cardiomyopathy. Am. J. Cardiol. 1984;53:320–323. doi: 10.1016/0002-9149(84)90446-6. [DOI] [PubMed] [Google Scholar]
- 164.Okruhlicova L., Tribulova N., Weismann P., Sotnikova R. Ultrastructure and histochemistry of rat myocardial capillary endothelial cells in response to diabetes and hypertension. Cell Res. 2005;15:532–538. doi: 10.1038/sj.cr.7290322. [DOI] [PubMed] [Google Scholar]
- 165.Silver M. D., Huckell V. F., Lorber M. Basement membranes of small cardiac vessels in patients with diabetes and myxoedema: preliminary observations. Pathology. 1977;9:213–220. doi: 10.3109/00313027709084812. [DOI] [PubMed] [Google Scholar]
- 166.Thompson E. W. Quantitative analysis of myocardial structure in insulin-dependent diabetes mellitus: effects of immediate and delayed insulin replacement. Proc. Soc. Exp. Biol. Med. 1994;205:294–305. doi: 10.3181/00379727-205-43710. [DOI] [PubMed] [Google Scholar]
- 167.Cameron D. P., Amherdt M., Leuenberger P., Orci L., Stauffacher W. Microvascular alterations in chronically streptozotocin-diabetic rats. Adv. Metab. Disord. 1973;2(Suppl. 2):257–269. doi: 10.1016/b978-0-12-027362-1.50033-9. [DOI] [PubMed] [Google Scholar]
- 168.Kilo C., Vogler N., Williamson J. R. Muscle capillary basement membrane changes related to aging and to diabetes mellitus. Diabetes. 1972;21:881–905. doi: 10.2337/diab.21.8.881. [DOI] [PubMed] [Google Scholar]
- 169.Eurich D. T., Majumdar S. R., McAlister F. A., Tsuyuki R. T., Johnson J. A. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 170.Masoudi F. A., Inzucchi S. E., Wang Y., Havranek E. P., Foody J. M., Krumholz H. M. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 171.Eurich D. T., McAlister F. A., Blackburn D. F., Majumdar S. R., Tsuyuki R. T., Varney J., Johnson J. A. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. Br. Med. J. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gangji A. S., Cukierman T., Gerstein H. C., Goldsmith C. H., Clase C. M. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389–394. doi: 10.2337/dc06-1789. [DOI] [PubMed] [Google Scholar]
- 173.Chan A. Y., Soltys C. L., Young M. E., Proud C. G., Dyck J. R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 174.McGuire D. K., Inzucchi S. E. New drugs for the treatment of diabetes mellitus: part I: thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–449. doi: 10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- 175.Hallsten K., Virtanen K. A., Lonnqvist F., Janatuinen T., Turiceanu M., Rönnemaa T., Viikari J., Lehtimäki T., Knuuti J., Nuutila P. Enhancement of insulin-stimulated myocardial glucose uptake in patients with Type 2 diabetes treated with rosiglitazone. Diabetes Med. 2004;21:1280–1287. doi: 10.1111/j.1464-5491.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 176.Giles T. D., Miller A. B., Elkayam U., Bhattacharya M., Perez A. Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction. J. Card. Failure. 2008;14:445–452. doi: 10.1016/j.cardfail.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 177.Nissen S. E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 178.St John Sutton M., Rendell M., Dandona P., Dole J. F., Murphy K., Patwardhan R., Patel J., Freed M. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes Care. 2002;25:2058–2064. doi: 10.2337/diacare.25.11.2058. [DOI] [PubMed] [Google Scholar]
- 179.Lago R. M., Singh P. P., Nesto R. W. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 180.Lincoff A. M., Wolski K., Nicholls S. J., Nissen S. E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA, J. Am. Med. Assoc. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 181.Dormandy J. A., Charbonnel B., Eckland D. J., Erdmann E., Massi-Benedetti M., Moules I. K., Skene A. M., Tan M. H., Lefèbvre P. J., Murray G. D., et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 182.Betteridge D. J., DeFronzo R. A., Chilton R. J. PROactive: time for a critical appraisal. Eur. Heart J. 2008;29:969–983. doi: 10.1093/eurheartj/ehn114. [DOI] [PubMed] [Google Scholar]
- 183.Home P. D., Pocock S. J., Beck-Nielsen H., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. Rosiglitazone evaluated for cardiovascular outcomes: an interim analysis. N. Engl. J. Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 184.Swedberg K., Pfeffer M., Granger C., Held P., McMurray J., Ohlin G., Olofsson B., Ostergren J., Yusuf S. Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm-Programme Investigators. J. Card. Failure. 1999;5:276–282. doi: 10.1016/s1071-9164(99)90013-1. [DOI] [PubMed] [Google Scholar]
- 185.Nystrom T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm. Metab. Res. 2008;40:593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 186.Nikolaidis L. A., Mankad S., Sokos G. G., Miske G., Shah A., Elahi D., Shannon R. P. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 187.Sokos G. G., Nikolaidis L. A., Mankad S., Elahi D., Shannon R. P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Failure. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 188.Fragasso G., Palloshi A., Puccetti P., Silipigni C., Rossodivita A., Pala M., Calori G., Alfieri O., Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J. Am. Coll. Cardiol. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 189.Lee L., Campbell R., Scheuermann-Freestone M., Taylor R., Gunaruwan P., Williams L., Ashrafian H., Horowitz J., Fraser A. G., Clarke K., Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 190.McCormack J. G., Barr R. L., Wolff A. A., Lopaschuk G. D. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 191.Patel A., MacMahon S., Chalmers J., Neal B., Woodward M., Billot L., Harrap S., Poulter N., Marre M., Cooper M., et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 192.Yusuf S., Teo K. K., Pogue J., Dyal L., Copland I., Schumacher H., Dagenais G., Sleight P., Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 193.Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G. Effects of an angiotensinconverting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 194.Lewis E. J., Hunsicker L. G., Clarke W. R., Berl T., Pohl M. A., Lewis J. B., Ritz E., Atkins R. C., Rohde R., Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 195.Shekelle P. G., Rich M. W., Morton S. C., Atkinson C. S., Tu W., Maglione M., Rhodes S., Barrett M., Fonarow G. C., Greenberg B., et al. Efficacy of angiotensin-converting enzyme inhibitors and β-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J. Am. Coll. Cardiol. 2003;41:1529–1538. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 196.Cohn J. N., Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 197.Wlodarczyk J. H., Keogh A., Smith K., McCosker C. CHART: congestive cardiac failure in hospitals, an Australian review of treatment. Heart Lung Circ. 2003;12:94–102. doi: 10.1046/j.1444-2892.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 198.Bakris G. L., Fonseca V., Katholi R. E., McGill J. B., Messerli F. H., Phillips R. A., Raskin P., Wright J. T., Jr, Oakes R., Lukas M. A., et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA, J. Am. Med. Assoc. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 199.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 200.Packer M., Coats A. J., Fowler M. B., Katus H. A., Krum H., Mohacsi P., Rouleau J. L., Tendera M., Castaigne A., Roecker E. B., et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 201.Nemoto O., Kawaguchi M., Yaoita H., Miyake K., Maehara K., Maruyama Y. Left ventricular dysfunction and remodeling in streptozotocin-induced diabetic rats. Circ. J. 2006;70:327–334. doi: 10.1253/circj.70.327. [DOI] [PubMed] [Google Scholar]
- 202.Fredersdorf S., Thumann C., Ulucan C., Griese D. P., Luchner A., Riegger G. A., Kromer E. P., Weil J. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc. Pathol. 2004;13:11–19. doi: 10.1016/S1054-8807(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 203.Poornima I. G., Parikh P., Shannon R. P. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ. Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 204.Barouch L. A., Berkowitz D. E., Harrison R. W., O'Donnell C. P., Hare J. M. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 205.Rutter M. K., Parise H., Benjamin E. J., Levy D., Larson M. G., Meigs J. B., Nesto R. W., Wilson P. W., Vasan R. S. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 206.Tenenbaum A., Fisman E. Z., Schwammenthal E., Adler Y., Benderly M., Motro M., Shemesh J. Increased prevalence of left ventricular hypertrophy in hypertensive women with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2003;2:14. doi: 10.1186/1475-2840-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yarom R., Zirkin H., Stammler G., Rose A. G. Human coronary microvessels in diabetes and ischaemia. Morphometric study of autopsy material. J. Pathol. 1992;166:265–270. doi: 10.1002/path.1711660308. [DOI] [PubMed] [Google Scholar]
- 208.Nunoda S., Genda A., Sugihara N., Nakayama A., Mizuno S., Takeda R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels. 1985;1:43–47. doi: 10.1007/BF02066486. [DOI] [PubMed] [Google Scholar]
- 209.Kawaguchi M., Asakura T., Saito F., Nemoto O., Maehara K., Miyake K., Sugai N., Maruyama Y. [Changes in diameter size and F-actin expression in the myocytes of patients with diabetes and streptozotocin-induced diabetes model rats] J. Cardiol. 1999;34:333–339. [PubMed] [Google Scholar]