Abstract
Background information. Centrosome duplication normally parallels with DNA replication and is responsible for correct segregation of replicated DNA into the daughter cells. Although geminin interacts with Cdt1 to prevent loading of MCMs (minichromosome maintenance proteins) on to the replication origins, inactivation of geminin nevertheless causes centrosome over-duplication in addition to the re-replication of the genome, suggesting that geminin may play a role in centrosome duplication. However, the exact mechanism by which loss of geminin affects centrosomal duplication remains unclear and the possible direct interaction of geminin with centrosomal-localized proteins is still unidentified.
Results. We report in the present study that geminin is physically localized to the centrosome. This unexpected geminin localization is cell-cycle dependent and mediated by the actin-related protein, Arp1, one subunit of the dynein–dynactin complex. Disruption of the integrity of the dynein–dynactin complex by overexpression of dynamitin/p50, a well-characterized inhibitor of dynactin, reduces the centrosomal localization of both geminin and Arp1. Enrichment of geminin on centrosomes was enhanced when cellular ATP production was suppressed in the ATP-inhibitor assay, whereas the accumulation of geminin on the centrosome was disrupted by depolymerization of the microtubules using nocodazole. We further demonstrate that the coiled-coil motif of geminin is required for its centrosomal localization and the interaction of geminin with Arp1. Depletion of geminin by siRNA (small interfering RNA) in MDA-MB-231 cells led to centrosome over-duplication. Conversely, overexpression of geminin inhibits centrosome over-duplication induced by HU in S-phase-arrested cells, and the coiled-coil-motif-mediated centrosomal localization of geminin is required for its inhibition of centrosome over-duplication. Centrosomal localization of geminin is conserved among mammalian cells and geminin might perform as an inhibitor of centrosome duplication.
Conclusions. The results of the present study demonstrate that a fraction of geminin is localized on the centrosome, and the centrosomal localization of geminin is Arp1-mediated and dynein–dynactin-dependent. The coiled-coil motif of geminin is required for its targeting to the centrosome and inhibition of centrosome duplication. Thus the centrosomal localization of geminin might perform an important role in regulation of proper centrosome duplication.
Keywords: actin-related protein 1 (Arp1), centrosome, dynamitin/p50, dynein–dynactin complex, geminin
Abbreviations: Arp1, actin-related protein 1; Az, sodium azide; CDK, cyclin-dependent kinase; Co-IP, co-immunoprecipitation; DAPI, 4′,6-diamidino-2-phenylindole; DOG, 2-deoxyglucose; GFP, green fluorescent protein; GST, glutathione transferase; HU, hydroxyurea; MCM, minichromosome maintenance protein; MEF, mouse embryonic fibroblast; pre-RC, pre-replication complex; siRNA, small interference RNA; TRITC, tetramethylrhodamine β-isothiocyanate
Introduction
Genome stability is maintained by high-fidelity replication of the genome and faithful separation of replicated DNAs into daughter cells. Regulation of DNA replication is achieved by proper licensing of the pre-RC (pre-replication complex), which involves the stepwise recruitment of Orcs, Cdc6, Cdt1 and MCMs (minichromosome maintenance proteins) on to the replication origins. In late mitosis and G1-phase, MCM2-7, a putative replicative helicase, is assembled on to replication origins to license DNA replication (Chong et al., 1995; Yankulov et al., 1999). Functional assembly of MCM proteins on to chromatin is dependent upon Cdc6 and Cdt1 (Tanaka et al., 1997; Maiorano et al., 2000). Once DNA replication is initiated, the replication licensing is inhibited by CDKs (cyclin-dependent kinases), Cdt1 degradation and the binding of the inhibitory protein geminin to Cdt1. Cdt1 is expressed in the G1-phase and is phosphorylated at the onset of S-phase, and is almost degraded through SCF (Skp1/cullin/F-box)-Skp2 and CUL4-DDB1-CDT2 ubiquitin E3 ligases (Nishitani et al., 2001; Liu et al., 2004). During S- and G2-phase, non-proteolytic Cdt1 is inactivated by binding to geminin, and consequently the inactivation of Cdt1 prevents MCMs reloading on to chromatin (McGarry and Kirschner, 1998; Wohlschlegel et al., 2000; Yanagi et al., 2002; Xouri et al., 2007). This process ensures that replication origins fire only once per cell cycle. Loss of geminin results in genomic over-replication (Melixetian et al., 2004; Zhu et al., 2004).
Centrosome duplication is strictly regulated in parallel with DNA replication (Hinchcliffe and Sluder, 2001). As the major MTOCs (microtubule-organizing centres) in animal cells, the centrosome duplicates during S-phase and plays a significant role in the assembly and bipolarity of the spindle (Nigg, 2002). A large set of proteins is localized on the centrosomes and are involved in centrosome duplication. Cytoplasmic dynein is a multisubunit microtubule motor complex that, together with its activator, dynactin, drives its cargo toward the minus ends of the microtubules and centrosomes. Dynactin has been proposed to function as a linker between dynein and its cargo (King and Schroer, 2000). Arp1 (actin-related protein 1; also known as centractin-α), the most abundant subunit in the dynactin complex, is in charge of microtubule transport of cellular organelles and centrosome-related proteins towards the minus end (Merdes et al., 2000; Xiang et al., 2000).
It has been reported that loss of geminin causes centrosome over-duplication in addition to genomic over-duplication, further evidence that geminin is an inhibitor of centrosome over-duplication (Tachibana et al., 2005), but the exact mechanism is unclear. The potential centrosomal proteins that directly interact with geminin are also unidentified. In the present study, we observed that geminin is localized to the centrosomes. The centrosomal localization of geminin is functional dynein–dynactin complex-dependent and the interaction of geminin with the dynein–dynactin complex is mediated by Arp1. The coiled-coil motif is required for centrosomal localization of geminin and the interaction of geminin with Arp1. The accumulation of geminin on centrosomes might shed light on uncovering the role of geminin in co-ordinating DNA replication and centrosome duplication.
Results
A fraction of geminin is localized on centrosomes in the cell cycle
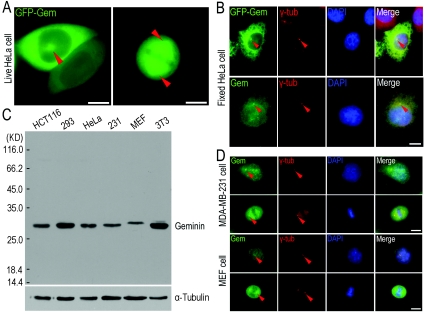
To study the speculated function of geminin on centrosome duplication, we cloned the human geminin gene, expressed the protein fused with GFP (green fluorescent protein) in HeLa cells and followed its localization. In the living cells, surprisingly, we observed that a fraction of GFP–geminin was localized on the aster-like structures in interphase and spindle fibres in mitosis (Figure 1A, indicated with arrowheads). To determine whether the aster-like structure was the centrosome, we fixed HeLa cells with ice-cold methanol and performed an immunofluorescence count staining using an antibody against γ-tubulin, a protein marker for centrosomes. The results showed that a fraction of GFP–geminin did co-localize with γ-tubulin at the centrosomes in interphase cells (Figure 1B, upper panel, indicated with arrowheads). To verify that the centrosomal localization of GFP–geminin was not an artefact due to overexpression of GFP–geminin, we performed co-immunofluorescence staining of endogenous geminin using a geminin-specific antibody with the antibody anti-γ-tubulin. As indicated in Figure 1(B) (bottom panel, indicated with arrowheads), the endogenous geminin was clearly detected co-localized with γ-tubulin on centrosomes.
Figure 1. Geminin localizes on centrosomes.
(A) A fraction of GFP–geminin localized to ‘aster-like’ structures in a live cell. HeLa cells were transfected with pEGFPC2-geminin vector using a standard calcium phosphate transfection protocol. The culture dish was placed on to a heated sample stage within a heated chamber (37°C) at 5% CO2. GFP–geminin-expressing living cells were imaged by a Zeiss200M fluorescence microscope (Axiovert 200M) with the plan APO 63′/1.35 objective. GFP–geminin is expressed and partially localized to ‘aster-like’ structures in interphase cells as well as spindle fibres in cells undergoing mitosis as indicated with arrowheads (red). Scale bar=10 μm. (B) Centrosomal localization of both exogenous GFP–geminin and endogenous geminin. GFP–geminin-expressing HeLa cells were fixed with ice-cold methanol and immunostained with a γ-tubulin antibody. After incubation with a TRITC-conjugated secondary antibody, cells were mounted with mowiol containing DAPI for staining of DNA. Staining for γ-tubulin indicated the presence of centrosomes. Endogenous geminin was examined by immunostaining with an anti-geminin antibody. A clear centrosome co-staining of γ-tubulin and geminin (indicated with arrowheads) was achieved. Scale bar=10 μm. (C) The endogenous geminin from different cell lines was probed using the same antibody against human geminin as used in Western blot analysis, suggesting that the anti-geminin antibody specifically recognized geminin in these cells. α-Tubulin was used as a loading control. The molecular mass in kDa is indicated on the left-hand side of the gel. 293, HEK (human embryonic kidney)-293 cells; 231, MDA-MB-231 cells; 3T3, NIH 3T3 cells. (D) Co-localization of geminin and Arp1 on centrosomes in MDA-MB-231 cells and MEF cells. MDA-MB-231 and MEF cells were fixed with ice-cold methanol and co-immunostained using antibodies against geminin and γ-tubulin (γ-tub). The similar centrosomal localization of both geminin and γ-tubulin (indicated with arrowheads, red) was observed in these cell lines as in (B). Scale bar=10 μm. Gem, geminin.
As geminin is a conserved protein among higher eukaryotes, we presumed that the centrosomal localization of geminin should also be conservative. To test this assumption, with Western blot analysis and immunostaining, we analysed two other cell lines, one is the MDA-MB-231 cell, a breast cancer cell line from humans, and the other is the MEF (mouse embryonic fibroblast) cell, an embryonic fibroblast cell line from mouse. As shown in Figure 1(C), the antibody raised for human geminin also cross-reacted with mouse geminin. Similar to the results in HeLa cells, a fraction of endogenous geminin was clearly co-localized with γ-tubulin on centrosomes in interphase and spindle fibres and poles in mitosis in both MDA-MB-231 and MEF cells (Figure 1D, indicated with arrowheads).
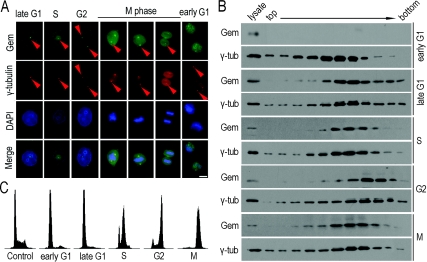
To determine the timing of geminin localization on centrosomes, we synchronized HeLa cells at anticipated times through double-thymidine arrest. The efficiency of cell synchronization was analysed by FACS (Figure 2C). Immunofluorescence labelling showed that geminin appeared on centrosomes in late G1-, S-, G2- and M-phase, and disassociated from centrosomes in early G1-phase (Figure 2A). Western blot analysis with an anti-geminin antibody of the purified centrosomes also showed that geminin was localized on to the centrosome from late G1- to M-phase, but not in early G1-phase (Figure 2B). These results indicate that centrosomal localization of geminin is in a cell-cycle-dependent manner.
Figure 2. Cell-cycle-dependent centrosomal localization of geminin.
(A) The centrosomal localization of geminin is accompanied with the progression of the cell cycle. Synchronized HeLa cells were fixed in ice-cold methanol and subjected to immunofluorescence staining using anti-geminin and anti-γ-tubulin antibodies. Geminin (Gem) locates on to centrosomes from late G1- to M-phase, and disassociates from centrosomes in early G1-phase (indicated with arrowheads). Scale bar=10 μm. (B) Centrosomes purified from synchronized HeLa cells were analysed by Western blotting. The absence of geminin (Gem) in purified early G1-phase centrosomes correlated with the results in (A), that geminin released from centrosomes in early G1 phase. γ-tub, γ-tubulin. (C) Synchronized HeLa cells in (A) and (B) were analysed by FACS. HeLa cells were arrested at late G1-phase by double-thymidine treatment. Cells were then released into fresh medium for 4, 10 and 16 h to enter S-, G2- and early G1-phase. M-phase cells were obtained by 24 h thymidine treatment followed by 12 h nocodazole treatment.
Arp1 is a potential protein which mediates centrosomal localization of geminin
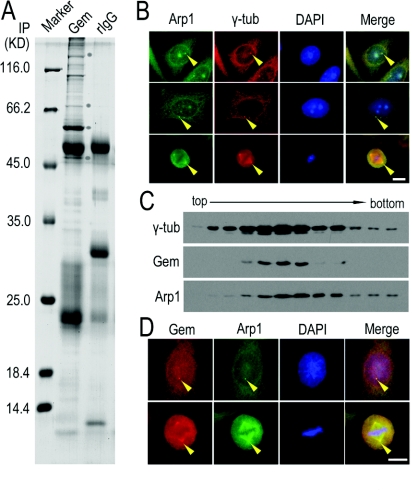
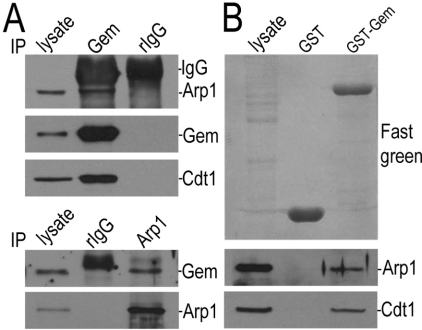
To search for potential geminin-binding proteins, we performed an immunoprecipitation assay using an anti-geminin antibody. The proteins co-immunoprecipitated with geminin were separated by SDS/PAGE and analysed by MS. We observed the presence of Arp1 and dynactin1 in the protein complex (Figure 3A). The interaction between geminin and Arp1 was further examined by Co-IP (co-immunoprecipitation) using anti-geminin and anti-Arp1 antibodies. Arp1 can be co-immunoprecipitated by geminin, and vice versa (Figure 4A). Arp1 could also be pulled down by exogenous GST (glutathione transferase)–geminin protein from HeLa cell lysate (Figure 4B). These results showed the direct interaction of geminin with Arp1. It is reported that Arp1 is the most abundant subunit of the dynactin complex, a cargo carrier toward the minus-end of microtubules (Fouquet et al., 2000). Arp1 is also a centrosomal protein and localizes on centrosomes and spindle in mitosis (Clark and Meyer, 1992). The results of the present study confirmed the localization of Arp1 on centrosomes and spindles (Figure 3B, indicated by arrowheads). We then examined the co-localization of geminin and Arp1 on centrosomes. We purified the centrosomes from HeLa cells through sucrose-density-gradient high-speed centrifugation and found that both geminin and Arp1 were present in the same fractions enriched in γ-tubulin (Figure 3C). These consistent results were obtained in the two other cell lines, MDA-MB-231 and MEF cells (Supplementary Figures S1A and S1C at http://www.biolcell.org/boc/101/boc1010273add.htm). We also co-stained the cells with anti-geminin and anti-Arp1 antibodies and found that the two proteins were co-localized on centrosomes in interphase cells and spindle or poles in mitotic cells (Figure 3D, indicated with arrowheads and Supplementary Figures S1B and S1D, indicated with arrowheads).
Figure 3. Arp1 as a potential centrosomal protein co-localized with geminin.
(A) Lysate from HeLa cells expressing exogenous GFP–geminin was immunoprecipitated (IP) using an antibody against geminin or rabbit IgG (rIgG) as a control. The proteins co-immunoprecipitated with geminin were separated on SDS/PAGE (10% gel) and stained with Coomassie Blue R-250. The protein bands were subjected to MS analysis. The bands indicated with a circle are (from the bottom to the top): dynactin 1 (p150Glued), Cdt1, GFP–geminin and Arp1 respectively. The molecular mass in kDa is indicated on the left-hand side of the gel. (B) Arp1 co-stained with γ-tubulin (γ-tub) in HeLa cells. HeLa cells were fixed and subjected to immunofluorescence staining using antibodies against Arp1 and γ-tubulin. The results showed that a fraction of Arp1 is located on centrosomes and Arp1 was well co-localized with γ-tubulin (indicated with arrowheads). Scale bar=10 μm. (C) Components of purified centrosomes from HeLa cells were examined for the co-existence of geminin (Gem) and Arp1. Fractions of centrosomes isolated from a discontinuous sucrose gradient were separated using SDS/PAGE (10% gel) and immunoblotted using antibodies against geminin and Arp1. γ-Tubulin (γ-tub) was immunoblotted as a positive control for centrosome purification. (D) Co-localization of endogenous geminin (Gem) and Arp1 was viewed by immunofluorescence staining using antibodies against geminin and Arp1 in HeLa cells fixed with ice-cold methanol. Both geminin and Arp1 were apparently co-localized in the centrosome in interphase and spindle poles in mitosis (indicated with arrowheads). Scale bar=10 μm.
Figure 4. Geminin directly interacts with Arp1.
(A) Geminin and Arp1 interacted with each other. In the upper panels, Arp1 was co-immunoprecipitated with geminin. Lysates of HeLa cells were immunoprecipitated (IP) with antibodies against geminin or rabbit IgG (rIgG) as a control. The co-existence of Arp1 and geminin was examined by immunoblotting analysis. Cdt1 was immunoblotted as a positive control for binding proteins of geminin. In the bottom panels, geminin was co-immunoprecipitated with Arp1. Lysates from GFP–Arp1-overexpressing HeLa cells were tested for immunosedimentation of geminin by Arp1 using an antibody against Arp1 or rIgG as a control. (B) In vitro pulldown assay for testing the association between Arp1 and GST–geminin. Purified GST–geminin was coupled to glutathione–Sepharose 4B beads and incubated with the lysate of HeLa cells for 2 h. The proteins on the beads were separated on SDS/PAGE (10% gel) and transferred on to a nitrocellulose membrane and stained with Fast Green (upper panel). The nitrocellulose membrane was then immunoprobed for Arp1 with a specific antibody. Cdt1 was immunoblotted as a positive control of geminin-associated proteins (bottom panels). Gem, geminin.
The centrosomal localization of geminin depends on the dynein–dynactin complex and microtubules
We then asked whether Arp1 plays a role in centrosomal targeting of geminin. If Arp1 is a carrier of geminin, centrosomal localization of geminin should be sensitive to overexpressed exogenous dynamitin/p50, a well-characterized inhibitor of the dynein–dynactin complex which regulates Arp1 centrosomal localization. We overexpressed exogenous dynamitin/p50 to inhibit the function of dynactin, and tested whether the centrosomal localization of geminin was affected. The results showed that the centrosomal targeting of geminin was gradually abolished by overexpression of dynamitin/p50 in a concentration-dependent manner, whereas GFP overexpression had no effect on the centrosomal localization of geminin (Figure 5A). Notably, along with the functional disruption of the dynein–dynactin complex by the overexpression of dynamitin/p50, the centrosomal targeting of Arp1 was also reduced (Figure 5B). Consistent results were also achieved in both MDA-MB-231 and MEF cells (Supplementary Figures S2A and S2B at http://www.biolcell.org/boc/101/boc1010273add.htm).
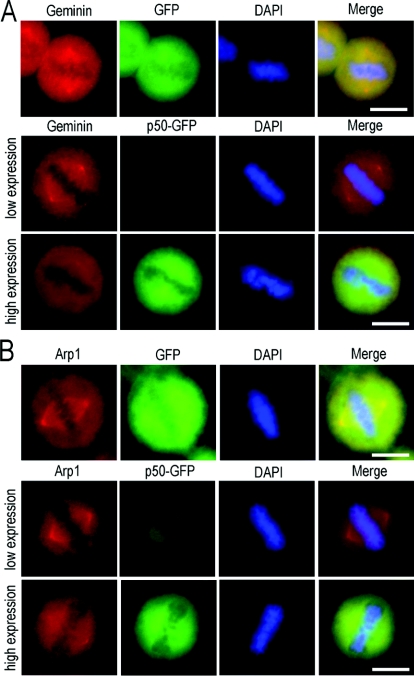
Figure 5. Disruption of the dynein–dynactin complex by overexpressed exogenous dynamitin/p50 impairs both geminin and Arp1 centrosomal localization.
GFP–dynamitin/p50 was introduced into HeLa cells using Lipofectamine™ 2000. At 24 h after transfection, the cells were fixed with ice-cold methanol and examined by immunofluorescence staining using antibodies against geminin and Arp1. Transfection of GFP was taken as a negative control. In GFP-expressing control HeLa cells, the centrosomal/spindle pole localization of both geminin and Arp1 was not affected, as indicated in the upper panels of (A) and (B). Similar results were obtained in low-level dynamitin/p50-expressing cells, in which both geminin and Arp1 were normally localized on centrosome/spindle poles [middle panels in (A) and (B)]. High expression of dynamitin/p50 in HeLa cells did impair centrosomal localization of both geminin and Arp1, as indicated in the bottom panels of (A) and (B). Scale bar=10 μm.
Arp1 is one subunit of dynactin, and targets its microtubule minus-end cargo transportation along microtubules. In consideration of spindle and spindle-pole localization of geminin (Figures 1A and 5A), we pursued the possibility that geminin transports to the centrosome along the microtubules. With the addition of nocodazole (20 μM) into medium for 30 min, microtubules were depolymerized and concomitantly geminin was lost from the centrosomes (Figure 6A). These results suggest that geminin targeting to the centrosome is dependent on microtubules.
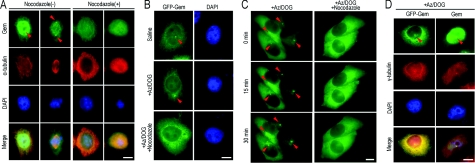
Figure 6. Dynein–dynactin-mediated centrosomal targeting of geminin is dependent on microtubules.
(A) Geminin accumulation on centrosomes depends on microtubules. Before the cells were fixed, nocodazole (20 μM) was added to the medium and cells were further incubated for 30 min. Co-immunofluorescence staining of geminin (Gem; green) and α-tubulin (red) was performed to examine altered localization of endogenous geminin when microtubules were depolymerized by nocodazole. As indicated with the arrowheads, centrosomal enrichment of geminin was inhibited after nocodazole treatment. Scale bar=10 μm. (B) Geminin accumulated on centrosomes after cellular ATP reduction. An ATP-inhibitor assay was performed as described in the Material and methods section. HeLa cells were transfected with GFP–geminin (GFP-Gem) and treated with either saline/glucose or saline/glucose plus 5 mM Az and 1 mM DOG and processed for microscopy. As indicated with arrowheads, after incubation for 30 min in Az/DOG, the accumulation of GFP–geminin on centrosomes was enhanced. When microtubules were depolymerized with 20 μM nocodazole, centrosomal enrichment of geminin was inhibited even in the presence of Az/DOG. (C) Live-cell analysis of geminin on centrosomes after Az/DOG treatment. Cells were imaged at 0, 15 and 30 min after Az/DOG was added to the medium. As indicated with arrowheads, GFP–geminin gradually accumulated on centrosomes. For microtubule-depolymerization assays, 20 μM nocodazole was added to the medium 30 min before Az/DOG treatment. The centrosomal localization of GFP–geminin was disrupted in the present of Az/DOG. Scale bar=10 μm. (D) Co-immunofluorescence staining of geminin (Gem) and γ-tubulin (red) showed geminin enriched on centrosomes after cellular ATP induction. The altered localization of endogenous geminin (right-hand panels) was consistent with that of GFP–geminin (GFP-Gem; left-hand panels) under the same treatment. Scale bar=10 μm.
Dynein is a microtubule minus-towards transport motor protein. It has been reported that dynein has good motility at low ATP concentrations at 5–10% of the normal 2–3 mM cellular ATP concentration, which is induced by chemicals such as Az (sodium azide) and DOG (2-deoxyglucose), whereas many other ATPases (such as microtubule plus-end transport motor proteins) lost the majority of their activity under the same conditions (Paschal and Vallee, 1987; Howell et al., 2001). Kinetochore components, such as Mad2 (mitotic arrest deficient 2), BubR1 [Bub (budding uninhibited by benomyl)-related 1] and CENP-E (centrosomal protein E), would be targeted on to the spindle poles through the dynein–dynactin complex after ATP reduction (Howell et al., 2001). If geminin was transported to the centrosome through the dynein–dynactin complex, the centrosomal concentration of geminin would be enhanced after cellular ATP reduction when microtubule plus-towards transport was impaired. Consistent with our hypothesis, after incubation with 5 mM Az and 1 mM DOG for 30 min at 37°C, in which cellular ATP was reduced to 5–10% of normal levels, both endogenous geminin and GFP–geminin were enriched at the centrosome in interphase cells (Figures 6B, 6C and 6D, indicated by arrowheads). As dynein is a minus-microtubule transport motor protein, the pathway would be interrupted when microtubules were depolymerized. Nocodazole was added into the medium for 30 min to depolymerize the microtubules before treatment with Az/DOG. We then observed that geminin was no longer enriched on centrosomes when the cells were subjected to treatment with Az/DOG (Figure 6B, indicated with arrowheads). Furthermore, we used real-time fluorescence microscopy to elucidate the dynamic behaviour of the centrosomal localization of geminin. Live-cell analysis also demonstrated that geminin concentrated on centrosomes was enhanced after ATP reduction and disassociated from centrosomes when microtubules were depolymerized (Figure 6C, indicated with an arrowhead). Our results demonstrated that centrosomal targeting of geminin was transported by the dynein–dynactin complex.
Taken together, these observations suggest that the centrosomal localization of geminin is dependent on the dynein–dynactin transport pathway mediated by Arp1 along microtubules.
The coiled-coil motif is required for the centrosomal localization of geminin and the interaction with Arp1
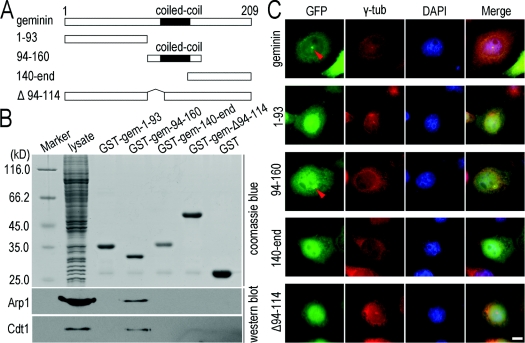
Geminin obtains its activity through a parallel coiled-coil homodimer, and point mutations that disrupt the dimerization abolish its interaction with Cdt1 and inhibition of replication (Saxena et al., 2004). To determine whether the coiled-coil motif is required for geminin binding with Arp1, four truncated geminin mutants were constructed (Figure 7A). In an in vitro pulldown assay, geminin mutants fused with GST proteins were purified and coupled on to glutathione–Sepharose 4B beads and were incubated with the lysate of HeLa cells for 2 h. The proteins on the beads were separated on SDS/PAGE (10% gel) and were transferred on to a nitrocellulose membrane and probed with an anti-Arp1 antibody. We found that only the GST–gem-94–160 mutant, which contains the coiled-coil motif, interacted with Arp1 (Figure 7B), whereas the depletion mutant GST–gem-Δ94–114, which has lost amino acid residues 94–114, did not interact with Arp1. As shown above, geminin recruitment to centrosomes was mediated by Arp1, and we therefore proposed that the coiled-coil motif may be required for centrosomal localization of geminin. Truncated mutants of geminin, amino acids 1–93, amino acids 94–160, amino acid 140–end and Δ94–114, each fused with a GFP tag were transfected into HeLa cells and subjected to an ATP-inhibitor assay as described in the Materials and methods section. As shown in Figure 7(C), only the mutant containing the coiled-coil motif (GFP–gem-94–160) was markedly recruited on to centrosomes after cellular ATP reduction. Mutants without the coiled-coil motif (GFP–gem-1–93 and GFP–gem-140–end) or lacking an intact coiled-coil motif (GFP–gem-Δ94–114) were unable to concentrate on centrosomes (Figure 7C). These results indicated that the coiled-coil motif is required for the geminin interaction with Arp1 and for the centrosomal localization of geminin.
Figure 7. The coiled-coil motif is required for geminin centrosomal localization and interaction with Arp1.
(A) Schematic diagrams of geminin and its truncated mutants. (B) The coiled-coil motif is required for geminin to interact with Arp1. For in vitro pulldown assays, four mutants of GST–geminin were purified and coupled on to glutathione–Sepharose 4B beads and incubated with the lysate of HeLa cells for 2 h. The proteins on the beads were separated on SDS/PAGE (10% gel) and subjected to Western blot analysis using antibodies against Arp1. Cdt1 was immunoprobed as a positive control. Coomassie Blue staining of the proteins represents the protein loading in the pulldown assay (upper panel). The molecular mass in kDa is indicated on the left-hand side of the gel. (C) The coiled-coil motif is required for centrosomal localization of geminin. HeLa cells were transfected with geminin or its mutants in the form of GFP-fusion proteins, and subjected to an ATP-inhibitor assay as described in the Materials and methods section. Only mutant 94–160 which contains the coiled-coil motif of geminin presents centrosomal enrichment in a similar manner to wild-type geminin. γ-Tubulin (γ-tub; red) was immunostained as a marker of centrosomes. Scale bar=10 μm.
Overexpression of geminin inhibits the centrosome over-duplication induced by HU (hydroxyurea)
To further investigate the role of geminin in centrosome duplication, we first silenced geminin with siRNA (small interfering RNA) to investigate whether knockdown of geminin resulted in centrosome over-duplication in MDA-MB-231 cells (Figures 8A and 8B), as reported in HCT116 and U2OS cells (Tachibana et al., 2005). MDA-MB-231, a cell line with a functional defective p53, similar to HCT116 (p53−/−) cells, have a defective G1/S checkpoint, and HU arrest in S-phase is capable of inducing centrosome over-duplication within one cell cycle (D'Assoro et al., 2004). In the present study, centrosome over-duplication was observed after introducing siGeminin into the MDA-MB-231 cells for 48 h (Figure 8A). In siGeminin-transfected cells, approx. 20% multiple centrosomes were observed, whereas in control cells only 5% multiple centrosomes were observed (Figure 8B). It was reported that loss of geminin leads to genome over-replication (Melixetian et al., 2004; Zhu et al., 2004; Machida and Dutta, 2007). If the centrosome over-duplication was induced by loss of geminin, but not DNA re-replication, overexpression of geminin might inhibit the centrosome over-duplication without DNA re-replication. It has been known that HU, an inhibitor of DNA synthesis, arrests cells at S-phase and leads to centrosome over-duplication without DNA re-replication (Figure 8C) (D'Assoro et al., 2004; Tachibana and Nigg, 2006). To test the effect of geminin on centrosome duplication, we transfected GFP–geminin into MDA-MB-231 cells pre-treated with HU. We observed that only 4% multiple centrosomes was observed in non-HU-treated control cells, whereas an 8-fold increase in multiple centrosomes was observed in HU-treated cells. In cells containing GFP–geminin, the ratio of over-duplicated centrosomes was greatly reduced to 5% (Figure 8E), which is near to the normal level. Figure 8(D) shows Western blot analysis performed to detect the expression levels of GFP–geminin and its mutants. It seems that the level of exogenous geminin is 10-fold greater than that of endogenous geminin. The results of the present study were consistent with the results from cell lines HCT116 and U2OS cells (Tachibana and Nigg, 2006).
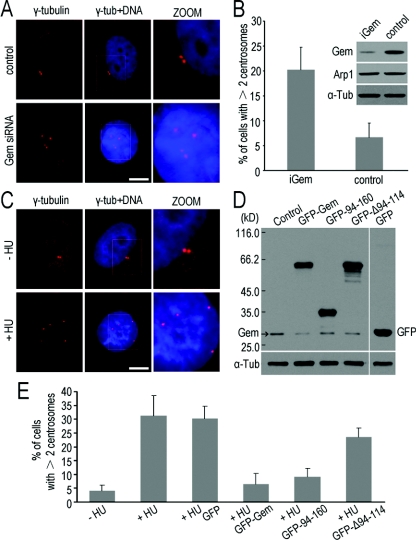
Figure 8. Geminin regulates centrosome duplication in MDA-MB-231 cells.
(A) siRNA of geminin resulted in centrosome over-duplication in MDA-MB-231 cells. Cells were transfected with siRNA targeting geminin (Gem siRNA) or non-specific siRNA as a control. Immunofluorescence staining of γ-tubulin (red) showed that the centrosomes were over-duplicated in geminin siRNA cells. Scale bar=10 μm. The cells with supernumerary centrosomes were counted and are shown in (B). The efficiency of geminin-knockdown was detected by Western blot analysis and is shown on the top right panel in (B). (C) Centrosomes were over-duplicated after being treated with HU in MDA-MB-231 cells. MDA-MB-231 cells were treated with/without 4 mM HU for 48 h and then immunofluorescence staining of γ-tubulin was performed to observe centrosomes. Scale bar=10 μm. (D and E) The coiled-coil motif is required for geminin to inhibit centrosome over-duplication in HU-arrested MDA-MB-231 cells. MDA-MB-231 cells were treated with 4 mM HU for 12 h and then transfected with geminin and its mutants as a GFP-fusion protein and cultured for another 48 h in the presence of HU to maintain the cells in S-phase. Cells were then either processed for immunofluorescence staining of γ-tubulin to observe centrosomes or Western blotted using anti-geminin or anti-GFP antibodies to exam the level of GFP-fusion proteins of geminin and its mutants. Note that over-duplication of centrosomes induced by HU was inhibited by overexpression of mutant 94–160, which contains the coiled-coil motif, as well as wild-type geminin. These results indicated that the coiled-coil motif is required for geminin to inhibit centrosome over-duplication. Supernumerary centrosomes were counted (E) and error bars represent 1 S.D.
To explore whether centrosomal localization is required for geminin inhibition of centrosome over-duplication induced by HU, mutants of geminin were introduced into MDA-MB-231 cells with HU-treatment. Expression of proteins was measured by immunoblotting using an anti-geminin antibody (Figure 8D). Only mutant GFP–gem-94–160 showed centrosome over-duplication inhibitor activity in a similar manner to wild-type geminin, whereas mutant GFP–gem-Δ94–114, lacking the intact coiled-coil motif, did not (Figure 8E). As mutant GFP–gem-94–160 contains the complete coiled-coil motif, we conclude that the coiled-coil motif of geminin is a prerequisite for the geminin interaction with Arp1 and centrosomal localization, and also for the inhibitor activity of centrosome over-duplication. These results suggest that the centrosomal localization of geminin defines an important role in centrosome duplication.
Discussion
DNA replication and centrosome duplication are two parallel key events for cell proliferation. Any regulation and co-ordination failure of these events would be a disaster to the cell life, or lead to genome instability. Eukaryotic cells have developed an elaborate mechanism to co-ordinate DNA replication and centrosome duplication and to limit the two key events to occur only once in one cell cycle (Hinchcliffe and Sluder, 2001; Bell and Dutta, 2002), although the detail of the mechanism is unclear. DNA replication is initiated by DNA replication licensing of the pre-RC and is tightly regulated by the DNA replication inhibitor geminin and oscillations of CDKs. Depletion of geminin not only leads to genome amplification (Melixetian et al., 2004; Zhu et al., 2004; Machida and Dutta, 2007), but also centrosome over-duplication and subsequent mitotic defect (Tachibana et al., 2005). Overexpression of geminin inhibits centrosome over-duplication caused by HU treatment (Tachibana and Nigg, 2006). It was also reported that Orcs and MCMs, members of the pre-RC, localize to the centrosome (Stuermer et al., 2007). But the possible direct interaction between geminin and centrosomal localization proteins is still unidentified. In the present study, we directly demonstrated that a fraction of geminin is physically localized to centrosomes. Through an ATP-inhibitor assay and disruption of the function of the dynein–dynactin complex by overexpressing its specific inhibitor dynamitin/p50, we further demonstrated that the centrosomal geminin fraction is transported along with microtubules by the dynein–dynactin complex, and the transportation mechanism is conservative, at least in mammalian cells.
Arp1 is a multifunctional protein involved in various cellular events. It is a subunit of the dynactin complex, and facilitates the centrosomal accumulation of some centrosome-associated proteins conducted by dynein–dynactin. It has also been proposed that Arp1 serves as a linker between the cargo protein and dynein–dynactin complex during the transportation of the cargo proteins to the centrosomes (Schroer et al., 1996). In the present study, we observed that geminin and Arp1 associated with each other and were co-localized on centrosomes. Functional disruption of the dynein–dynactin complex by overexpressing dynamitin/p50 not only resulted in disassociation of geminin, but also Arp1 from the centrosomes. These results might suggest that the centrosomal geminin is transported to centrosomes by the dynein–dynactin complex mediated by Arp1.
The coiled-coil motif of geminin is required for its dimerization and interaction with Cdt1 and inhibition of DNA replication (Benjamin et al., 2004; Saxena et al., 2004). The results of the present study show that the coiled-coil motif is also required for the geminin interaction with Arp1 and its centrosomal localization. The coiled-coil-motif-mediated centrosomal localization of geminin is also required for its inhibition of centrosome over-duplication. We propose that geminin plays a different role through the coiled-coil motif and might be a linker between DNA replication and centrosome duplication.
Taken together, the present study raised a notion that geminin plays roles in the cell cycle, not only preventing DNA over-replication through an extensively elucidated DNA replication inhibitor to inhibit Cdt1, but also prohibiting centrosome over-duplication possibly through interfering locally with the function of one or more centrosomal components, which is so far uncharacterized. We propose that, similar to the regulation by geminin of DNA replication initiation, the regulation by geminin of centrosome duplication is achieved through transportation of a fraction of this protein to the duplicating centrosomes to bind and inhibit the centrosome duplication initiator(s) once the centrosome duplication is initiated. However, the coiled-coil domain is a kind of domain which meditates potential protein–protein interactions, the detailed mechanism of interaction between geminin and Arp1 is still to be clarified, and the downstream targets need to be identified. Furthermore, the final characterization of the initiator(s) is needed for finally solving the mechanism of centrosome duplication regulation by geminin. Although the regulation of centrosome duplication has not yet been rigorously defined, our results favour an hypothesis that geminin might directly co-ordinate the two key events of DNA replication and centrosome duplication.
Materials and methods
Plasmids and antibodies
Human geminin (GenBank® assession number: NM_015895) and Arp1 (GenBank® assession number: NM_005736) were cloned from a cDNA library using RT (reverse transcriptase)–PCR and subcloned into pET28a for protein purification and pEGFPC2 for transfection. All sequences were confirmed by DNA sequencing. Primers for geminin are: sense, 5′-CCCGAATTCATGAATCCCAGTAT-3′ (EcoRI site underlined) and antisense, 5′-GGGGTCGACTCATATACATGGCTTT-3′ (SalI site underlined). Primers for Arp1 are: sense, 5′-TCCGAATTCCTATGGAGTCCTACGATGA-3′ (EcoRI site underlined) and antisense, 5′-CGGCTCGAGTTAGAAGGTTTTTC-3′ (XhoI site underlined). Recombinant His-tagged geminin was expressed in bacteria BL21 and purified with Ni-NTA (Ni2+-nitrilotriacetate) resin (Qiagen) and rabbits were immunized to raise antiserum. Antibodies were purified from the antiserum by affinity chromatography with HBr-activated Sepharose 4B (Amersham Biosciences) coupled with His-tagged geminin following the manufacturer's protocol. Anti-Arp1, α-tubulin and γ-tubulin antibodies were purchased from Sigma–Aldrich. An anti-Cdt1 antibody was purchased from Bethyl Laboratories. FITC- and TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated donkey anti-mouse IgG, and FITC- and TRITC-conjugated donkey anti-rabbit IgG were purchased from Jackson Laboratories.
Cell culture and transfection
Cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Invitrogen) supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C, 5% CO2. For transfection, HeLa cells were plated and cultured in 35-mm culture dishes for 20 h and transfected with recombined plasmid using a standard calcium phosphate transfection protocol. MDA-MB-231 and MEF cells were transfected with a pEGFP-P50 vector using Lipofectamine™ 2000 (Invitrogen) following the manufacturer's protocol.
For siRNA transfection, siRNA oligonucleotides targeting geminin were chemically synthesized as described previously (Tachibana et al., 2005) and transfected into MDA-MB-231 cells using Lipofectamine™ 2000.
Immunofluorescence microscopy
Cells were cultured on cover slides in 35-mm dishes for 24 h before fixation. Cells were fixed with ice-cold methanol for 5 min, and then the cells were directly incubated with the indicated primary antibodies overnight at 4°C. After rinsing with PBS, the cells were incubated with the fluorescence-labelled secondary antibodies for 1 h at room temperature (23°C). The cover slides were mounted with mowiol (Sigma–Aldrich) containing 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole; Sigma–Aldrich) for DNA staining. Images were captured on a Zeiss Axioskop fluorescence microscope (Axiovert 200M) coupled with a cooled charge-coupled device camera (AxioCam MRM, Zeiss) and were processed using the AxioVision program.
Protein Co-IP and pulldown assays
For protein Co-IP, asynchronized cells were suspended in IP buffer [0.5% Nonidet P40, 50 mM Hepes (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.5 mM sodium orthovanadate, 1 mM PMSF, 10 μg/ml aprotinin and 5 μg/ml pepstatin A] on ice for 15 min and centrifuged at 15500 g for 15 min. The supernatant was incubated with protein-A beads coupled with the indicated antibodies for 2 h at 4°C. The beads were recovered and washed with IP buffer and harvested by brief centrifugation and suspended in SDS-sample buffer. Pulldown assays were performed as described previously (Sugimoto et al., 2004). Asynchronized cells were suspended in pulldown assay buffer [20 mM Tris/HCl (pH 7.4), 0.05% Triton X-100, 5% glycerol, 150 mM NaCl, 1 mM DTT (dithiothreitol), 0.5 mM sodium orthovanadate, 1 mM PMSF, 10 μg/ml aprotinin and 5 μg/ml pepstatin A] on ice for 20 min and centrifuged at 15500 g for 15 min. The supernatant was incubated with glutathione–Sepharose 4B beads coupled with GST–geminin or GST–geminin mutants and GST protein respectively for 2 h at 4°C. The beads were recovered and washed with pulldown assay buffer and harvested by brief centrifugation and suspended in SDS-sample buffer.
Centrosome isolation
Centrosome isolation from cultured cells was performed as described previously (Mitchison and Kirschner, 1986). Briefly, 1 h before harvest, HeLa cells were incubated with 0.2 μM nocodazole and 1 μg/ml cytochalasin D at 37°C. Cells were then in turn washed with TBS [10 mM Tris/HCl (pH 7.4) and 150 mM NaCl] and 1/10 TBS/8% (w/v) sucrose, and then lysed in buffer A [1 mM Hepes (pH 7.2), 0.5% Nonidet P40, 0.5 mM MgCl2, 1 mM 2-mercaptoethanol, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin A] and centrifuged at 2500 g for 10 min to remove cell debris, nuclei and chromatin. The lysate were filtered through a nylon mesh (125 μm) and adjusted to a final concentration of 10 mM Hepes. The sample was treated with 2 units/ml DNase I for 30 min on ice and centrifuged with a 60% (w/v) sucrose cushion at the bottom of centrifugation tube for 30 min at 10000 g. The centrosome-containing sucrose cushion was transferred into a Beckman polyallomer centrifuge tube previously padded with a discontinuous sucrose gradient buffer consisting of 100 μl of 70% (w/v) sucrose solution, 60 μl of 50% (w/v) sucrose solution and 60 μl of 40% (w/v) sucrose solution and centrifuged at 35000 rev./min for 1 h (TLS55 rotor; Beckman Optima™). The sedimented centrosomes in the sucrose solution were divided into equal volumes of 100 μl. SDS sample buffer (6×) was added and the centrosomal components were separated on SDS/PAGE (10% gel), followed by Western blot analysis.
ATP-inhibitor assay
An ATP-inhibitor assay was performed as described previously (Howell et al., 2001). Briefly, cells were rinsed in saline [140 mM NaCl, 5 mM KCl, 0.6 mM MgSO4, 0.1 mM CaCl2, 1 mM Na2HPO4, and 1 mM KH2PO4 (pH 7.3)] to remove culture medium and placed in either saline/glucose (saline with 4.5 g/l D-glucose) or saline/glucose plus 5 mM Az and 1 mM DOG for 30 min at 37°C. Cells were then processed for microscopy. For the microtubule-depolymerization assays, 20 μM nocodazole was added to the medium 30 min before Az/DOG treatment.
Online data
Acknowledgments
We thank Dr Hui Zhang (Nevada Cancer Institute, Las Vegas, Nevada, U.S.A.) for his critical reading and constructive suggestion for this work, and other members in our laboratory for helpful comments prior to submission.
Funding
This work was supported by funds from the National Natural Science Foundation of China [grant numbers 30721064, 30225016, 30330200], the National Basic Research Programme of China [grant numbers 2004CB720003, 2006CB0D0100] and the National Key Scientific Programme of China [grant number 2007CB914502].
References
- Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- Benjamin J.M., Torke S.J., Demeler B., McGarry T.J. Geminin has dimerization, Cdt1-binding, and destruction domains that are required for biological activity. J. Biol. Chem. 2004;279:45957–45968. doi: 10.1074/jbc.M407726200. [DOI] [PubMed] [Google Scholar]
- Chong J.P., Mahbubani H.M., Khoo C.Y., Blow J.J. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- Clark S.W., Meyer D.I. Centractin is an actin homologue associated with the centrosome. Nature. 1992;359:246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- D'Assoro A.B., Busby R., Suino K., Delva E., Almodovar-Mercado G.J., Johnson H., Folk C., Farrugia D.J., Vasile V., Stivala F. Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene. 2004;23:4068–4075. doi: 10.1038/sj.onc.1207568. [DOI] [PubMed] [Google Scholar]
- Fouquet J., Kann M., Soues S., Melki R. ARP1 in Golgi organisation and attachment of manchette microtubules to the nucleus during mammalian spermatogenesis. J. Cell Sci. 2000;113:877–886. doi: 10.1242/jcs.113.5.877. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe E.H., Sluder G. ‘It takes two to tango’: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 2001;15:1167–1181. doi: 10.1101/gad.894001. [DOI] [PubMed] [Google Scholar]
- Howell B.J., McEwen B.F., Canman J.C., Hoffman D.B., Farrar E.M., Rieder C.L., Salmon E.D. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.J., Schroer T.A. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Liu E., Li X., Yan F., Zhao Q., Wu X. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J. Biol. Chem. 2004;279:17283–17288. doi: 10.1074/jbc.C300549200. [DOI] [PubMed] [Google Scholar]
- Machida Y.J., Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano D., Moreau J., Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- McGarry T.J., Kirschner M.W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Melixetian M., Ballabeni A., Masiero L., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K. Loss of geminin induces rereplication in the presence of functional p53. J. Cell Biol. 2004;165:473–482. doi: 10.1083/jcb.200403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Heald R., Samejima K., Earnshaw W.C., Cleveland D.W. Formation of spindle poles by dynein/dynactindependent transport of NuMA. J. Cell Biol. 2000;149:851–862. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T.J., Kirschner M.W. Isolation of mammalian centrosomes. Methods Enzymol. 1986;134:261–268. doi: 10.1016/0076-6879(86)34094-1. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Taraviras S., Lygerou Z., Nishimoto T. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 2001;276:44905–44911. doi: 10.1074/jbc.M105406200. [DOI] [PubMed] [Google Scholar]
- Paschal B.M., Vallee R.B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Saxena S., Yuan P., Dhar S.K., Senga T., Takeda D., Robinson H., Kornbluth S., Swaminathan K., Dutta A. A dimerized coiled-coil domain and an adjoining part of geminin interact with two sites on Cdt1 for replication inhibition. Mol. Cell. 2004;15:245–258. doi: 10.1016/j.molcel.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Schroer T.A., Bingham J.B., Gill S.R. Actin-related protein 1 and cytoplasmic dynein-based motility: what's the connection? Trends Cell Biol. 1996;6:212–215. doi: 10.1016/0962-8924(96)20014-5. [DOI] [PubMed] [Google Scholar]
- Stuermer A., Hoehn K., Faul T., Auth T., Brand N., Kneissl M., Putter V., Grummt F. Mouse pre-replicative complex proteins colocalise and interact with the centrosome. Eur. J. Cell Biol. 2007;86:37–50. doi: 10.1016/j.ejcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Sugimoto N., Tatsumi Y., Tsurumi T., Matsukage A., Kiyono T., Nishitani H., Fujita M. Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. J. Biol. Chem. 2004;279:19691–19697. doi: 10.1074/jbc.M313175200. [DOI] [PubMed] [Google Scholar]
- Tachibana K.E., Nigg E.A. Geminin regulates multiple steps of the chromosome inheritance cycle. Cell Cycle. 2006;5:151–154. doi: 10.4161/cc.5.2.2363. [DOI] [PubMed] [Google Scholar]
- Tachibana K.E., Gonzalez M.A., Guarguaglini G., Nigg E.A., Laskey R.A. Depletion of licensing inhibitor geminin causes centrosome overduplication and mitotic defects. EMBO Rep. 2005;6:1052–1057. doi: 10.1038/sj.embor.7400527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Knapp D., Nasmyth K. Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi: 10.1016/s0092-8674(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel J.A., Dwyer B.T., Dhar S.K., Cvetic C., Walter J.C., Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- Xiang X., Han G., Winkelmann D.A., Zuo W., Morris N.R. Dynamics of cytoplasmic dynein in living cells and the effect of a mutation in the dynactin complex actin-related protein Arp1. Curr. Biol. 2000;10:603–606. doi: 10.1016/s0960-9822(00)00488-7. [DOI] [PubMed] [Google Scholar]
- Xouri G., Squire A., Dimaki M., Geverts B., Verveer P.J., Taraviras S., Nishitani H., Houtsmuller A.B., Bastiaens P.I., Lygerou Z. Cdt1 associates dynamically with chromatin throughout G1 and recruits geminin onto chromatin. EMBO J. 2007;26:1303–1314. doi: 10.1038/sj.emboj.7601597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi K., Mizuno T., You Z., Hanaoka F. Mouse geminin inhibits not only Cdt1–MCM6 interactions but also a novel intrinsic Cdt1 DNA binding activity. J. Biol. Chem. 2002;277:40871–40880. doi: 10.1074/jbc.M206202200. [DOI] [PubMed] [Google Scholar]
- Yankulov K., Todorov I., Romanowski P., Licatalosi D., Cilli K., McCracken S., Laskey R., Bentley D.L. MCM proteins are associated with RNA polymerase II holoenzyme. Mol. Cell. Biol. 1999;19:6154–6163. doi: 10.1128/mcb.19.9.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Chen Y., Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol. Cell. Biol. 2004;24:7140–7150. doi: 10.1128/MCB.24.16.7140-7150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.