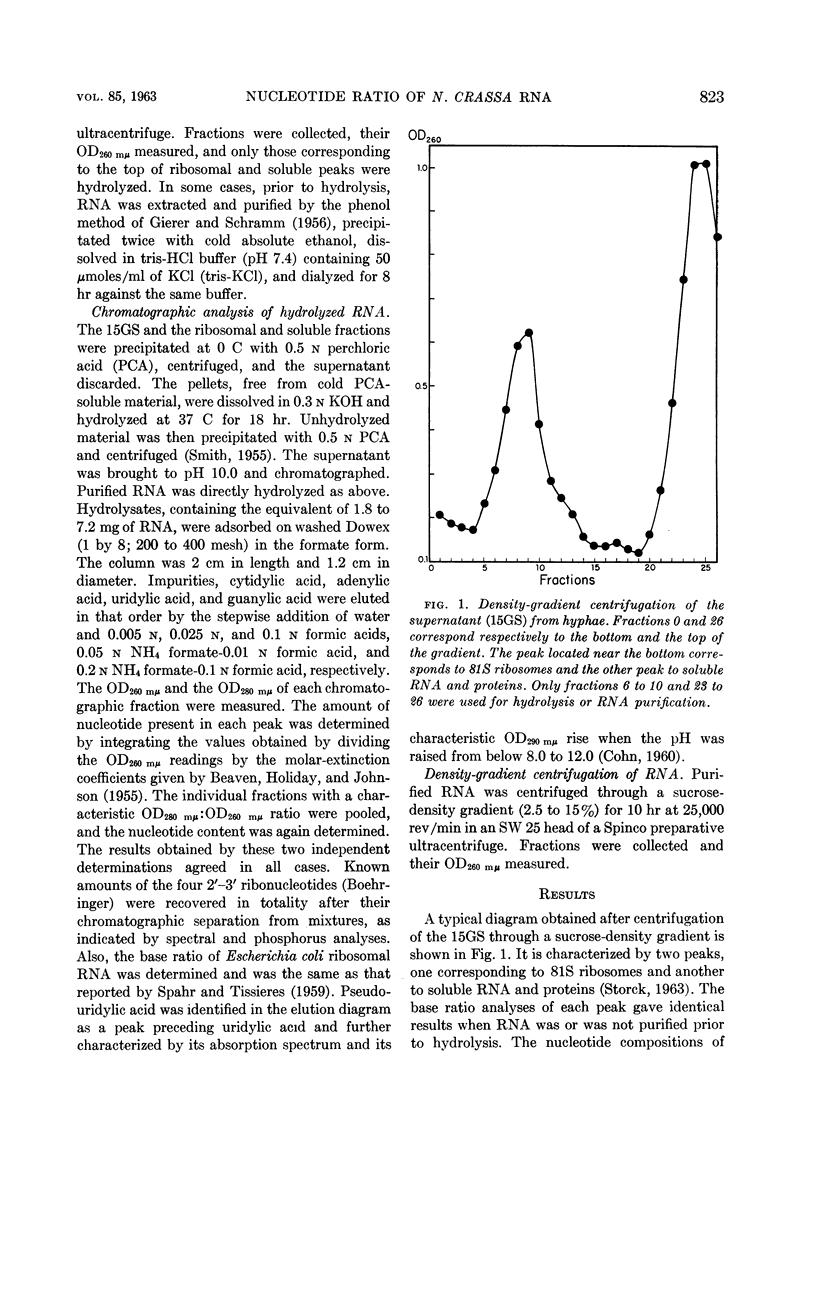
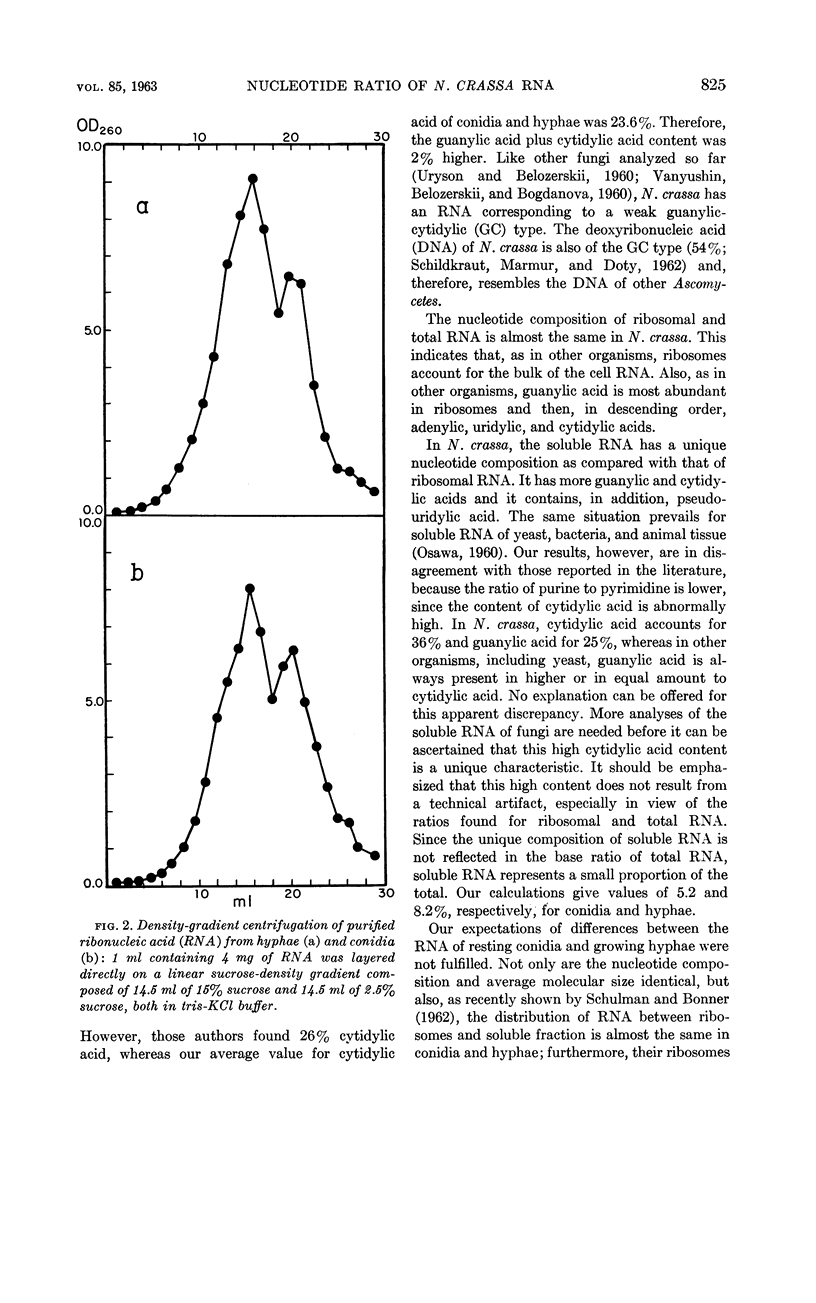
Abstract
Henney, H. (The University of Texas, Austin) and R. Storck. Nucleotide composition of ribonucleic acid from Neurospora crassa. J. Bacteriol. 85:822–826. 1963.—The nucleotide composition of total, ribosomal, and soluble ribonucleic acid (RNA) from the conidia and the hyphae of Neurospora crassa was determined. The corresponding RNA classes from the two morphological types had the same base ratio. Total, ribosomal, and soluble RNA from hyphae contained, respectively, 51.0, 50.2, and 61.6% guanylic acid (G) plus cytidylic acid (C), and from conidia, 50.6, 49.9, and 62.1%. The proportion of nucleotides in ribosomal RNA was in close agreement with that reported for ribosomal RNA from other organisms. Soluble RNA contained 2.8% pseudouridylic acid, 36.0% C, and 25.5% G, and differed from soluble RNA from other sources in its high C content. Centrifugation of purified RNA from conidia and hyphae, through a linear sucrose-density gradient, yielded identical sedimentation profiles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN W. E. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J Biol Chem. 1960 May;235:1488–1498. [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KOEHLER J. K. Variation of yeast ribosomes with nutritional state. Nature. 1962 May 26;194:757–759. doi: 10.1038/194757a0. [DOI] [PubMed] [Google Scholar]
- LOUDERBACK A. L., SCHERBAUM O. H., JAHN T. L. The effect of temperature shifts on the budding cycle of Saccharomyces cerevisiae. Exp Cell Res. 1961 Nov;25:437–453. doi: 10.1016/0014-4827(61)90293-2. [DOI] [PubMed] [Google Scholar]
- OSAWA S. The nucleotide composition of ribonucleic acids from subcellular components of yeast, Escherichia coli and rat liver, with special reference to the occurrence of pseudouridylic acid in soluble ribonucleic acid. Biochim Biophys Acta. 1960 Aug 12;42:244–254. doi: 10.1016/0006-3002(60)90788-5. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SCHULMAN H. M., BONNER D. M. A naturally occurring DNA-RNA complex from Neurospora crassa. Proc Natl Acad Sci U S A. 1962 Jan 15;48:53–63. doi: 10.1073/pnas.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORCK R. Characterization of ribosomes from Neurospora crassa. Biophys J. 1963 Jan;3:1–10. doi: 10.1016/s0006-3495(63)86800-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESE C. R. Unusual ribosome particles occurring during spore germination. J Bacteriol. 1961 Nov;82:695–701. doi: 10.1128/jb.82.5.695-701.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. G. Macromolecular constituents of proliferating and nonproliferating yeast extracts. Arch Biochem Biophys. 1956 Jul;63(1):100–105. doi: 10.1016/0003-9861(56)90013-3. [DOI] [PubMed] [Google Scholar]



